Research Article, Int J Cardiovasc Res Vol: 5 Issue: 3
T1-Mapping in Daily Cardiac Magnetic Resonance Imaging Practice: Combined Use of Native T1 and Extracellular Volume Quantification
| Jonathan Nadjiri1*, Albrecht Will1, Eva Hendrich1, Cornelia Pankalla1, Andreas Greiser2, Stefan Martinoff1 and Martin Hadamitzky1 | |
| 1Institut für Radiologie und Nuklearmedizin, Deutsches Herzzentrum München, Technische Universität München, Lazarettstrasse 36, 80636 Munich, Germany | |
| 2Siemens Healthcare, Henkestrasse 127, 91052 Erlangen, Germany | |
| Corresponding author : Dr. med. Jonathan Nadjiri Deutsches Herzzentrum, Lazarettstrasse 36, 80636 München, Germany Tel: +49-89-1218-4555 Fax: +49-89-1218-4553 E-mail: j.nadjiri@gmx.net |
|
| Received: December 13, 2015 Accepted: April 14, 2016 Published: April 20, 2016 | |
| Citation: Nadjiri J, Will A, Hendrich E, Pankalla C, Greiser A, et al. (2016) T1-Mapping in Daily Cardiac Magnetic Resonance Imaging Practice: Combined Use of Native T1 and Extracellular Volume Quantification. Int J Cardiovasc Res 5:3. doi:10.4172/2324-8602.1000264 |
Abstract
T1-Mapping in Daily Cardiac Magnetic Resonance Imaging Practice: Combined Use of Native T1 and Extracellular Volume Quantification
Introduction: Dual T1-mapping allows for a comprehensive assessment of myocardial tissue by combining detection of edema in the native scan and quantification of extracellular volume (ECV) after administration of Gadolinium (Gd). Recent studies proved the diagnostic value of T1-mapping in different pathologies. The aim of this study was, to evaluate the practicability and robustness of T1-mapping in assessing common pathologies in daily cardiac magnetic resonance (CMR) practice.
Methods: From October 2012 to October 2013, we investigated 136 consecutive patients undergoing clinically indicated CMR examination by performing additional T1-mapping measurements. We used a Modified-Look-Locker-Inversion-Recovery (MOLLI) sequence with 3 inversion pulses and a 4-(1)-3-(1)-2 readout pattern. For extracellular volume calculation a second scan was performed 10min. after administration of 0.2mmol/kg body weight gadopentetate dimeglumine. Diagnosis was based on clinical information and standard CMRsequences comprising native T2-weighted dark-blood turbo spin echo (TSE) sequences, pre- and early post-Gd T1-weighted darkblood TSE sequences and Late Gadolinium Enhancement. The study population comprised a control group, patients with acute and chronic myocarditis, patients with acute and chronic infarction, patients with dilated and hypertrophic cardiomyopathy, patients with aortic stenosis and patients with amyloidosis or sarcoidosis.
Results: Native T1 showed a significant difference when compared with a control in acute myocarditis, acute myocardial infarction, hypertrophic and dilated cardiomyopathy, and amyloidosis. ECV showed significant differences to the control group in all cohorts of pathologies. Particularly high native T1 values were observed in acute myocarditis, acute myocardial infarction, hypertrophic cardiomyopathy and amyloidosis, a high ECV was found in acute and chronic myocarditis, acute and chronic myocardial infarction, sarcoidosis and amyloidosis.
Conclusion: Native T1-mapping and ECV correlated well with myocardial alterations in commonly diagnosed cardiac disorders. It proved reliable and robust in daily clinical practice and allows for a good differentiation between normal findings and common pathological CMR diagnoses. The combined use of native T1 and ECV quantification is a promising approach for comprehensive assessment of the myocardium and may improve diagnostic accuracy of CMR in myocardial disease.
Keywords: Cardiac magnetic resonance imaging; T1-mapping; Extracellular volume; Cardiomyopathies
Keywords |
|
| Cardiac magnetic resonance imaging; T1-mapping; Extracellular volume; Cardiomyopathies | |
Abbreviations |
|
| ACC: American College of Cardiology; AHA: American Heart Association; CMR: Cardiac Magnetic Resonance (Imaging); DCM: Dilated Cardiomyopathy; ECG: Electrocardiography; ECV: Extracellular Volume; EF: Ejection Fraction; FOV: Field of View; HCM: Hypertrophic Cardiomyopathy; IR: Inversion Recovery; LGE: Late Gadolinium Enhancement; MOLLI: Modified Look-Locker Inversion (Recovery); ms: Milliseconds; ROC: Receiver-Operator-Characteristic; SD: Standard Deviation; SSFP: Steady State Free Precession; T: Tesla; TE: Echo Time; TR: Repetition Time; TSE: Turbo Spin Echo | |
Introduction |
|
| In clinical practice, cardiac magnetic resonance (CMR) plays an important role in assessing cardiomyopathies and acute myocardial damage such as myocardial infarction and inflammation. In such cases it is not uncommon that final treatment decisions are based on CMR results. Technical developments established certain sequences in today’s CMR routine protocols for detecting cardiomyopathies and acute myocardial damage such as T2-weighted dark-blood imaging to assess edema, native and contrast enhanced T1-weighted imaging for anatomic evaluation, detection of dysplasia and hyperemia, cine sequences to determine cardiac function and Late Gadolinium Enhancement (LGE) to assess scar tissue and fibrosis [1]. | |
| Those techniques are well established and allow for high specificity and sensitivity for certain cardiac disorders, for example in detection of myocarditis or infarction [2-4]. Diagnostic performance of these established sequences in other cardiac disorders varies; in some disorders e.g. for amyloidosis, diagnosis can be challenging with these techniques [5,6]. Additionally, these techniques have also technical limitations. T2-weighted imaging using dark-blood turbo spin echo sequences (TSE) is known to be prone to motion artefacts and signal drop outs, particularly in patients with tachycardia, commonly occurring in myocarditis or acute myocardial infarction [7]. Furthermore, proof of edema in T2-imaging requires tissue for comparison, which might also be compromised and lead to false negative results [8,9]. Even though, LGE imaging is a wellestablished method to detect localized myocardial fibrosis or scar tissue, respectively, the ability of LGE imaging to assess diffuse fibrosis is limited [10,11]. Although CMR has been recognized to be a non-invasive test capable of assessing cardiac function, anatomy, perfusion and viability [12,13], in some situations CMR results can be inconclusive and hard to interpret. Therefore, the role of CMR is regarded to be depending on availability and local expertise [1]. | |
| To overcome some of these limitations, recently, T1-mapping sequences emerged as promising alternative. Mapping sequences allow for a per voxel calculation of the absolute relaxation-time eliminating the need of comparison with assumed healthy tissue. Native T1-mapping is able to detect edema, hemorrhage, siderosis, lipid and protein deposition as well as fibrosis [14,15]. Additionally the extracellular volume (ECV) can be measured by combining native T1-mapping and contrast-enhanced T1-mapping, providing additional information regarding extracellular disease [16]. | |
| The aim of this study was to evaluate feasibility and reliability of T1-mapping and ECV assessment in daily practice and to assess their characteristics in common cardiac diseases. | |
Methods |
|
| From October 2012 to October 2013, we performed additional native and contrast-enhanced T1-measurements in all consecutive patients undergoing clinically indicated contrast-enhanced CMR examination for myocardial assessment. Excluded were patients undergoing myocardial stress test, assessment of myocardial tumors or hemodynamic evaluation of shunts or valvular heart disease. Only patients with clear CMR-diagnosis were eligible for the study population. | |
| Patients with unclear diagnosis were excluded from study population after scan. CMR was performed on a 1.5T system (Magnetom Avanto, Siemens Medical Solutions, Erlangen, Germany) equipped with a dedicated cardiac phased-array surface coil as described before [17]. For image acquisition, patients were positioned in supine position, and images were acquired at repeated end-expiratory breathholds with ECG gating. | |
| CMR-sequences | |
| Routine examination comprised T2-weighted dark-blood turbo spin-echo (TSE) sequences, pre and early post gadolinium T1-weighted TSE sequences, cine-SSFP sequences, and inversion recovery spoiled gradient echo sequence for LGE: | |
| T1: T1-weighted multislice spin-echo images were acquired ECG-triggered in short-axis orientations with gapless left ventricular coverage (acquisition matrix, 192 × 126; slice thickness, 8mm; echo time (TE) 6 milliseconds (ms); repetition time (TR) 1 RR interval. Measurement was conducted with identical parameters before and one minute after intravenous bolus administration of contrast dye. | |
| T2: Dark-blood T2-weighted TSE sequence was acquired in short-axis orientations with gapless left ventricular coverage: Field Of View (FOV) 340 × 276 mm2, matrix 128 × 192, (TE) 99 ms, (TR) 2 RR intervals until a maximum heart rate of 100 ms or 3 RR-intervals at higher heart rates. | |
| Cine: Parameters for cine balanced-SSFP sequences imaging were: Matrix 256 × 256, FOV 340 × 340 mm2, TE 1.2 ms, TR 50.76 ms, asymmetric echo, segments adjusted to heart rate, slice thickness 8mm. The cine images were acquired in short axis orientations and as a 2-, 3- and 4-chamber view. | |
| LGE: Scar tissue was assessed 15 min after injection of 0.2 mmol/ kg body weight of dimeglumingadopentetat (Magnevist, Bayer HealthCare Pharmaceuticals, Berlin, Germany after expiration of patent protection Magnograf, Marotrast GmbH, Jena, Germany) on the T1-weighted inversion-recovery gradient echo sequence. Pulse sequence parameters were: Slice thickness 8mm, excitation every second heart beat, (TR) 6.0 ms, (TE) 3.37 ms, acquisition matrix: 168 × 256, FOV 340 × 276 mm2, flip angle 30°. The LGE images were acquired in short axis orientations and in a 4-chamber view. | |
| Based on the findings in these sequences, medical history and clinical information patients were categorized into cohorts of the following pathologies: acute and chronic myocardial infarction, acute and chronic myocarditis, dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy (HCM), sarcoidosis and amyloidosis. Patients were categorized into a specific group only if they had typical findings regarding to their pathology. Patients with uncertain diagnosis or more than one cardiac disease (e.g. ischemic and HCM or ischemic and acute or chronic myocarditis) were categorized as nonspecific and excluded. Individuals were classified as control only if imaging showed no alterations at all; there was no medical history of cardiac disease for those patients. | |
| Patients having more than one pathology viz., additional heart valve abnormalities, very rare conditions, borderline pericardial effusion or known clinical infections were classified as nonspecific. Patients in the control group with the highest and the lowest T1 relaxation-times were considered as outliers and excluded. | |
| Patients categorized as sarcoidosis and amyloidosis had firm diagnosis in patient’s history or positive biopsy results. | |
| Patients were categorized in cohort of HCM if LV wall thickness was >15 mm and either eccentric hypertrophy or mid-myocardial fibrosis were present. | |
| DCM was diagnosed if ejection fraction was <40% and end diastolic volume was >231 ml for male patients and >193 ml for female patients without any further evidence of other cardiac disorders. Acute myocarditis was diagnosed as described before [2] and optimized to our environment: Edema was assumed in case of T2 signal intensity was 2 SD above remote myocardium or T2 signal intensity was 2.5 fold higher in myocardium compared to skeletal muscle. | |
| Patients were categorized into cohort of aortic stenosis in case of diagnosis in medical records including echocardiographic confirmation. | |
| Infarction was diagnosed in case of subendocardial involvement of LGE; existence of edema assessed as described above led to sub categorization acute and chronic. | |
| Chronic myocarditis was diagnosed in case of subepicardial LGE in anterior, lateral or inferior wall, without existence of edema. | |
| T1 mapping: For T1-mapping, we used a Modified-Look-Locker- Inversion-Recovery (MOLLI) prototype sequence (Siemens WIP 448B) with 3 inversion pulses and a 4-(1)-3-(1)-2 readout pattern, Matrix 192 × 124, FOV 224 × 279 mm2. T1-mapping was repeated 10min after application of contrast media for ECV calculation as described below. Sequences were conducted as described by Kellmann et al. [18]. | |
| Images were acquired in short-axis orientations with gapless left ventricular coverage and a 4-chamber view. | |
| Post processing | |
| Assessment of T1 relaxation-time and ECV values was based on 3 short axis slices being representative for apical, mid and basal myocardium and 1 long axis view using the AHA/ACC 17-segment model. Assessment of those segments was done by segment or, if circu mscribable, by lesion in diseases associated with regional alterations like infarction, myocarditis and sarcoidosis. Analysis was done for the whole heart in diseases associated with global cardiac alterations such as sarcoidosis, amyloidosis, aortic stenosis, DCM and HCM. | |
| ECV: ECV was determined as described before [19], using the following method: | |
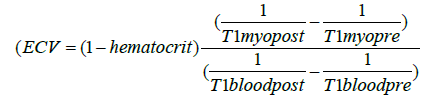 |
|
| In this formula “myo” equals myocardium, “pre” indicates native measurements while “post” stands for contrast enhanced values. | |
| Statistical analysis | |
| Categorical variables were expressed as frequencies and percentages, continuous variables were expressed as mean ± SD. Comparison was done using Student t-test. Statistical significance was accepted for 2-sided p values of <0.05. The statistical package R version 3.2.2 [20] was used for statistical analysis. For ROC-analysis the package pROC was utilized [21]. | |
Results |
|
| Within one year, 279 patients underwent clinically indicated CMR. Out of these, 81 patients were excluded because the scan was performed for another reason than myocardial assessment. 62 had to be excluded, because they were categorized as nonspecific. | |
| The remaining 136 patients were included into the analysis. A detailed breakdown of the study population is provided in Table 1, image examples of the pathologies are illustrated in Figure 1. Demographics and left ventricular parameters are provided in Table 2. | |
| Table 1: Description of 1-year CMR collective including study population. | |
| Table 2: Characteristics of 1-year CMR study collective. | |
| Figure 1: Common cardiac pathologies in native and contrast enhanced T1-mapping, T2w TSE and LGE. | |
| In the control group, mean native T1 was 908 ms ± 31 ms and ECV was 26.0% ± 2.2%. Based on these results we defined threshold values of two (T1: 970 ms; ECV: 30%) and three (T1:1000 ms; ECV: 33%) standard deviations above control for further analysis. | |
| Significant differences in native T1-mapping in comparison to control group were observed in acute myocarditis (1045 ms ± 65 ms, p=0.0002), acute myocardial infarction (1081 ms ± 105 ms, p<0.0001), DCM (952 ms ± 50 ms, p=0.0016), HCM (1001 ms ± 64 ms, p=0.015) and amyloidosis (1078 ms ± 47 ms, p=0.021). ECV was significantly increased in all patient groups compared to the control group. Highest values for ECV were observed in acute myocarditis (43.3% ± 14.7%, p=0.008), acute (54.1% ± 12.8%, p<0.0001) and chronic (35.22% ± 12.1%, p=0.013) myocardial infarction, sarcoidosis (46.05% ± 12.4%, p=0.005) as well as in amyloidosis (57.5% ± 9.2%, p=0.027). | |
| Values are illustrated in detail in Table 3 und Figure 2. | |
| Table 3: Values of ECV and native T1 values in common pathologies in CMR. | |
| Figure 2: Comparison of mean native T1-values and ECV of common pathologies in study population. Cutoff for native T1 and ECV were 2 SD above control; 970ms, 30.4% (red lines).The asterisk marks groups with a significant difference in comparison to control. | |
| Additionally, ROC analysis was done to distinguish between acute and chronic myocarditis, as well as acute and chronic infarction by native T1 values (Figure 3a and 3b). | |
| Figure 3: ROC-Curve of T1 values for detection of acute versus chronic myocarditis (A) As well as acute versus chronic infarction (B). 2 SD=970 ms; 3 SD=1000 ms. | |
| As shown on Figure 3a, diagnostic performance of T1 mapping was moderate to distinguish between acute and chronic myocarditis. Diagnostic value of T1 mapping to differentiate between acute and chronic infarction was even more limited as shown in Figure 3b. | |
| Sensitivity and specificity for two and three standard deviations, as well as optimized thresholds are provided in Table 4 for native T1 and in Table 5 for measured ECV in evaluated pathologies. | |
| Table 4: T1 relaxation times as threshold for detection of acute and chronic infarction and myocarditis 2 SD=970 ms, 3 SD=1000 ms. | |
| Table 5: ECV values as threshold for detection of acute and chronic infarction and myocarditis 2 SD=30.4%, 3 SD=32.6. | |
| Over all gender specific differences were not significant for ECV and T1 relaxation-times. Mean values were 942 ms and 949 ms for T1 relaxation-times and ECV of 29.9% and 29.6% for male and female subjects, respectively, (p=0.4 for T1 relaxation-times, p=0.66 for ECV). | |
Discussion |
|
| T1-mapping and ECV correlated well with myocardial alterations in commonly diagnosed cardiac disorders. | |
| It proved reliable and robust in daily clinical practice and allowed for a good differentiation between normal findings and common pathologic CMR diagnoses. | |
| This study demonstrates the limited potential of native T1 mapping to distinguish between acute and chronic myocardial alterations. | |
| Range of normality | |
| As recommended by Moon et al. [15], we used the results of control patients to define threshold values in order to differentiate between normal and abnormal T1-mapping results. Using the commonly used confidence interval for normal values of two standard deviations around the mean, T1 relaxation-values lower than 850 ms and higher than 970 ms and ECV values lower than 22% and higher than 30% should be regarded as abnormal in our setting. | |
| In literature, an upper threshold value for T1-mapping with MOLLI of 990 ms is suggested [8], which is comparable to our results given the known dependency of the method on scanner hardware and sequence setting [15], and the reported sensitivity and specificity on detecting changes in myocarditis of around 90% are in agreement to our findings. However in a review of control groups, the relaxation times presented by this study seem to be systematically a little bit lower in general [22]. This is very likely a consequence of the used shortened readout pattern used in this study (4-(1)-3-(1)-2) which is supposed to be less heart rate depended compared to the original readout pattern (3-(3)-3-(3)-5) [23-25]. | |
| Results from our control group indicate that, there is no significant difference in ECV and T1 relaxation-times in male and female patients. Furthermore results indicate that age has no effect on T1 relaxation-times. This supports the role of T1-mapping as a reliable tool in daily practice. | |
| Native T1-mapping | |
| As expected, native T1 relaxation-times were prolonged in pathologies associated with cell edema indicating acute tissue damage, in which increased signal intensities in T2 imaging can be observed, namely acute myocarditis [2] and acute myocardial infarction [26]. Elevation of T1 relaxation-times in these pathologies was highly significant and well above the limit of normality resulting in excellent sensitivity and specificity. | |
| In contrast to T2-weighted imaging, T1 relaxation-times are prolonged in myocardial disease associated with fibrosis or increased extracellular volume in the absence of acute inflammation, too. Longest T1 relaxation-times were observed in chronic myocardial infarction and amyloidosis. In these two pathologies, T1 relaxation is consistently above the limit of normality with sensitivities and specificities sufficient for a robust detection and exclusion of pathology. As reported before myocardial alterations appearing in amyloidosis can be detected by native T1 mapping alone as the extracellular amyloid deposition causes a massive prolongation of T1 relaxation times [5,15,27]. | |
| Dilated and hypertrophic cardiomyopathies also consistently showed prolonged T1 relaxation- times significantly higher than control group. These findings are in accordance to current literature as DCM and HCM are associated with increased T1 relaxation times [28,29]. | |
| For chronic myocarditis, sarcoidosis and aortic stenosis only, inconsistent prolongation of T1 relaxation-times was observed. In these pathologies, native T1-mapping can therefore only be used to raise suspicion of, but not to rule out, myocardial disease. | |
| The optimal threshold to differentiate the different pathologies from normal myocardium varied for the different pathologies with the lowest threshold of 925 ms for dilated cardiomyopathy and the highest threshold of 962 ms for acute myocardial infarction; only amyloidosis has consistently very high native T1 values, shifting the optimal cut-off to 1013 ms. Therefore it is reasonable to use a threshold of 2 SD above average as threshold for pathology; using 3 SD reduced sensitivity without any change in specificity. | |
| The fact that T1 relaxation-times are prolonged not only in myocardial inflammation but also in chronic fibroses limits its use for differentiation between acute and chronic pathology, for which T2 imaging often is used. While the values for sensitivity and specificity of 90% and 78% respectively for differentiation of acute from chronic myocarditis were acceptable for clinical use, the commonly used thresholds result in an inacceptable low specificity for differentiation of acute myocardial infarction from a chronic process. In this situation, a much higher threshold of 1100 ms has to be used to gain a reasonable accuracy. | |
| Extracellular volume | |
| As expected, ECV was markedly increased in pathologies consistently showing scar, fibrosis or otherwise increased extracellular volume. Values above 50% were observed in acute or chronic infarction and amyloidosis; in sarcoidosis ECV usually was above 40%. | |
| In acute myocarditis, where extracellular space is increased due to interstitial edema after myocardial damage [2], ECV was markedly increased, too. But it showed a certain extent of variability resulting in an optimal threshold for differentiation from normal myocardium of 30.3%, just above the 2 SD above average normal. Taking into account the importance of this diagnosis and the fact that specificity decreases only marginally when compared with a threshold of 3 SD, it seems reasonable to use 30% ECV as general upper limit of normality. This threshold can be supported by a paper by Radunski et al. who describes an optimal diagnostic performance to detect myocarditis with a threshold of 29% [30]. | |
| Daily use | |
| Retrospectively, diagnosis would have been facilitated in several cases by native T1-mapping because the extent of myocarditis was underestimated in some cases with standard sequences. | |
| Although the number of patients in this study was too small for a statistical analysis, T1-mapping seemed more robust than dark-blood T2-imaging and was better to be interpreted particularly in patients with tachycardia or arrhythmia where T2-weighted imaging was compromised by motion and off-resonance artifacts. | |
Conclusion |
|
| By assessing both myocardial inflammation and increased intracellular volume, the combination of native T1-mapping and assessment of ECV after gadolinium application allows for a robust and reliable assessment of pathologies commonly seen in daily practice. The method proved quite immune to artifacts and showed a very high sensitivity even in subtle changes. Compared to T2- imaging, it was of limited use in the differentiation between acute and chronic infarction. Overall T1-mapping seems to be a promising approach for comprehensive assessment of the myocardium and may improve diagnostic accuracy of CMR. | |
Limitations |
|
| Except from amyloidosis or sarcoidosis, diagnosis was not proven by endo-myocardial biopsy, but on clinical data and conventional CMR alone. | |
| Patients of the control group underwent CMR with a clinical indication; nevertheless, there was no medical history of cardiac disease for those patients. | |
| This study covers several common entities in a single-center approach but was not powered to allow a superiority analysis in the different pathologies. | |
Compliance with Ethical Standards |
|
| Conflict of interest | |
| Andreas Greiser is a full-time employee of Siemens Healthcare. | |
| Ethical approval | |
| All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. | |
| This is a retrospective study. All examinations were clinically indicated. | |
References |
|
|
|
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 



