Review Article, J Immunol Tech Infect Dis Vol: 2 Issue: 4
38 Years of Hybridoma Technology as a Source of Analytical Tools for Immunodiagnostics of Infectious Diseases. Some Contributions Developed in Cuba to Improve the Original Procedures and Future Prospects
| Anselmo J Otero-González1*, Irelio Rodríguez-Matheu2, Octavio Luiz Franco3 and Jorge Sarracent-Pérez4 |
| 1Center of Protein Studies, Faculty of Biology, Havana University, Havana City, Cuba |
| 2Department of animal cell culture, National Centre for Scientific Research, Havana City, Cuba |
| 3Center for Proteomic and Biochemical Analysis, Postgraduate Genomic and Biochemical Sciences, Catholic University of Brasília, Brasília-DF, Brazil |
| 4Vice-direction of Parasitology, “Institute of Pedro Kourí” of Tropical Medicine, Havana City, Cuba |
| Corresponding author : Anselmo J Otero-González, Mic, PhD, ScD Center of Protein Studies, Faculty of Biology, Havana University, Calle 25 entre J e I. Vedado, Plaza, Havana City, Cuba Tel: (537) 832 4830 Fax: (537) 832 1321 E-mail: aoterog@infomed.sld.cu |
| Received: August 25, 2013 Accepted: October 22, 2013 Published: October 25, 2013 |
| Citation: Otero-González AJ, Rodríguez-Matheu I, Franco OL, Sarracent-Pérez J (2013) 38 Years of Hybridoma Technology as a Source of Analytical Tools for Immunodiagnostics of Infectious Diseases. Some Contributions Developed in Cuba to Improve the Original Procedures and Future Prospects. J Immunol Tech Infect Dis 2:4. doi:10.4172/2329-9541.1000117 |
Abstract
38 Years of Hybridoma Technology as a Source of Analytical Tools for Immunodiagnostics of Infectious Diseases. Some Contributions Developed in Cuba to Improve the Original Procedures and Future Prospects
In 1975 George Köhler and César Milstein, with a remarkable article on hybridoma technology published in Nature, opened a new era in biology with enormous implications for analysis in biomedicine and biotechnology. It remains unquestionably important nowadays and it is basically a multifaceted methodology including procedures for antigen preparation, immunization, somatic cell fusion and cloning, cryopreservation, immunochemical screening, functional and biochemical characterization and industrial scaling up. Over the years, some improvements in high efficiency monoclonal antibody generation and production have been achieved all over the world. In this review some contributions to this technology developed in Cuba from 1982 and 2000 are re-visited and updated.
Keywords: Hybridoma technology; Impact and improvements; Monoclonal antibodies
Keywords |
|
| Hybridoma technology; Impact and improvements; Monoclonal antibodies | |
Introduction |
|
| It is more than three and a half decades since George Köhler and César Milstein, with their seminal article published in Nature on August 7, 1975 [1], opened up new vistas in biology with enormous implications for analysis in biomedicine and biotechnology at that time, since then, and into the future. While there was evidence of cells similar to those later known as hybridomas [2,3] it was not until that moment that a practical, reproducible and efficient procedure to generate continuous cell lines producing preconceived specificity antibodies had been described. Monoclonal antibodies (Mabs) have already started a real revolution in areas such as detailed antigenic characterization, detection and isolation of antigens in complex mixtures, the elucidation of mechanisms of action, as well as numerous applications in immunodiagnostics, immunotherapy and imaging [4]. But without any doubts one of more remarkable and significant impacts of monoclonal antibody irruption is in the area of detection and control of infectious agents (Figure 1). | |
| Figure 1: Contribution of monoclonal antibodies generated by hybridoma technology to immune detection and monitoring of infectious agents and/or its metabolic products by 3rd generation immunoassays. | |
| As it was realized later, although it could be inferred from the trajectory and the line of thinking of the authors, the result they achieved was not the originally intentioned. They were awarded the Nobel Prize in Physiology and Medicine in 1984 (shared with Niels Jerrne) for the description of the basis of hybridoma technology and its global impact for biomedicine. Milstein has stated that he firmly believes that if in one corner of the laboratory they had not been studying the system of immune genes in mutant plasmacytomas and in the opposite corner the expression of the diversity of antibodies on lymphocytes in mice, it is unlikely that they would have developed the procedure that became such a new and important methodology for biology [5]. | |
| The basic technological antecedents of hybridoma production are found in the availability of experimental mouse plasmacytomas [6], in the effectiveness of a selective medium for removing non-hybridized cells [7] and in effective methodologies for the fusion of somatic cells [8]. | |
| This culture procedure, mixing primary with continue cell line, which requires experience and manual skills in animal cell culture, rapidly surpassed in scope the simple generation of Mabs, so that soon lymphokine secretor T hybridomas [9] and plasmacytomamacrophage hybrids expressing properties of phagocytic cells [10] were generated, as well as the possibility of generating hybridomas from other species such as rat and rabbit [11,12]. | |
| The discovery of the extraordinary potential of the technology of hybridomas resulted in the availability of large quantities of Mabs. For the first time these biological reagents could be standardized with constant structure and affinity. This goal was impractical with traditional polyclonal preparations, which have slowly been displaced in immunoassays, immunotherapy and affinity chromatography [13]. | |
| Monoclonal antibodies have helped to reveal the structural bases of the diversity of antibodies. Comparison of families of immunoglobulins derived from a simple gene line showed the importance of somatic mutations in antibody class diversity and specificity [14]. | |
| There is no doubt that another of the most spectacular uses of Mabs is taking place in the diagnosis and treatment of cancer, whether of murine, human, chimerical or recombinant origin. This battery of monoclonals has been used as serological and immunohistological markers, for radiolocation, for in vitro treatment of bone marrow (grafting of “healthy” cells) and serotherapy with immunotoxins, among other uses [15]. | |
| In Cuba a remarkable effort has been made since 1982 for generating useful mouse monoclonal antibodies by the hybridoma technology for diagnostic, therapeutic and imaging purposes [16-19]. | |
| The technology of hybridomas is multifaceted and includes procedures for preparing antigens (purification), immunization, fusion of somatic cells in mixed culture and cell cloning (both difficult and ponderous, requiring much practical skill and knowledge of cell culture), cryopreservation, immunochemical, biochemical and functional screening (passing through the purification of antibodies) for characterization and finally, the scaling up of cellular production for industrial fermenters. | |
| There is a continuous process of improvement of the overall methodology, which has seen the replacement of the original plasmacytoma secretory cell line [1] by one in which the secretion of immunoglobulins has been suppressed and the replacement of the Sendai virus by polyethylene glycol as fusogenic agent [20]. In this paper we re-visit and update some of the contributions made in Cuba in the first 20 years of use of this technology to generate Mabs with biomedical and biotechnological application and present our point of view as to the immediate future of these procedures known as hybridoma technology. For this purpose we would like to sub divide the technology on some of its components: | |
| Cell culture optimization | |
| Plasmacytoma aptitude for fusion experiments: Key actors in this technology are the parental tumor cell lines directly responsible for the proliferative immortality and large antibody production of generated hybridomas. The fact that multiple myeloma disease was explained as a neoplasm of antibody-producing cells, and that each tumor is the result of a population from a single cell secreting an antibody with unique idiotypic specificity was the basis for these populations to be selected in the 1960s to investigate the structure of antibodies originating the famous Potter mouse myeloma for the NIH myeloma induction program in USA [6]. For many years it was impossible, in a practical way, to induce specific antibody-secreting tumors by immunizing mice because malignancy is a random event and therefore generation of specific cell line in this way is very rare [21]. | |
| The physiological condition of cells involved in the generation of hybridomas conferring essential proliferative capacity on them must be taken into account for successful cell fusion. The spleen lymphoblast from immunized animals, as one of the parent components in fusion, are usually freshly harvested primary cells and must therefore preserve the proliferative ability which they had in the natural environment from which they were obtained. This condition is closely related to the concept of viability, as the ability to produce cell colonies [22]. | |
| Plasmacytomas are currently kept frozen, and they are thawed and reentered in culture condition few days before a cell fusion experiment. There is a common tendency to sub-cultivate plasmacytoma cells to be used in a fusion experiment by growing them as ascites in the peritoneum of isogenic mice with the aim of increasing its viability and its fusion capacity [23]. This procedure also virtually eliminates any underlying mycoplasma infection, which is completely incompatible with the generation of the hybridomas by the abduction of nucleotide precursor. A simple and popular procedure is thawing the plasmacytoma three days prior to the fusion experiment and changing the medium 24 hours before use, to ensure an appropriate “exponential phase” at the time of fusion [24]. In any case, it is reasonable to try to keep a culture of plasmacytomas in exponential growth phase, or close to it, as a criterion of physiological excellence, in order to achieve abundant and healthy hybridoma colonies. | |
| The exponential phase of growth is an event that was observed for the first time in agitated liquid cultures of micro-organisms and that has been mathematically characterized in its application for industrial cultures. In the case of almost synchronous unicellular growth and under unrestricted growth conditions different from those obtained by the depletion of substrate, the growth rate can be expressed on the basis of a limiting substrate as: dX/d/dT= f (X)(S) where X is the mass of cells per unit of volume and S, the concentration of the limiting substrate. The specific growth rate is defined by μ=x2 dX/dt=d lnX/ dt which is called the equation of exponential growth, where μ is independent on the cell mass and that is constant during the exponential phase. In the middle of the 20th century Jacques-Lucien Monod brilliantly linked multi-enzymatic kinetics with cell kinetics on continuous microbial fermenter processes, with his famous equation: | |
| Μ=μmax(s/Ks + s). These are key parameters in continuous fermentation theory and practice [25]. | |
| Mammalian cells, because their origin, have a heterogeneous behavior for in vitro culture with regard to the individual cell size, natural growth asynchronies, and cell density dependent growth inhibition. Subsequently, mathematical modeling of its growth is complex and multifactorial. If it is assumed that at time t=0, all cells have the same individual size and T0 is the average generation time: | |
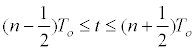 |
|
| The number of cells that will be found in the nth interval of division will be: | |
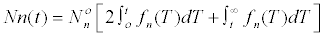 |
|
Where 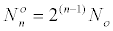 is the number of cells at the beginning of
the range. is the number of cells at the beginning of
the range. |
|
| Finally: | |
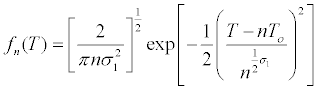 |
|
| In which it is assumed that the distribution of generation time is normal with an average T0 and the Fn (T) describes the frequency of cells dividing in an interval given in an initially synchronous population [26]. | |
| Regarding animal cells in culture and in particularly with mammalian cells, the existence of an exponential phase is a controversial topic, especially for static anchored or semi-adhesive cell cultures because of the number of cells that remain at a given time of growth in a state of a living but not growing quiescence. Currently, growth quickly leaves the exponential phase [27]. In the case of cells growing in suspension into bioreactors, like recombinant CHO cells, the situation is similar to what it happens with microorganisms in fermenters. Therefore the determination of the exact moment for the achievement of the exponential phase is crucial to estimate the scaling efficiency in productions of molecules associated with cell growth [28]. Viable cell counts, packed cell volume, ratio of intracellular nucleotides, analysis of cell cycle, the rate of oxygen intake on line and the optical density of the turbidity of the culture medium are currently used for the evaluation. | |
| In general, the concept of exponential growth in mammalian cells in cultivation is based on the apparent linearity of the semilogarithmic graphics of the number of cells (or cellular components directly related to the growth) and the time. This method of graphical analysis has been considered unreliable for estimating an actual exponential growth [29]. This subject has been re-examined with the much more sensitive method of Smith [30] in which the specific growth rate refers to the number of cells that, at any given time, is plotted against time. Exponential growth behaves, according to this treatment, as a horizontal line. From 125 sets of kinetic data treated with this procedure only 11 showed a real exponential phase, which exemplifies how rare this behavior is, particularly with adherent and semi-adherent mammal cell lines. Rather than speak of an exponential phase, it should be considered an exponential instant that will be highly dependent on the duplication time for mammalian cells in culture, which is between 10 and 24 hours. Therefore, if all environmental conditions are favorable it should be expected that this exponential moment could be achieved between 48 and 72 hours after seeding. The weakness of this approach is that a subsequent point in the curve kinetics is required to estimate the specific speed of growth, and therefore the horizontal behavior indicating the exponential growth. An event like this in which for a bacteria like E. coli can have 19 minutes as a generation time, for an animal cell culture, may easily require 24 hours for estimating the right moment of exponential behavior as a measurable point. It is in this situation in which a rapid and appropriate vital staining to estimate the number of cells produced in culture would be more practical for gauging the ideal condition of a cell population for a biotechnology action (a fusion experiment for example). | |
| The metabolic reduction of colored formazan tetrazolium salts (originally insoluble) has been used for many years for the histochemical location of enzymatic activity [31-33], and its colorimetric detection, after the appropriate extraction, has been used for the cell growth of microorganisms and higher cells [34-36]. | |
| In 1986 the use of 2-3-5 triphenyltetrazolium chloride (TTC) was introduced in the department of Animal Cell Culture, National Center for Scientific Research of Cuba (CNIC) [37] to estimate aptitude of plasmacytomas for somatic cell fusion experiments. At that time a soluble formazan derivative, MTT (bromide salt of 3-(4,5-dimetiltiazol-2-il)-2, 5-difenyltetrazolium) used to have three years published by Mosmann [38] as a simpler alternative to estimate the vitality of populations of animal cells. However, this reagent was not commercially available and its preparation in the laboratory was out of our abilities in chemical synthesis. In fact the procedure and commercial kit to evaluate the animal cell proliferation based in MTT reagent recommended by the American Type Culture Collection (ATCC) was not available until 1989. Later, more expensive derivatives appeared which produce directly soluble formazan in the culture medium, thus eliminating the need for an additional step for solubilizing [39]. | |
| While the procedure reported in the article by Otero et al. [37] was not new in its year of publication with respect to the dye, it was novel to evaluate the plasmacytoma physiological state and its associated capacity to generate viable hybridomas. Later innovations regarding the solubility of formazan reinforce the validity of the original intention, using tetrazolium salts to estimate the optimal condition of plasmacytomas used in the experiments of somatic fusion with the aim of obtaining monoclonal antibodies. | |
| Growth promotion of mouse hybridomas: Since the beginning of the 70s it has been well known that the basis of growth control of animal cells in vitro is related to phenomena such as cell anchorage to surfaces where they are grown, the dependency of factors present in the animal serum added to the culture medium, morphological changes associated with circumstantial or definitive stages and the role of calcium in the transformation of a given cell mass [40]. | |
| The original idea related to the importance of physical contact between cells to control the growth in vitro avoiding the overlapping of cell layers, a phenomenon that can also be seen in the natural tissue architecture, was rapidly surpassed when researchers understood the importance of soluble factors, which depend on cell density and whose controlling action could be reproduced with their addition even in early stages of the culture [41]. | |
| In the control of mammalian cell growth several polypeptides are involved, some of them, with hormonal action, as well as compounds of low molecular mass [42] all of them acting through specific receptors. During the 1980s several reports of factors that influenced the growth of the various normal or transformed cell types in culture appeared. In 1985 Arden and his colleagues found an appropriate cell system to detect what has was called hybridoma growth factor (HGF) [43]. It is based on a hybridoma cell line that is very sensitive to this factor and evaluated in a system of high density growth by the incorporation of tritiated thymidine. The HGF is produced in large quantities by the monocytes and macrophages and not by the T lymphocytes. It is a polypeptide of 26 KDa with an almost identical sequence to that of interferon ß2. HGF has almost 30% homology with Interleukin 1 and it has been found that the cells do not need to be stimulated for their segregation, but that they definitely require the presence of animal serum as an additive in the culture medium, which may be supplemented by purified bovine proteins but not by other proteins such as ovalbumin or gamma human globulin. The production of HGF in vitro begins two hours after inoculation and is completed within 24 hours of culture [44]. | |
| In the early 1980s, the laboratories in Cuba generating mouse Mabs were divided into two styles in how was the growth promotion of the generated hybridomas. The first one added a suspension of normal mouse spleen cells to the fused cells suspension and the second applied a mouse peritoneal macrophage feeder layer to the plates a day or a few hours before the fusion. This last variant has a number of advantages, such as: | |
| • Segregation of interleukins 1 and 6 by macrophages that promotes B cell growth during blastic transformation. | |
| • Phagocytosis of cellular debris resulting from the destructive of action of HAT selective medium. | |
| • Control of latent mycoplasma infection which is frequently asymptomatic and lethal for new hybridomas by capturing thymidine, a key reagent for the success of the selective system. | |
| However, this practice implies, in fact, an additional primary cell culture for an already expensive and complicated hybridoma technology that consumes animals, culture media, fetal bovine serum (FBS), laborious handling and, finally, the macrophage feeder layer is an additional source of contamination. | |
| For these reasons, the conditioned medium (TMCS) of transformed macrophages J774.A1 was selected for exploring if it was possible to replace such feeder layer as a growth promoting source for newly formed hybridomas and also as a supporting additive for cloning hybridomas by the limiting dilution technique [45]. | |
| The D4 system developed at the National Institute for Oncology and Radiobiology, Havana, Cuba [46] for the evaluation of mouse B cell growth factors is based on a hybridoma which is highly dependent on the human endothelial cell conditioned medium of (HECS) [47]. It shows a very effective response in the presence of this additive, which was used as a control in the experimental evaluation of our TMCS. | |
| The evaluation of TMCS kinetic by the cell line J774.A1 was assessed with and without the presence of fetal bovine serum in the culture medium with respect to the D4 system in high cell density. A maximal growth promotion activity was found at 48 hours after confluent monolayer independent of the presence or not of the FBS. This activity declined thereafter. This is important because it shows that the promotion is not due to remnants of the fetal serum used in the production of the promoter. | |
| This premise was the basis for the evaluation of TMCS in the absence of FBS. In order to discard the possible influence of FBS traces for achieving the macrophage confluence, the monolayer was washed exhaustively before starting the TMCS production for the next 48 hours. In the D4 system controlled with HECS, which is produced with 30% human serum, TMCS at 12.5% was more effective than the FBS10%, showing a characteristic of dose-effect behavior. | |
| The cloning capacity of TMCS at different concentrations in the D4 system at low cell density was expressed in terms of cloning efficiency on the of basis of FBS at 5% that, alone, was not capable of generates colonies. TMCS at low-density cell conditions was able to promote cell growth with a maximum at 48 hours after the confluence of macrophage monolayer at a concentration of 12.5%. The same design was applied for evaluating the growth promotion for a hybridoma that was unrelated to the D4 system when TMCS at 12.5% was added immediately after a cell fusion experiment. | |
| Finally, the effectiveness of TMCS to promote the growth of newly formed hybridomas was compared with the activity of a feeder mixture of mouse spleen cells. We found that 48 h- TMCS at 12.5% enhanced hybridoma growth as effectively as did the spleen cell mixture, with the advantage of using a liquid filtered supplement instead of an extra primary culture suspension. | |
| In 1985 we had two good reasons to suppose that the growth promotional effect of TMSC was not due to the hybridoma growth factor HGF (Il-6). Firstly Arden and co-workers found that the HGF is not produced by macrophages in the absence of bovine serum as it was in our case, and secondly, the promoter effect found for us in the TMCS was similar to that produced by purified mouse IL-1 in comparison to the HECS of endothelial cells [46]. | |
| At the same time Sugasawara and collaborators [48] had assessed the growth promotion of the conditioned medium of primarily cultivated macrophages and the supernatant of three lines of transformed macrophages lines, including J774, but they did not focus on the nature of the molecules involved. On the other hand, Rathjen and Geczy [49] confirmed the qualities of macrophage conditioned medium from this transformed line when they boosted their production with LPS. Years later, Liu et al. [50] shown that the requirement of FBS in the medium for the generation of hybridomas could be replaced entirely with 300 units/mL of HGF (IL-6). The use of purified Interleukin 6 (HGF) or Interleukin 1 (IL-1) as additive to promote growth, cloning, and expansion of hybridomas B of mouse in the culture medium is significantly expensive in comparison with the use of a liquid non-purified additive produced in absence of FBS and added at 12.5% as in the case of TMCS. The company Sigma Chemical Co. (USA) was offering, for many years, a product with the following description: ¨CONDITIONED MEDIUM FROM J774A.1CELLS, HYBRI-MAX¨. Cat Number M 8782, prepared using a mouse macrophage cell line, J774A.1, grown in IMDM with 2% FBS. The success of this product shows the relevance of this research by Otero et al. at the beginning of the 80s [45]. | |
| Primary screening of hybridoma supernatants | |
| The primary evaluation of cell culture supernatants from hybridomas depends on the detection of the desired specificities in 24 or at the most 48 hours so one can discard hybridoma producer colonies of no interest [51]. Regarding this matter, the following premise should be consistently observed. If analytical purposes are in mind, the screening method for investigating the desired specificities in hybridoma supernatants should be as close as possible to the analytical system to which the Mab (or Mabs) are intended. In other words, if you want to use a Mab in an ELISA type immunoassay, that antibody should be tested by an ELISA and not be screened by immunofluorescence or another technique far from this detection principle. | |
| High affinity antibodies should be available if a highly sensitive analytical test is needed (detectability). This circumstance must be taken into account in the design and implementation of the immunization protocol. On the other hand, the affinity of an antibody can be modified post - immunization, mimicking what the Immune System does in vivo through the mechanism of somatic hypermutation. This can be achieved by cloning and expressing the variable regions in filamentous phages especially designed to show the specific protein on its surface to be matched to the antigen. Running repeated sessions of antigenic confrontation has achieved a kind of selection in vitro in favor of variable regions with greater affinity [52]. The primary screening of the hybridomas can give a preliminary idea of the affinity of each immunoglobulin by testing serially diluted supernatants. | |
| An immunoassay to detect mouse Mabs in hybridoma supernatants should meet certain analytical requirements: | |
| a. Have enough sensitivity to detect antibodies in supernatants in a 100 μL hybridoma culture which means sub-microgram thirdgeneration immunoassays. Agglutination tests and others with low sensitivity are discarded. Other analytical qualities should be investigated in secondary analysis [53]. | |
| b. Be able to detect, at least, all subclasses of IgG using a conjugate with antibodies from another species that recognizes the whole molecule of mouse IgG. If IgM class Mabs are desired a enzyme conjugated with antibody anti-mouse μ chain should be used. | |
| c. Capability for automation in microtitre plates for automatic readers. Indirect immunofluorescence is an unavoidable exception for non-purified cell surface antigens. | |
| d. Use of appropriate analytical controls: | |
| • Positive: a similar Mab-producing hybridoma supernatant (epitope or antigen) if available, or mouse hyperimmune serum from the animal selected as spleen donor in the cell fusion experiment. | |
| • Negative: supernatant of hybridoma producing irrelevant Mab specificity or plasmacytoma culture supernatant. | |
| The Ultra Micro Analytical System (SUMA™, by its abbreviation in Spanish) was developed in Cuba in the 1980s [54] as a fluorescence ultra-micro immunoassay (10 μL) for the massive and quantitative screening of Alpha-Fetoprotein (AFP) in serum of pregnant women for the detection of fetal neural tube defects. Today its use has been extended to many applications [55]. The analytical platform of this sensitive immunoassay was appropriate for the screening of the first Mab generated against AFP in Cuba. | |
| This analytical micro system had, in 1982, a number of convenient aspects for its use as support for Mab screening: | |
| a. 10 μL of reaction volume which allows multiepitopic analysis or serial dilution with the same supernatant sample for the primary evaluation. | |
| b. Saving reagent usage (antigen and conjugate). | |
| c. Increased sensitivity by fluorimetric amplification which is very useful for detecting weak positive antibodies as well as a preliminary estimate of the affinity of the antibody before trials. | |
| d. Efficient automation inherent from the system. | |
| With these characteristics in mind and with the first results obtained with the use of SUMA system for screening Mabs [56] it became clear that even with the use of a highly demanding “cut off” value as a criterion of possibility (3 or 4 times the average of fluorescence of the negative controls) it was possible to discriminate weak positive supernatants. This consideration becomes relevant since it is common that a wide “grey area” in the primary search includes signals for samples in which the positivity is uncertain and requires costly confirmation (time and materials). This assay demonstrated that the negative samples were distributed closely around the level of “blank” and reasonably far from the value of the control sample considered as a weak positive. | |
| This assay was designed as a triple antibody ELISA because even though human alpha-fetoprotein is a globular structure, apparently not deformable when coated on polystyrene, a well characterized rabbit monospecific antiserum to AFP was available whose benefits of specificity and sensitivity were used as a reagent for capture. It should be noted, with regard to the accuracy, that this older SUMA system version used plates of polyvinyl chloride coated with polystyrene. Now the system uses ultramicro plates completely made from polystyrene [56]. | |
| The SUMA system for detecting Mabs allowed an initial discrimination of positive supernatants, which was subsequently confirmed in secondary screenings. This fact demonstrates the feasibility of the use of the SUMA platform for the design and implementation of ELISA immunoassays for the screening of monoclonal antibodies. It was the first implementation of the SUMA system in connection with hybridoma technology. | |
| Quantification of mouse monoclonal antibodies | |
| The quantification of monoclonal antibodies from hybridoma supernatants and ascitic fluids not only represents a parameter of production quality control but also an effective control of hybridoma clonality [57]. It has become an indispensable tool for the assessment of therapeutic antibodies, which are or will be administered to humans to find and destroy cancer cells, infectious agents, autoimmune or allergic lymphoid sub-populations as well as to produce the necessary immunosuppression for organ transplant procedures [58]. | |
| Indeed, the widespread use of hybridoma technology for the generation of monoclonal antibodies has created a need for simple, rapid, sensitive and specific methods for the quantification of immunoglobulins in hybridoma culture supernatants. An accurate determination of the level of antibodies is critical to understand the effect of the physical parameters on hybridoma growth and the segregation of immunoglobulins. This aspect is particularly important because of the widespread use of fermenters for the large scale production of Mabs in controlled culture media with or without animal serum. The determination of immunoglobulin concentration in supernatants is also necessary when trying to establish reproducible conditions in different immunoassays where the availability of antibody comes only from culture supernatants. The usual Mab concentration levels in supernatants for stationary cultures range from 5 to 50 μg/mL, depending on the clone and cell density. Bioreactors usually provide much higher concentrations of antibodies [59]. | |
| A particular hybridoma may lose its ability to express immunoglobulin which is clonally distributed in the original mouse repertoire and recovered by hybridoma technology. That loss can occur immediately after a certain number of subcultures. In some cases it becomes unproductive in only one generation. On the other hand, a mutant non-producing clone with higher growth rate can develop accumulating cell mass in further generations, with a consequent gradual loss in the productive capacity of the desired antibody. This will be expressed as a slow decreasing in the concentration of antibodies in the culture medium in a specified period of time. | |
| Most of the ELISA type immunoassays reported to quantify immunoglobulins of mouse IgG class [57,60-62] use antibodies of another species, specific for subclasses, thereby introducing the selection criterion within the quantification. Such subclass antispecies antibodies can be individually conjugated or revealed by a second antibody of another species conjugated to the enzyme. This feature makes them particularly expensive. | |
| The novelty of the sandwich ELISA to quantify mouse Mabs of IgG class reported by Vilaseca et al. [63] is that it does not require a different species antibody specific for each subclass as often found in commercial kits. They are usually more difficult to obtain because the immunization of rabbits, sheep or goats is needed with purified mouse IgG subclasses. A representative polyclonal preparation against the 4 main subclasses obtained from a hyper immune serum obtained by immunizing with whole mouse IgG is appropriate to develop this test. Of course it must be taken into account that standard curves must be set up with the monoclonal antibodies of each specific subclass because only the fraction of polyclonal antibodies that recognize the subclass of interest will act in the same way as for the correspondent standard antibody. In this way only monoclonal standard will be different for each subclass whereas coating and conjugated polyclonal antibodies are universal for each subclass determination. | |
| This approach is also appropriate when attempting to quantify antibodies in the serum of patients with autoimmune diseases or anticancer immunotherapy. Most of these antibodies are IgG class and subclasses 1, 2a and 2b for the particular test that is applicable, using the corresponding subclass in the standard curve. Such an assay was able to avoid interference with the naturally produced human serum proteins, including immunoglobulins, and to maintain the required sensitivity for a pharmacokinetic monitoring. | |
| Although the immunotherapy of cancer with Mabs has focused on the antibodies of the IgG class and its subclasses and fragments, it is possible that antibacterial, antifungal, anti-protozoa and antiviral immunotherapy will go through clinical trials that involve the use of antibodies for the mouse IgM class because of its multifaceted ability and power for multi-binding recognition. As an alternative, plant IgG recombinant fragments that are appropriate to achieve a rapid infiltration into tumors may not be sufficiently efficient to neutralize pathogens directly or trigger an appropriate biological activity. | |
| Human IgM has been quantified by a simple and robust assay that does not require sophisticated equipment and that can be used to diagnose sleeping sickness. A high concentration of IgM in the cerebrospinal fluid occurring due to a massive synthesis of intrathecal IgM is a marker for the diagnosis of meningoencephalitis caused by Trypanosoma [64]. An ELISA test has also been developed to study the distribution of native human IgM in tumor-xenografted SCID mice as an alternative to the conventional radioisotopic analytical techniques [65]. | |
| ZeptoMetrix Corporation from New York, USA offers an immunoassay type ELISA (IMMUNO-TEK) announced as fast and easy to determine IgM of mouse hybridoma supernatant, ascites and other biological fluids. It is particularly useful for monitoring the generation, production and purification of mouse monoclonal antibody in less than two hours, with a quantification range between 7.8 and 125 ng/mL. The conjugate is based on a polyclonal anti mouse IgM conjugated with horseradish peroxidase and tetramethylbenzidine as a co-substrate. | |
| The tests developed by Vilaseca et al. [63] and by Fraga et al. [66] offer a package of analytical tools developed in the Hybridoma Unit at the Institute of Tropical Medicine in Havana in the 1990s to deal mainly with the generation of monoclonal antibodies against infectious agents and its quantification for diagnostic purposes. Although the generation of therapeutic monoclonals has a particular conception and special approaches and designs, the analytical tools for its quantification can be shared if they are compatible with the scenarios (serum or tissue) where they have to act and be evaluated. In this case, although it was not possible for us to monitor mouse IgM in humans, it was possible to have an appropriate and well qualified analytical tool. | |
| Table 1 summarizes chronologically, the contribution of authors towards hybridoma technology in two decades of endeavors and developments. | |
| Table 1: Contributions developed in Cuba towards Hybridoma Technology from 1982 to 2000. | |
Prospects |
|
| Hybridoma technology, originally established in 1975 [1], remains remarkably significant for generating monoclonal antibodies against antigens of academic, clinical and diagnostic relevance [67]. Novel improvements are emerging as advanced methodologies in the frame of hybridoma technology as B cell targeting and fusion with plasmacytoma cells by electrical pulses [68], multitargeting [69] and stereospecific targets [70] that may be useful for simultaneous generation of Mabs, selective generation of stereospecific Mabs and also to more reliable production of human monoclonal antibodies. | |
| With the advent and consolidation of technologies for the production of recombinant antibodies [71], the classical technology for generating monoclonal antibodies seemed to lose significance and scope. However, it soon became clear that the fragments of immunoglobulin obtained by genetic recombination have their specific field of application [72] and what is most important, that there are therapeutic applications for which complete human antibodies are required. The application of the classical technology of somatic cell fusion for human plasmacytomas and immune cells [73] still has major disadvantages, such as the lack of human parent cell line stability, the limited availability of human specific immune cells, as well as the low affinity of generated antibodies. | |
| In 1996 high affinity monoclonal antibodies specific for human CD4 from transgenic mice expressing human immunoglobulins were reported [74]. The impact of this article was not only that human antibodies from transgenic mice can be obtained but that these generated monoclonals were high-affinity (Ka between 109- 1010 Mol-1) and that they are derived from more than a lineage of B cell. This result was a significant step demonstrating the usefulness of miniloci transgenic mice for generating human monoclonal antibodies. | |
| At that time there were several approaches to obtain human monoclonal namely: minilocus, transgenic mice, filamentous phages libraries and cultivation in vitro of human lymphocytes. In all cases the isolation of monoclonal antibodies started as a two-stage process: first, the generation of a repertoire of Mabs with different specificities, and second, the selection of those Mabs that recognize the antigen of interest. It is important that the obtained antibodies have appropriate characteristics regarding antigen binding, as they are used for therapeutic use in humans both for identifying tissue and neutralizing dangerous external targets. The latter represents the physiological function of the humoral immune response and for this purpose vertebrates have developed mechanisms to improve the affinity of the generated antibodies. Lines of mice containing miniloci of human immunoglobulins in place of the corresponding loci of mouse ones have been created using the transgenic approach. The primary intention is to take advantage of the process of diversification and selection of the immune system of the mouse in order to obtain high-affinity human monoclonal antibodies [75]. | |
| It is this aspect which is crucial for immunotherapy, where hybridoma technology seems to revive. Two recent examples reinforce this idea: | |
| • In May 2010 the drug Denosumab (Prolia; Amgen) was authorized by the European Commission for the treatment of post-menopausal women with high risk of fractures suffering from osteoporosis and for the treatment of the loss of bone mass associated with hormone therapy in men with cancer of the prostate with high risk of fractures. Shortly afterwards, the drug was approved by the FDA for women. Denosumab is essentially a human immunoglobulin (IgG2) monoclonal generated from transgenic mice by cell fusion technology and binds with high specificity and affinity for the ligand RANK preventing interaction with this receptor on the surface of osteoclasts and their precursors. Thus, the antibody inhibits the formation, function and survival of osteoclasts to the detriment of bone resorption and in favor of the increase in bone mass and trabecular strength [76,77]. | |
| • A recent and promising clinical trial showed that the Ipilimumab, a human monoclonal antibody with immunomodulatory properties that recognizes the antigen 4T of cytotoxic cells (CTLA4) led to an increase in the survival of 3.7 months in patients with advanced melanoma. The antibody joined CTLA4, a key negative regulator of the activation of T cells. These results open the hope for an improvement in the treatment of patients with metastatic melanoma for which there is currently no treatment that improves survival [78]. Ipilimumab (developed by Madarex in conjunction with Bristol-Myers-Squibb) is a fully human monoclonal antibody (IgG1) developed through the hybridoma technology from transgenic mice, and that enhances the immune response against the cancer cells. With this drug, a monotherapeutic anti-tumour activity has been shown, or in combination with vaccines against cancer [79,80]. | |
| Advances in immunotherapy appear to confirm the original idea that to be effective in interacting with the immune system in cancer patients [81], the antibody must be completely human and generated by an animal, taking advantage of the mechanisms of maturation evolutionarily developed with this purpose [82] and there is not, so far, a better experimental scenario for this goal than the well-established and optimized hybridoma technology developed by Kohler and Milstein [1]. | |
| It is extremely remarkable that 26 out of the 28 therapeutic Mabs that have been approved by the FDA in USA now days have been obtained from hybridomas, some of them as chimeras or reshaped for humanization. Transgenic humanized mouse hybridoma strains essentially carry on the natural recombination and in vivo affinity maturation with a great repertoire capacity for high-affinity antibodies to any antigen. For them the hybridoma technology will open new vistas for the more efficient generation of fully human Mabs with a clear benefit towards the development and application of Mabs as therapeutics for cancer and infectious diseases [83]. | |
References |
|
|
|

