Research Article, Vector Biol J Vol: 1 Issue: 2
Study on Larvicidal Effects of Essential Oils of Three Iranian Native Plants against Larvae Anopheles stephensi (Liston)
| Hanieh Torabi Pour*, Mansoureh Shayeghi, Hassan Vatandoost and Mohammad Reza Abai | |
| Department of Department of Medical Entomology, School of Public Health,Tehran University of Medical sciences, Tehran, Iran | |
| Corresponding author : Hanieh Torabi Pour Hanieh Torabi Pour, Department of Medical Entomology School of Public Health, Tehran University of Medical sciences, Tehran, Iran Tel: ++98 21 8889 6696 E-mail: hanitorab@yahoo.com |
|
| Received: February 01, 2016 Accepted: May 11, 2016 Published: May 18, 2016 | |
| Citation: Torabi Pour H, Shayeghi M, Vatandoost H, Abai MR (2016) Study on Larvicidal Effects of Essential Oils of Three Iranian Native Plants against Larvae Anopheles stephensi (Liston). Vector Biol J 1:2. doi:10.4172/2473-4810.1000109 |
Abstract
Objective: To determine the larvicidal activity of three medicinal plants essential oils against larvae Anopheles stephensi
Methods: The larvicidal effects of essential oils of seven medicinal plants collected from different parts of Iran including Carum carvi (seeds), Artemisia dracunculus (branches and leaves) and Rosmarinus officinalis (branches and leaves) were determined. The essential oils were hydro-distilled for 3-5 h using a Clevenger typeapparatus in Ecotoxicology Laboratory, School of Public Health (SPH), Tehran University of Medical Sciences (TUMS). The antilarvae tests of EOs were carried out according to WHO’s guidelines at Bioassay Laboratory at adjacent of Culicidae Insectary of SPH, TUMS.
Results: The 50% and 90% of lethal concentrations of studied plant EOs were 21.59 and 72.44 ppm for C. carvi, 1.33 and 4.12 ppm for A. dracunculus, 93.22 and 229.29 ppm for R. officinalis, respectively. The chemical constituents of EOs were settled by GCMS analysis and the main constituents with larvicidal effect were determined.
Conclusion: The results revealed the high larvicidal potential of EOs of A. dracunculus and C. carvi and possible cultivation of latter plants, the identification of chemical compounds, trade formulation, semi-field and field evaluations of EOs are strongly recommended at malarious area, southern Iran.
Keywords: Anopheles stephensi; Medicinal plants; Essential oils; Larvicidal effects; malaria; Iran
Keywords |
|
| Anopheles stephensi; Medicinal plants; Essential oils; Larvicidal effects; malaria; Iran | |
Introduction |
|
| By considering the fact that using chemical larvicidal material in order to control the immature flies’ population has destructive consequences on the environment and the process of using these material makes some anopheles’ species resistant to these pesticides so the researches are in the direction of extracting larvicidals from the nature specially plants. | |
| Because of this fact, a wide range of researches have been carried out concerning the effects of lavicidals on anopheles’ larvae. In recent years various studies on the poisonous effects of Iranian EOs including Alliaceae, Apiaceae, Asteraceae, Cupressaceae, Graminaceae, Lamiaceae, Lauraceae, Myrtaceae, Pedaliaceae, Rutaceae, Scrophulariaceae, Verbenaceae, Zingiberaceae against various types of insects have been reported [1]. | |
| The larvicidal effects of three species of Iranian medicinal plants including Carumcarvi, Artemisia dracunculus, and Rosemarinus officinalis against the larva of the main Malaria vector in Iran Anopheles stephensi have been studied in this research. C. carvi is a plant of Apiaceae family. This plant is native to central mountains of Iran especially near Kerman. | |
| a) The most important reported effects of C. carvi are: Anti- Worm, Anti-anemia, anti-microbial, anti-histamine, anti-cancer, anti-inflammatory, antispasmodic, carminative, anti-fungal, chloretic, diuretic, digestive, expectorant, larvicide, muscles relaxant, stimulant and stomach tonic [2]. R. officinalis is of Lamiaceae plants. It is native to Mediterranean Europe and Near East which is being planted in Iran too. Its extract is insect repellent [3]. | |
| b) The most important reported effects of R. officinalis are: Analgesic, anti-Alzheimer’s, anti-arrhythmic, anti-bacterial, antiatherosclerotic, anti-cancer, anti-vascular fragility, anticonvulsants, anti-edema, anti-swelling, anti-mutagen, anti-oxidants, anti-toxin, anti-prostaglandin, anti-fever, antiseptic, anti-spasmodic, antivirus, anti-distention, anti-fungus, insecticides, sudorific, stimulant, digestive, diuretic, Hepato protectors and stomach tonic. R. officinalis also has a pesticide effect [4]. A. dracunculus of Asteraceae family which does not grow naturally in Iran and it can be found in planted and cultivated form [5]. This plant is being cultivated is many areas of Iran, some say that its origin is the North and the West of America. | |
| c) The most important reported effects of A. dracunculus are: Allergen, analgesic, antibacterial, anti-fragility of capillary vessels, anti-inflammatory, anti-fever, anti-spasmodic, anti-distention, diuretic, fungicide, hypnotics, stomach tonic, anti-worm, anticancer and stomachic [6]. This research aims to study the larvicidal effects of the EOs of three plant species (Carum carvi, Artemisia dracunculus, Rosemarinus officinalis) by exposing Anopheles stephensi to the prepared logarithmic concentrations of these EOs in laboratory condition and in case of acceptable results these effective, cheap and easy to available larvicides could be used to control Malaria disease. | |
Methods |
|
| Collecting and preparing the plants | |
| This part includes extracting the EOs of the plants in the study including: Artemisia dracunculus which was planted in Iran, Rosemarinus officinalis (was collected from Tehran in fresh form then was dried) and Carum carvi (which was collected from Jiroft in Kerman province) and also it included identifying the physical and chemical features of these plants and their components and studying the larvicidal effects of them on Anopheles stephensi. The said plants were collected in 2013 spring then were dried in shadow based on their main organs and when they were fully dried the final identification of the said species was carried out. | |
| Rearing of mosquito larvae | |
| Rearing and maintaining mosquito larvae was carried out in the temperature of 29 ± 2ºC and relative humidity of 70 ± 10% and Lightdark cycle of 16h light and 8h was performed in Culicidae insectarium of the School of Public Health Tehran University of Medical Sciences. The larvae of the late 3rd stage or early 4th stage of Anopheles stephensi were used for larvicidal tests. | |
| Extracting the essential oil by distillation with water method | |
| In order to extract the EO of any target plant in this study, first it was taken to Pesticides Chemistry Lab and was identified then it was relented. An amount about 30 gr of the plant was weighted and put in a 1l Laboratory flask then about 600 cc distilled water was added to the flask and after that the Clevenger was mounted on the flask and the heater of the Clevenger was turned on and after 4 hours it was turned off. The collected EO in the calibrated part of the Clevenger was put in small glassy container special for EO collecting then it was dehydrated with Sodium Sulfate and was put in the refrigerator up to the test time. | |
| Biological tests (larvicidal) | |
| The standard WHO method for biological tests was used. The overall temperature of the lab (28ºC), test period (24h) and the number of larvae (25 in each 400 cc beaker) has to be constant. The best age range of the larvae for the tests are the larvae of the late 3rd stage or early 4th stage range and preferably dechlorinated water should be used in the tests. At least 5 logarithmic concentrations should be made of the EO. In order to find the suitable concentration first the concentrations should be chosen in a larger domain and based on the results the concentration domain becomes narrower. Usually the concentration in which has the 50% relative mortality and two concentrations lower than it and two concentrations upper than it are used to draw that regression line diagram. In each test 5 concentrations of pesticide and for each concentration 4 repetitions and in general 2 witnesses are considered. In all tests, for all the present material, we had witness but we did not have it for the larvicidal pesticide [7]. | |
| Method of EO logarithmic concentration making | |
| The extracted EO of the plants: C. carvi, A. dracunculus, R. officinalis were used to carry out biological tests. For each concentration of the said Eos in the test at least 4 repetitions were considered. In order to evaluate the Larvicidal Effects of each EO the mortality indicator after 24h was used. The tests by five logarithmic concentrations including 80,40,20,10,5 ppm for C. carvi, 10,5,2.5,1.25,0.625 ppm for A. dracunculus and 20,40,80,160,320 ppm for R. officinalis were carried out and for each concentration 4 repetitions were carried out simultaneosly. For each series of the tests two series of witnesses (including 1 ml Ethanol in 249 cc dechlorinated water) were also considered. For C. carvi, because of the fact that the mortal concentration of 100% of larvae has been 80 ppm, we start the logarithmic concentrations from 5 ppm and with a sequence, make it twice and for A. dracunculus the mortal concentration of 100% of larvae has been 10 ppm, we start the logarithmic concentration from 0.625 and make it twice in a sequence and also for R. officinalis the mortal concentration of 100% of larvae has been 320 ppm and we start the logarithmic concentration at 20 ppm and in a sequence we make it twice. | |
| Methods of making concentrations are will be discussed below | |
| Larvicidal sensitivity test: The method of the test like the larva test method was WHO method and the tests were carried out in a 400 ml glassy beaker. First the various logarithmic concentrations were produced from the target EO in the before said order then 22 400 cc beakers and 22 50 cc beakers have to be prepared (for each 4 concentration should be done, two repetitions for witness are considered too). Using a graduated cylinder 224 cc dechlorinated water to each 400 cc beaker and 25 cc dechlorinated water to each 50 cc beaker was added. Then 25 larvae of late 3rd stage or early 4th stage which certainly had been fed up with food (fish food) were used. | |
| Then we transferred the larvae to each 50cc beakers and added 1 cc of the EO with the desired logarithmic concentrations to each 400 cc beakers of the same concentration and were mixed what was in the beakers. 1 cc Ethanol was added to the witness beakers instead of the EO. Then we transferred the contents of the 50cc beakers to 400cc beakers and cover the beakers were covered with plate and after 24h the test results were read. | |
| Chemical analysis: The EO of the studied plants after being prepared, were injected to GC-MS apparatus for the components analyses. The used GC was of VARIAN CP-3800 type and its column was 30m in length and inner diameter of 0.25 mm and thickness of 0.25 μm and it was a VF-5MS type too. | |
| The temperature plan of the column was: The initial oven temperature was 50ºC and remained in this temperature for 1 min then the temperature increased to 300ºC by the rate of 10ºC in a minute, the temperature of the injection chamber was 260ºC, and Helium was used as the carrier gas with a flow of 1 mm in a minute. | |
| In model of mass set: VARIAN SATURN 2200 the type of column was VF-5MS. The length of GC column was 30 mm, the inner diameter of the column was 0.25 mm, the thickness of the layer was 0.25 μm and the initial temperature of the oven was 50ºC. The final column temperature was 300ºC, the injection mode was split and the carrier gas was He. | |
| Statistical analysis | |
| The test results after 24h were read as the following way: the number of alive larvae, the number of dead larvae, the number of moribund larvae, number of larvae and the total number and the results were used to draw the mortality tables. The mortal quantities of 50% and 90% of EOs (LC50 and LC90) and the level of confidence of 95%, the equation of the regression line will be estimated using a regression probit analysis [8]. | |
| When the mortality of the witness group is less than 5% then the resulted data of biometric tests have been correct but if the mortality of the witness group is between 5% to 20% they have to be corrected using the following formul,called Abbot: | |
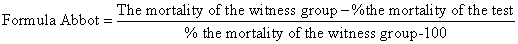 |
|
Results |
|
| The resulted data of the larvivcidal activity of the plants in our test showed that: | |
| Rosemarinus officinalis EO in 320 ppm concentration, Carumcarvi EO in 80 ppm concentration and Artemisia dracunculus EO in 10 ppm concentration show mortality effect of 100%. By decreasing the concentrations into 20 ppm for Rosemarinus officinalis, 5 ppm for Carumcarvi and 0.625 ppm for Artemisia dracunculus mortality became close to zero. | |
| The 50% and 90% of lethal concentrations of studied plant EOs were 21.59 and 72.44 ppm for C. carvi, 1.33 and 4.12 ppm for A. dracunculus, 93.22 and 229.29 ppm for R. officinalis, respectively. Lethal dose of 50% and 90% (LC50 and LC90 ) and other statistical data related to biochemical tests of larvicidal effects of EOs after 24h contact with age 4 larva of A. stephensi have been mentioned in the following Table 1. | |
| Table 1: Lethal concentration (LC50 and LC90) and associated parameters of 24 h bioassay tests of essential oil of(Carumcarvi, Artemisia dracunculus and Rosmarinus officinalis) against 3rd-4th instar larvae of Anopheles (Cellia) stephensi (Liston, 1901). |
|
| Also the equation of the regression line of each EO can be seen in the diagrams, the said equation is which is derived from the following Figures 1-3. | |
| Figure 1: Equation and regression line and lethal concentration (LC50) of essential oil of seeds Carumcarvi L. (Family: Apiaceae ) against 3rd-4th instar larvae of Anopheles stephensi. | |
| Figure 2: Equation and regression line and lethal concentration (LC50) of essential oil of stems and leaves Artemisia dracunculus L. (Family: Asteraceae ) against 3rd-4th instar larvae of Anopheles stephensi. | |
| Figure 3: Equation and regression line and lethal concentration (LC50) of essential oil of flowers and leaves Rosmarinus officinalis (Family: Lamiaceae) against 3rd-4th instar larvae of Anopheles stephensi. | |
| GC-MS results of the EO of the tested plants | |
| Types and percentage of the chemical components in the EO of each plant was distinguished by GC-Mass method. Knowing the forming components of EO is necessary for purification of the effective material and correct formulation of the larvicide. | |
| Based on the results of the main chemical components of GC-MS analysis of Carum carvi were: 37.2% drima-7,9 (11)-diene, 9% ((E)-2-hexenal), 11.1% trans-carveol, 7.6% AR-curcumene, 6% benzyl-benzoate, 4.4% Cadalene, 4% Geraniol, 3.9% a-cadinene, 5.7% Hexadecanol (Table 2). | |
| Table 2: The GC-MS analysis of the plants EO (Carumcarvi, Artemisia dracunculus, Rosemarinus officinalis). | |
| The Main chemical components of Artemisia dracunculus were: 85.9% (Z)-3-hexenol, 9.4% Isovaleric acid and 2.9% Hexadecanol (Table 2). | |
| The Main chemical components of Rosemarinus officinalis were:46.8% ((E)-2-hexenal), 12.4 (Linalool), 10.3% (a-ylangene), 5.9% Geraniol, 3.4% (Carvacrol), 2.9% (α-campholenal) and 2% (Isovaleric acid) (Table 2). | |
Discussion |
|
| The results shows that each 3 tested EOs have larvicidal effects against A. stephensi larvae and A. dracunculus in comparison with the other two plants in this test is the most effective one which has the biggest mortality with the lowest concentration LC50. The resulted data of the larvicidal activities of the studied plants says that R. officinaliis EO with 320 ppm concentration and C. carvi with 80 ppm concentration and A. dracunculus with 10 ppm concentration show the mortality effect of 100%. By decreasing the concentrations into 20 ppm for R. officinalis and 5 ppm for C. carvi and 0.625 ppm for A. dracunculus respectively, the mortality moved in the direction to LC50 of the studied plants in this biological test were LC50= 21.59 ppm and LC90=72.44 ppm for C. carvi and LC50=1.33 ppm and LC90 =4.12 ppm for A. dracunculus and LC50=93.22 ppm and LC90 =229.29 ppm for R. officinalis. | |
| In this study the larvicidal effect of 3 species of medicinal plants against the larva of the main Malaria vector in Iran A. stephensi was studied and based on the results all of the studied plants were effective against A. stephensi larva and after 24h showed the larvicidal effects. The most effective plant among these three was A. dracunculus. | |
| In a study by Pitasawat et al. [9] the larvicidal effect of C. carvi against vector mosquitos of A. dirus and A. aegypti was studied. In this study C.carvi EO in the following concentrations: LC50=72.28 ppm, LC95=104.69 ppm and LC99 =128.87 ppm against A. dirus and LC50=54.62 ppm, LC95=90.06 ppm and LC99=119.21 ppm against A. aegypti was tested. | |
| In another study which was carried out by Yu et al. [10]. about the larvicidal activity of the R. officinalis components, the chemical components and their activity against the sensitive species to DDT, resistant to DDT and the Culex quinquefasciatus larvae collection were studied. The average lethal dose (LC50) of R. officinalis EO against the sensitive species to DDT, resistant species to DDT and Cu quinquefasciatus species were 30.6 ppm, 26.4 ppm and 38.3 ppm respectively. | |
| In a study by Govindarajan [11] the lethal effects of R. officinalis against two mosquito species: Culex tritaeniorhynchus and Anopheles subpictus were studied. The results of R. officinalis test against C. tritaeniorhynchus were LC50= 115.38 (85.27-144.10) ppm and LC90= 211.53 (176.37-280.69) ppm and against An. Subpictus were LC50= 64.50 (49.96-79.16) ppm and LC90= 113.74 (95.58-149.57) ppm. | |
| In another study which was carried by Conti et al. [12] about the lethal activity of R. officinalis against A. albopictus the highest dose was 300 ppm and the lethal level of R. officinalis was 51.7%. The results of the lethality test for R. officinalis against A. albopictus was LC50 > L250. | |
| In a study in 2013 in Iran by Valizadeganon Aphis gossypii Glove aphid, the Fumigant toxicity of A. dracunculus EO on the adult pest was studied. The results suggested that A. dracunculus EO after 24h shows a dramatic mortality on the pest in the study. The LC50 in the adult male aphids was 18.63 μL/L in the air [13]. | |
Recommendations |
|
| The results of this study show that the extracted EOs of the medicinal plants could be thoroughly studied as the substitutes for usual pesticides because based on the results which are a proof for the resistance of Malaria vectors to Pyrethroid insecticides i.e. the last resort of synthetic pesticides to control Malaria vectors. So the need for a conscious and purposeful seek to achieve the rare opportunities for introducing effective EOs in controlling immature flies is an innovation in controlling flies and paves the way for the commercialization of the product and also by considering the obvious effect of the EOs of the studied plants, distinguishing the effective components of these EOs, larvicidal features and formulating them for the field evaluation and use against Malaria vectors is suggested. | |
Acknowledgments |
|
| The authors of this article appreciate the Department of Medical Entomology and Vector Control of School of Public Health Tehran University of Medical Sciences for their cooperation during this research. | |
References |
|
|
|
