Research Article, J Fashion Technol Textile Eng Vol: 2 Issue: 2
Functional Finishing in Polyamide Fabrics using Dye/ Inorganic Hybrid Materials comprising Silica and Heteroaryl 1,3,4-Thiadiazole Dye
| Ming-Shien Yen*, Mu-Cheng Kuo, and Chien-Wen Chen | |
| Department of Materials Engineering, Kun Shan University, Tainan 71003, Taiwan, ROC | |
| Corresponding author : Dr. Ming-Shien Yen Department of Materials Engineering, Kun Shan University, Tainan 71003, Taiwan, ROC Tel: +886 62050349; Fax: +886 62050349 E-mail: yms0410@mail.ksu.edu.tw |
|
| Received: May 12, 2014 Accepted: June 25, 2014 Published: June 30, 2014 | |
| Citation: MS, Kuo MC, Chen CW (2014) Functional Finishing in Polyamide Fabrics using Dye/Inorganic Hybrid Materials comprising Silica and Heteroaryl 1,3,4-Thiadiazole Dye. J Fashion Technol Textile Eng 2:2. doi:10.4172/2329-9568.1000109 |
Abstract
Development of Aloe Gel Coated Single Jersey Fabric for Dermatitis
The awareness of health and hygiene for consumers has increased the demand for antimicrobial textiles. Whilst in the past it was predominantly technical textiles which had antimicrobial finishes in particular to protect against bacteria and fungi; now-a-days textiles worn close to the body have been developed for a variety of different applications as far as medical and hygienic tasks. Antimicrobial finish on fabrics can minimize the transfer of microorganisms onto the wearer by creating a physical barrier. The various medicinal plants found in nature exhibit excellent anti-microbial properties. A novel attempt has been imparted in this research work to develop medicinal herb Aloe barbadensis gel extracts treated single jersey cotton knitted fabrics using alternate medical concepts to cure atopic dermatitis. It involves the applications of aloe gel extracts of the plant onto cotton knitted fabric by optimizing finish process conditions such as concentration, time and temperature by Box and Behnken statistical method.
Keywords: Tetraethoxysilane; 1,3,4-thiadiazole; Hybrid; Water-repellent; Polyamide
Keywords |
|
| Tetraethoxysilane; 1,3,4-thiadiazole; Hybrid; Water-repellent; Polyamide | |
Introduction |
|
| As consumer demand grows for higher comfort and quality, traditional single-function materials are being eliminated gradually and replaced by multifunctional, comfortable, and environmentally friendly materials, including advanced technical textiles [1]. Comfortable clothing is generally resilient, quick-drying, water vapor-permeable, cool-feeling, water-repellent, soil-resistant, and crease-resistant. In particular, functions such as water-repellency [2-6], oil-repellency, and soil resistance are increasingly becoming a focus of consumer interest. | |
| To achieve materials with these qualities, fabrics can be treated with silanes or woven with a silane-treated component to form an outer layer with very low surface tension, resulting in water and oil repellency and other effects. Typically, the desired water contact angles are obtained by treatment with fluorochemicals alone. However, fluoroalkyl compounds of C6 materials are expensive, environmentally questionable, and pose a potential risk for human health in cases of skin contact [7-9]. It is also known that some of the azo dyes and their intermediates also have environmental and hazard for human health [10-12]. Enhancing water-repellency through solgel modification has been introduced as an alternative approach [4,13,14]. The sol-gel method [15,16], which is generally applied to functionalized silica materials, has become an important processing technique because of its simplicity. Organic or inorganic sol-gels are created by first mixing monomer or precursor; subsequent hydrolysis, condensation, and polymerization steps afford various hybrid materials [17,18]. Polymer/silica nanocomposites can be made by in situ polymerization of silica precursors, the most widely used of which are tetraethoxyorthosilicate (TEOS) and tetramethoxyorthosilicate (TMOS). Alkoxysilane- containing polymers [19–25] have also been used as silica precursors. | |
| Heterocycles have been used to fabricate a diverse variety of dyes. It has been claimed that dye manufacturing was the first area to exploit heterocyclic amines on an industrial scale [26]. Most heterocyclic dyes of technical interest for textile applications are derived from diazo components, consisting of five-membered rings containing one sulfur heteroatom. The amino function group directly attached to the heterocyclic compound (possessing sulfur atom), which may be derivatized to a diazo component. The ring may also possess one or more nitrogen heteroatoms. These diazo components are coupling with the coupling component to produce red through to purple, blue and green mono-azo dyes to meet rigorous technical and economical requirements [27,28]. Heteroaryl dyes, derived from 2-amino-thiadiazole, have been described previously [29]. Dyes derived from 2-amino-1,3,4-thiadiazole are of particular interest for creating brilliant shades of red and the thiadiazole classes of diazo components have been examined extensively [30,31]. | |
| In view of the above discussion, a sol-gel method was used to modify covalently heteroaryl 1,3,4-thiadiazole azo compounds with vinyltriethoxysilane (VTES) and tetraethoxysilane to create a series of dye/inorganic hybrid materials. The characteristics of polyamide 6 (PA-6) fabric that had been dyed with these hybrid materials were also evaluated. Fabrics modified with the silica-modified dye showed significant enhancements in water-repellency. Dyeing and finishing were also achieved in a single bath, thus lowering processing costs. | |
Experimental Details |
|
| Analytical instruments | |
| All melting points are uncorrected and in °C. FTIR spectra were recorded on a Bio-Rad Digilab FTS-40 spectrometer (KBr); 1H nuclear magnetic resonance (NMR) spectra were obtained on a BRUKER AVANCE 400MHz NMR spectrometer, and chemical shifts are presented in δ ppm using TMS as an internal standard. The 29Si NMR spectra were collected using a BRUKER AVANCE 400 MHz NMR spectrometer at 78.49 MHz, with a recycle time of 60 s., and the number of scans is 914. Mass spectra were obtained using a Finnigan TSQ-700 GC/LC/MS spectrometer. The color strength and the levelness of the processed textiles were measured on a Hunter Lab Corporation spectrocolorimeter (Mini Scan XE Plus/Color Flex) (4000S, D/8). Water contact angle measurements were acquired with a water contact angle meter (Sigma CAM100). Wash fastness was evaluated following AATCC Test Method 61-2001 test No. 2A, using an AATCC Standard Instrumental Logic Art, LA-650 Infrared Dyer. Evaluation of rubbing fastness was performed in accordance with AATCC Test Method No. 8-2004 using a rubbing machine (HT- 8031). Water-repellency (spray test) was measured in accordance with AATCC Test Method No. 22-2004. | |
| Materials | |
| Vinyltriethoxysilane, tetraethoxysilane, p-nitro-aniline, formic acid and thiosemicarbazide were purchased from Acros Co., Ltd., Belgium. Thiourea, sulfuric acid, hydrochloric acid, sodium nitrite, sodium acetate, sodium hydroxide and p-nitroaniline were purchased from Hayashi Pure Chemical Co., Ltd., Japan. NP-9 (Taiwan Surfactant Co.) is a non-ionic surfactant agent regards as the detergent agent. Scoured and bleached plain-weave polyamide 6 fabrics [ends (120)*picks (73)/(70d/24f)*(160d/72f)] were used throughout this experiment and supplied by Everest Textile Industry Co., Ltd., Taiwan. | |
| Preparation of intermediates 3 | |
| A finely ground powder of thiosemicarbazide 4.55 g (0.05 mole) was added to a mixture of formic acid 3 mL (0.058 mole) and 36.5% hydrochloric acid (5 mL) and stirred for 30 min at 80°C. Then the solution was refluxed to 100°C for 5h and cooled to 30°C. The resulting solution was diluted on its pH was raised to 7 with aqueous ammonia to get the intermediates 2-amino-1,3,4-thiadiazole (3) [32]. Tables 1 and 2 present the physical and spectral data of these compounds. | |
| Table 1: Characterization data for intermediates and dye derivatives 3, 5, 7, 9. | |
| Table 2: FTIR spectral data of intermediates and dye derivatives 3, 5, 7, 9. | |
| Preparation of the Dye 5 | |
| A finely ground powder of p-nitro-aniline 4 (1.38 g, 0.01 mole) was added to a mixture of 36.5% hydrochloric acid (12 mL) and stirred for 20 min. Sodium nitrite (0.72 g, 0.0105 mole) was added in portions to 98% sulfuric acid (5 mL) at 10°C and stirred for 1 h at 60-65°C. The solution was cooled to below 5°C, and then the finely ground derivatives were slowly added; the mixture was stirred for an additional 1H at 5-10°C until it was clear. The resulting diazonium solution was used immediately in the coupling reaction. A clear mixed solution of the coupling component 2-amino-1,3,4-thiadiazole 3 (1.0 g, 0.01 mole) and 10% sodium carbonate was stirred. The diazonium salt solution was added slowly so that the temperature did not rise above 5°C, while maintaining pH at 5-6 by addition of sodium acetate or 40% sodium hydroxide solution. The mixture was then stirred for 2 h at 0-5°C. The resulting product was filtered, washed with water and recrystallized from ethanol to give dye 5. | |
| Preparation of the Precursor 7 | |
| Precursor 7 was prepared by the reaction of dye 5 (2.22 g, 0.01 mole) followed by the addition of vinyltriethoxysilane 6 (9.5 g, 0.05 mole) in 80 mL ethanol with stirring at 65°C for 4 h at an adjusted pH of 4-5. | |
| Preparation of Hybrid Materials 9 | |
| Scheme 1 presents the route for synthesizing coupling components and bis-hetaryl monoazo dye. Hybrid material 9, the symbol description is the same as 9a1, was prepared by the condensation of precursor 7 (4.12 g, 0.01 mol) and tetraethoxysilane (2.08 g, 0.01 mol) in 80 mL ethanol with stirring at 65°C for 2 h by adding hydrochloric acid (0.365 g, 0.01 mol) and 5 mL of water. The hybrid materials 9a1-9a4 were prepared using different molar ratios of precursor 7 to tetraethoxysilane during hydrolysis-polycondensation; the molar ratios were 1:1, 1:2, 1:3 and 1:4. | |
| Scheme 1: Synthesis of the hybrid materials 9. | |
| Polyamide Fabrics Process | |
| Treatment of the PA-6 fabrics was performed using the “two dips, two nips” padding method with a pickup of 80% using a padder (Rapid Labortex Co., Ltd.). The typical padding solution was prepared as follows: fabrics were dipped for 3 min in an ethanol solution containing the required mass of hybrid materials. A padding-dryingcuring finishing procedure was used to disaggregate the agglomerated particles into well dispersed colloidal particles. Silica-modified fabric samples were then padded and nipped to remove excessive liquid and to obtain a percent wet pickup of 80% using a padder with a setnipping pressure. The processed fabric was pre-dried in an oven at 70°C for 2 min. Then, the processed fabrics were cured at 145°C for 2 min in a preheated curing oven (Chang Yang R3). The fabrics were then rinsed with the detergent agent NP-9 1 g/L at 60°C for 10 min to thoroughly remove any hybrid material residue and dried at 70°C for 5 min prior to being evaluated. | |
| Determination of Color Strength and Related Parameters | |
| Reflectance values of the treated samples were measured using a UV-Vis spectrophotometer (UV-1201, Shimadzu) at λmax, and K/S values were determined using the Kubalka–Munk equation given below [33]: | |
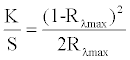 , , |
|
| where K is the coefficient of absorption, S is the coefficient of scattering, and Rλmax is the reflectance value of the fabric at peak wavelength. | |
| Color differences and relative color strengths between those fabrics dyed with the silica hybrid materials and undyed fabrics were obtained through the following relationships: | |
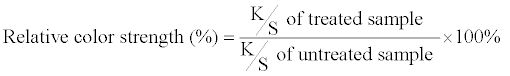 |
|
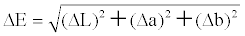 , , |
|
| where L is the lightness or shade of the dye, ‘a’ is a measure of redness or greenness, and ‘b’ is a measure of yellowness or blueness. Note that ΔL = Ldyed – Lundyed, Δa = adyed – aundyed, and Δb = bdyed – bundyed. | |
| The color difference between the largest and smallest values on the same fabric was obtained, and these values indicate the color levelness of the fabrics. According to the regulation of National Bureau of Standards (N.B.S.), it is acceptable for the industrial application when this value is less than 2.0. | |
Results and Discussion |
|
| FTIR analyses | |
| The FTIR spectrum of the intermediate 3 showed bands at 3094, 3305 cm-1 corresponding to the presence of an amino group. The spectrum also showed C-H group absorption peaks at ~2975 cm-1. Figure 1 shows the FTIR spectrum of dye 5 indicated the presence of an N-H group and C-H groups, with absorption peaks at approximately 3096 and 3305 cm-1, respectively. The deviation of the amino group absorption peaks at 3450 cm-1 indicates that some of the dye had reacted with VTES via an addition reaction [34]. The appearance of an Si-OR absorption peak around 1026 cm-1 shows that VTES was able to convert the primary amine to a secondary amine, with a corresponding absorption peak around 3451 cm-1. Thus, it can reasonably be assumed that reactions occurred between some of the dye and VTES. The FTIR spectrum of the hybrid material 9 includes an absorption peak from the converted secondary amine. The strong Si-O peak at 1067 cm-1 indicates dissociation of the NH2 bond. The Si-C bond at ~1242 cm-1 reveals that, following this dissociation, the CH2 groups linked to the Si-O bond and prompted the formation of a Si-O-Si network. This interaction was enhanced by increasing the TEOS concentration, as evidenced by associated increases in the absorption strength of the Si-O-Si peak at ~1068 cm-1. The structure of the Si-O-Si bond was further characterized by 29Si NMR. | |
| Figure 1: FTIR spectra of dye 5, precursor 7 and hybrid materials 9a3. | |
| 1H NMR and 29Si NMR spectra analyses | |
| The 1H NMR spectra of compounds 3 and 5 each contain a 2H singlet at 7.49 ppm, indicating the presence of an amino group at the 2-position of the 1,3,4-thiadiazole ring. In 3, a 1H singlet at 8.28 ppm indicates a methylene group at the 5-position of the same ring. Compound 5 also yielded doublets at 7.85 ppm and 8.23 ppm that were attributed to protons at the 3- and 5-positions and at the 2- and 6-positions of the phenyl ring, respectively. All data were consistent with the expected structure of the 1,3,4-thiadiazole compounds. | |
| 29Si NMR [35] is often used to characterize the structures formed by Si hydrolysis. While the FTIR results above indicated the formation of Si-O-Si bonds by a sol-gel reaction, solid-state 29Si NMR provided additional information on the structure of the silica and the extent of the Si-OH condensation reaction. Figure 2 shows the 29Si NMR spectra of simple dye and VTES contain absorption peaks at -71.41 ppm (T2) and -80.44 ppm (T3), corresponding to Si-OR following the hydrolysis of VTES. Figure 3 illustrates the 29Si NMR spectra of processed hybrid dye formed with various proportions of VTES and TEOS exhibited peaks at -101.38 ppm (Q3), caused by the absorption of (H-O)Si(-OSi≡)3 structures, and at -110.05 ppm (Q4) due to the absorption of Si(-OSi≡)4 structures. At molar ratios up to 1:10:15 dye: VTES: TEOS, the intensities of the Q3 and Q4 absorption peaks changed only slightly. Adding TEOS in excess of the 1:10:15 ratio significantly increased the Q3 and Q4 absorptions. The strength of the Q4 peak indicates a high degree of silicon condensation. | |
| Figure 2: 29Si NMR spectra of precursor 7. | |
| Figure 3: 29Si NMR spectra of hybrid materials 9 with variation ratios of VTES/ TEOS. | |
| Color strength and dyeing fastness | |
| The color strength and levelness of polyamide fabrics dyed with the various modified dye are shown in table 3. Dye containing a nitrosubstituted benzene ring as the diazo component exhibited greater color strength. The dyeing levelness of the dye and all of its hybrid materials ranged from 0.5 to 0.8, all of which were acceptable. | |
| Table 3: Color strength and levelness of precursors and hybrid materials processed on PA-6 fabrics. | |
| The rubbing fastness of 7 and silica-modified dye 9 are shown in table 4. Dry rubbing fastness was generally grade 4, while wet rubbing fastness was grade 3-4. Washing fastness was generally rated grade 3–4 because of Van der Waals forces between the azo dye and the amide groups on the polyamide fiber stems; no strong bond existed between the dye and the fabric. However, after dyeing the fabric with derivative 9, the Si–O hydrolysis produced a network structure, allowing the hybrid molecules to more strongly adhere to the fabric surface. Thus, the hybrid materials demonstrated greater washing fastness. | |
| Table 4: Washing and rubbing fastness of dye, precursors and hybrid materials processed on PA-6 fabrics. | |
| Water-repellency and the contact angle | |
| Water-repellent sol–gel coatings were prepared by dip-coating fabrics with silica sols 7 that had been treated with two types of hydrophobic additives: alkyltrialkoxysilanes with 1,3,4-thiadiazole azo dye and various diazonium compounds. The processed fabric was placed in a 6-inch-diameter frame, and 250 mL of water was splashed onto it through a funnel. After 25–30 s, the fabric was turned upsidedown, and the backside of the fabric was gently tapped to disengage excess water droplets. This procedure was repeated three times; average values are given in table 5. Dyeing the polyamide fiber with the azo dye did not significantly improve water-repellency. However, the VTES precursor slightly improved the water-repellent properties of the processed fabric. Although the average rating was only grade 2–3, it improved to grade 3–4 after the addition of TEOS, a significant increase. | |
| Table 5: Surface hydrophobicity evaluation of dye, precursors and hybrid materials processed on PA-6 fabrics. | |
| The contact angle of the unprocessed fabric which dyed with dye 5 was approximately zero. Figure 4 shows the contact angles of the processed fabrics before and after 20 washing cycles. After dyeing the fabric with derivatives 9a1–9a4, leading to a lowering of surface tension of the fibre surface contact angle increased to 127–133°. Thus, water droplets cannot easily wet the hybridized VTES/TEOS-modified surface, further indicating an increase in water-repellency. The processed fabrics were then washed 20 times with water and waterrepellency was retested. Contact angles after washing were generally 114–120° (Table 6), indicating that water-repellency was maintained even after 20 wash cycles. The differences between the contact angles of 9a1–9a4 before and after the washing treatment were ranging from 13° to 14°. These slight differences between the contact angles indicate that the different processing treatments to which the PA-6 fabrics are subjected do not significantly affect the water repellency. | |
| Figure 4: Contact angle analysis of PA-6 fabrics 9a1–9a4. | |
| Table 6: Surface hydrophobicity evaluation of hybrid materials processed on PA-6 fabrics after 20 wash cycles. | |
Conclusions |
|
| A series of heteroaryl 1,3,4-thiadiazole azo dye was prepared. VTES/TEOS were added in various ratios to produce a series of functional hybrid materials via a sol-gel method. FTIR and NMR analyses confirmed that the silicon in the hybrid material can indeed bond with the 1,3,4-thiadiazole dye. This study evaluated the application properties of 1,3,4-thiadiazole azo dye modified with a siloxane precursor. Test results showed that for dyed fabrics after finishing, washing fastness was generally rated grade 3–4, dry rubbing fastness grade 4–5, and wet rubbing fastness grade 3–4. The water-repellency of polyamide fabrics dyed with the unmodified 1,3,4-thiadiazole dye was rated grade 1–2, indicating negligible waterrepellent effects. In contrast, fabrics dyed with the silica-modified dye exhibited significantly enhanced water-repellency rated grade 3–4. The water contact angle on fabrics dyed with the modified dye was 127–133° after finishing and decreased to 114–120° after 20 washing cycles. This result implied that Si-gel could enhance the contact angle of the treated PA-6 fabrics and improve its water-repellent property. | |
Acknowledgments |
|
| The authors thank the National Science Council of the Republic of China, Taiwan, for financially supporting this research under Contract No. NSC-98- 2622-E-168- 007-CC3. | |
References |
|
|
|