Review Article, Int J Cardiovasc Res Vol: 9 Issue: 2
Anticoagulation in Patients with Atrial Fibrillation After a Transcatheter Aortic Valve Replacement: A Review
Garly Saint Croix1*, Jason Galo2, Sharde Chambers2 and Nirat Beohar1
1Division of Cardiology, Columbia University, Mount Sinai Medical Center of Florida, United States
2Department of General Internal Medicine, University of Miami, Miami, FL, United States
*Corresponding Author: Dr Garly Saint Croix
Columbia University, Division of Cardiology at Mount Sinai Medical Center of Florida, Miami Beach, FL 33140, Florida, United States
Tel: +17868126137
E-mail: saintcroix.garly@gmail.com
Received: February 19, 2020 Accepted: February 24, 2020 Published: March 02, 2020
Citation: Saint Croix GR, Galo J, Chambers S, Beohar N (2020) Anticoagulation in Patients with Atrial Fibrillation After a Transcatheter Aortic Valve Replacement: A Review. Int J Cardiovasc Res 9:2. doi: 10.37532/icrj.2020.9(2).396
Abstract
Transcatheter Aortic Valve Replacement (TAVR) is now the treatment of choice for patients with severe aortic stenosis regardless of their surgical risk. A significant portion of TAVR patients have concomitant Atrial Fibrillation (AF) leading to worse prognosis. Studies about the safety and efficacy of anticoagulation in this group are scarce. Hence, this review aims to assess the differential clinical outcomes of the type of anticoagulation on patients who underwent TAVR with concomitant AF. We performed a systematic literature review to present the data from identify randomized and nonrandomized clinical studies that reported anticoagulation for AF after a TAVR. In addition, we consulted the literature to check expert’s opinion on the subject and summarized them in this review.
Keywords: TAVR; Aortic stenosis; Anticoagulation; Stroke; Bleeding
Introduction
According to the US Census, there are over 47 million people who are 65 years and older living currently living in the United States, which translates to about 15% of the general population [1]. Up to 1.5 million are estimated to be living with Aortic Stenosis (AS), with the highest prevalence among those who are 80-89 years old with natural course of the disease expected to progress as the affected population continues to age [2]. With such a high burden of aortic valvular disease, the advent of TAVR has become an unprecedent era of cardiology. Currently, TAVR is a low-risk and common procedure with significant decrease of morbidity and mortality over the last decades confirmed by positive outcomes for patients even with low surgical risk according to PARTNER 3 and Evolut Low Risk trials [3,4]. Without intervention, patients with symptomatic severe AS have an average life expectancy of 1-2 years [4]. Those with severe features and who are asymptomatic will die in 75% of the cases or have symptoms in 5 years [5]. Furthermore, the same population of severe aortic valve stenosis who needs valve replacement usually have concomitantly a high prevalence of AF. According to a registry analysis, 40% of patients undergoing TAVR have pre-existing AF and 8 to 15% of TAVR cases develop new-onset AF in the post-op setting [6]. This leads to an increase of risk for major cardiovascular and thromboembolic adverse events [7]. The literature is scarce regarding management of AF with TAVR and there are no specific guideline recommendations to follow. This situation leads to uncertainty regarding the use of anticoagulation in these patients. Therefore, giving the importance of this topic because of the thrombo-embolism risk burden especially when a debilitating stroke event occurs, the aim of this review is to determine the different options for antithrombic therapy, assess and recommend the best strategy to pursue the ideal benefits for these patients .
TAVR and Risk of Thromboembolic Events
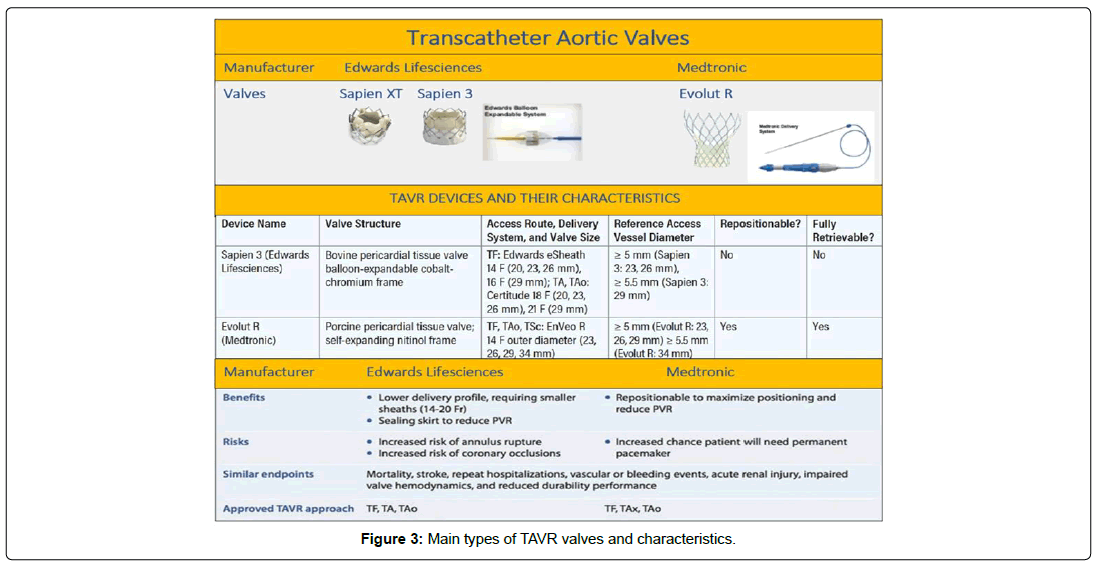
Several advancements have been made after the introduction of TAVRs in humans in 2002 by Dr. Cribier in France [8]. Since then, prosthetic valve modifications have been implemented to reduce perioperative morbidity and mortality as well as thromboembolic complications related to intervention in a severe aortic valve stenosis. Currently, although there are a lot of prosthetic aortic valves in the market, two main ones remain balloon expandable (SAPIEN VT and SAPIEN 3) and self-expandable valves (Evolut and Evolut R) [9]. Though each valve type functionally reinstitutes appropriate flow gradient and opening apparatus for cardiac outflow, there are certain advantages and disadvantages to each. The Edward Sapiens valve is a porcine type, has a balloon mounted delivery system to provide radial force against the annulus and the leaflets, requiring rapid ventricular pacing to be implemented. It cannot be retrieved once deployed, it depends on aortic elasticity and has less potential for atrio- ventricular block. On the other side, the Medtronic Core valve is a bovine type, has a self-expendable delivery system that relies on passive radial force to seal the annulus and the leaflets, allowing proper delivery in beating heart. Reposition of this valve is possible after deployment, it involves ascending aortic support and has more potential to create atrio-ventricular block [10]. Thus, there are many deciding factors that are taken into consideration when choosing a specific valve type for TAVRs, including the annular apparatus/burden of calcification of the aorta, proximity of the aortic root and Left Ventricular Outflow Tract (LVOT) to the aorta, coronary obstruction/occlusion, and paravalvular leaks/regurgitations [11]. However, there is no headto- head randomized study between the 2 valves and both of them have comparable safety and efficacy features, reassuring long-term outcomes after 5 years although degenerative changes start to occur around 10 years after placement [12]. Nonetheless, considering an AF perspective, several studies of the aortic root and LVOT to the aorta, and calcification of the valve have shown a selective advantage of selfexpandable valves over balloon-expandable valves in patients at risk for AF or with close proximity for embolization [12,13]. However, in the absence of evidence-based medicine, it becomes unclear to confirm which valve is better than the other in inducing AF as well as reducing the risk of stroke, TIA and other thrombo-embolic events.
The incidence of AF has not been documented to be related to the type of valves inserted [13]. The choice study showed a lower incidence of aortic regurgitation with balloon-expandable valves compared to self-expandable valves though data seems to be lacking regarding long-term mortality benefit [14]. Although this study is one of the rare comparing balloon vs self-expandable valves, this prospective study didn’t assess outcomes of AF patients treated with aortic valve replacement.
The rate of stroke and thromboembolic events associated with TAVRs 30 days after surgery leading to both clinical and subclinical strokes and coronary obstruction can be up to 15.9% [15]. This means that stroke remains one of the most common complications of TAVR and is associated with increased mortality [16]. The etiology of post- TAVR stroke is most commonly debris embolization during the procedure. The histopathologic analysis of the debris varies, though thrombus formation occurs in almost every case [17]. Though stroke is a devastating complication of TAVR, there is little experience with early intervention to improve outcomes and using a carotid sentinel to catch potential distal debris didn’t show additional benefits [18]. Atrial Fibrillation may occur frequently during the peri-procedural period of TAVR and its incidence doubles the risk of stroke in this population [19]. Thus, intra- and postprocedural antithrombotic therapy is required, although the regimen that best minimizes thromboembolic events and bleeding complications has yet to be defined. Patients undergoing TAVR with comorbid conditions requiring oral anticoagulation or individuals who develop AF pose unique challenges. Antiplatelet and anticoagulant therapy should then be carefully balanced (Table 1) [20].
| Variable | Apixaban (n=141) | Vitamin K Antagonist (n=131) | P Value |
|---|---|---|---|
| Primary safety endpoint: composite of all-cause mortality, all stroke, life-threatening bleeding, acute kidney injury, coronary obstruction, major vascular complication, and valve dysfunction requiring intervention | 13.5% (19) | 30.5% (40) | <0.01 |
| Acute kidney injury stages 2 and 3 | 2.1% (3) | 8.4%(11) | <0.01 |
| Life-threatening bleeding | 3.5% (5) | 5.3% (7) | <0.01 |
source: (Seeger et al.)
Table 1: Apixaban vs. vitamin-K antagonist: 30 day outcomes.
Atrial Fibrillation, Burden and Types
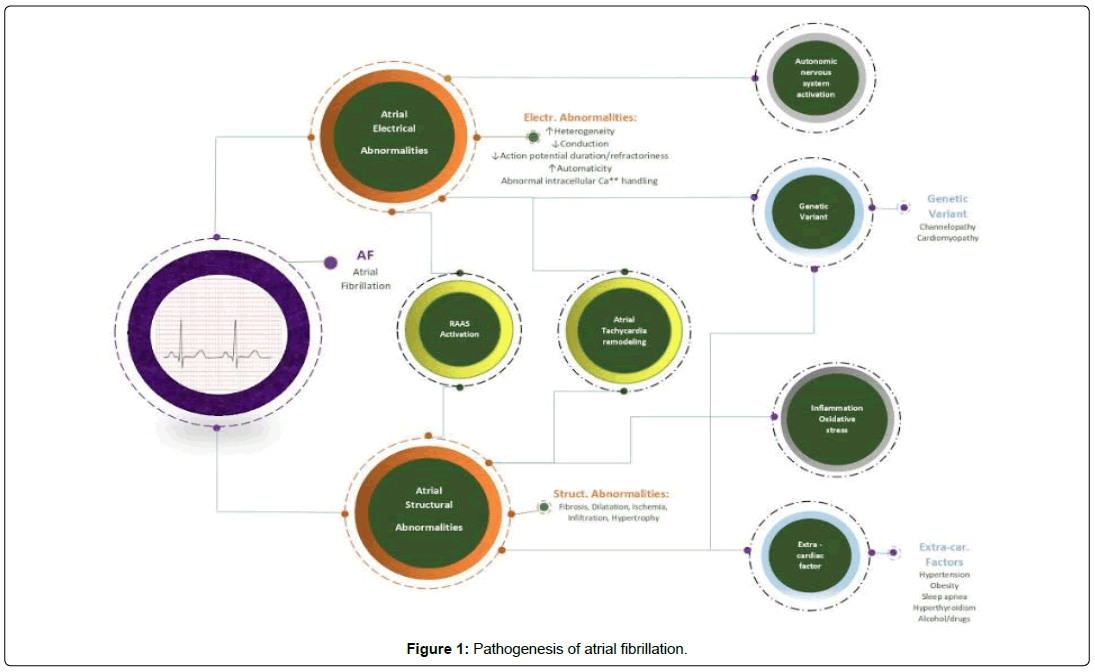
Atrial Fibrillation is a subtype of supra ventricular tachycardia that causes an irregularly irregular heart rhythm. AF is the most common sustained arrhythmia characterized by documentation of absolutely irregular RR intervals and no discernable, distinct p waves lasting to at least 30 seconds. This leads to disorganized, rapid, and irregular atrial activation leading to irregular ventricular response [21]. Furthermore, prevalence increases with age and approximately 70% if individuals with AF are between 65 and 85 years of age and the prevalence of AF is 9% in patient aged 80 years old and higher [22]. In all age groups, males are more commonly affected than females. Despite a high prevalence of risk factors, African Americans tend to have lower AF incidence compared to Caucasians (Figure 1). The American Heart Association and American College of Cardiology further classified AF as follows: Paroxysmal Atrial Fibrillation: intermittent in nature, terminating spontaneously or within 7 days of treatment. Persistent Atrial Fibrillation: Failure to terminate in 7 days. Long lasting Atrial Fibrillation: Lasting for more than 12 months. Permanent Atrial Fibrillation: Persistent AF where rhythm strategy is no longer pursued [23]. Another classification of AF is non-valvular vs valvular. The latter defines whether the disease is related to valvular problems, replacement or repair.
Atrial Fibrillation and TAVR Patients
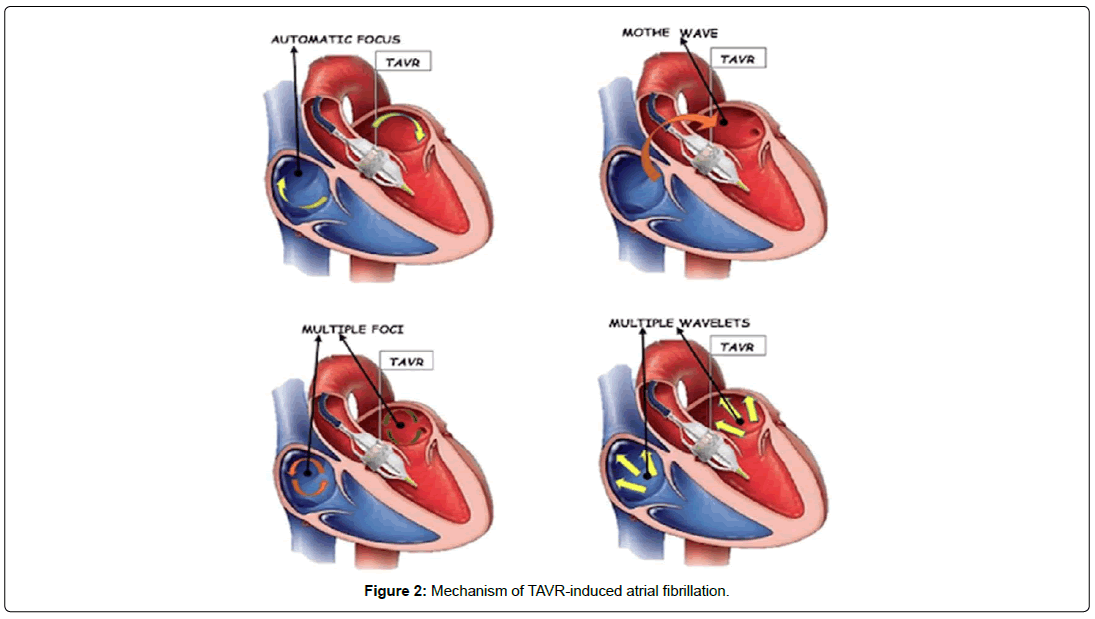
Given AF and aortic stenosis are strongly age-dependent, overlap between the 2 conditions can occurs. Hence, AF has been shown to coexist in up to 50% of patients with symptomatic aortic stenosis undergoing aortic valve replacement . Atrial Fibrillation can be a pre-existing comorbidity in patients undergoing TAVR, but it can often present after the procedure [24]. It may also occur frequently during the peri- procedural period [25]. After TAVR, AF is the most common reason a patient will require anticoagulation. A metaanalysis concluded that AF is common in TAVR population with 33.1% of the overall study population of 11,033 patients. 26.2% of them had AF prior TAVR, 6.9% of them developed new onset AF [26]. PARTNER 1 trial compared trans-catheter versus surgical aortic valve replacement in high-risk patients and found 40% of the total patients had pre-existing AF [27] This highlights the significant prevalence of AF in patients before they undergo aortic valve replacement. The incidence of new-onset AF after trans-arterial valve replacement is also important. According to expert opinion, TAVR can induce mechanical and electrical changes during deployment leading to AF by different mechanism as shown in (Figure 2). A study looking at outcomes data from 13,356 patients undergoing TAVR across the U.S. found that 1,138 patients, or 8% developed AF for the first time after the procedure [28]. Other studies have shown the prevalence of pre-existing AF to be also around 40%, but the incidence of newonset AF could be as high as 15%. Furthermore, patients who undergo TAVR may develop structural valve dysfunction, myocardial injury be related to leaflet thrombosis and is a culprit of stroke formation [29]. Atrial fibrillation, whether it is pre-existing or new onset, is associated with worse outcomes in TAVR patients including risk of cerebrovascular events and death. The impact of new AF on post-TAVR patient showed an increased risk of stroke at early (odds ratio (OR) 2.1, p<0.0001) and late follow up (OR 1.92, p<0.0001) compared to sinus rhythm. In addition, according to the same study, there was more risk of bleedings (OR 1.65, p=0.002) and increased risk of late stroke (OR 1.3 p 0.03) in the group with pre-existing AF in TAVR. The impact of newonset AF is significant, as these patients experienced a 37 percent higher risk of death after one year, regardless of the type of AF (Table 2).
| Variable | New-onset AF (n=1,138) | No AF (n=12,418) | P Value |
|---|---|---|---|
| In-Hospital mortality: | 7.8% | 3.4% | <0.01 |
| In-Hospital stroke | 4.7% | 2.0% | <0.01 |
Rate of stroke 1 year hazard ratio: 1.50; 95% CI: 1.14 - 1.98
Rate of bleeding 1 year hazard ratio: 1.24; 95% CI: 1.10 – 1.40
Source: (Vora et al.)
Table 2: New onset atrial fibrillation after TAVR registry 2011-2015.
Risk factors associated with the development of new-onset AF post-TAVR included female gender, older age, and presence of chronic obstructive pulmonary disease. TAVR that was not performed via the trans-femoral route was also associated with a greater incidence of AF. Furthermore, these patients seem to be at an increased risk of both bleeding and stroke. The risk of stroke is highest during the sub-acute phase (days 1-30) after the procedure but is also higher at 12 months. There has been so far a large, single-center, prospecting study evaluating the use of novel oral anticoagulants (apixaban) and an ongoing trial using edoxaban in patients with pre-existing AF who undergo TAVR [30]. In one of these studies comparing warfarin against apixaban, safety endpoints measures were significantly lower in patients receiving anticoagulation with apixaban compared to warfarin (13.5% vs 30.5%, p=<0.01) (Table 1). A study evaluating the impact of continued versus interrupted anticoagulation with warfarin versus novel oral anticoagulants (NOAC) and found that the rate of early safety events was the lowest in NOAC (13.2%) and not increased in continued warfarin (19.7%) compared to interrupted warfarin (23.1%). In addition, all-cause 1-year mortality was 20.1% in interrupted warfarin, 13.7% in continued warfarin and 8.8% in NOAC. In addition, there is currently an ongoing randomized clinical trial assessing the benefits of edoxaban in patients undergoing TAVR with concomitant AF, which will be the first of its kind (Table 3).
| Variable | iVKA (n=299) | cVKA (n=117) | NOAC (n=182) | P value |
|---|---|---|---|---|
| Early safety at 30 days: composite of all-cause mortality, all stroke, life-threatening bleeding, acute kidney injury stages 2 and 3, coronary obstruction requiring intervention, major vascular complication, and valve dysfunction requiring repeat procedure | 23.1% (69) | 19.7% (23) | 13.2% (24) | 0.029 |
| Renal failure at 30 days | 15.8% (46) | 9.5% (11) | 7.7% (14) | 0.020 |
| 1-Year mortality | 20.1% | 13.7% | 8.8% | 0.015 |
the 1 year mortality significant benefit of noac was driven largely by a lower cardiovascular mortality compared to ivka and cvka (p=0.004).
Source: (Mangner et al.)
Table 3: NOAC vs. Interrupted/continued vitamin-K antagonist in Afib w TAVR: 30 day 1-year outcomes.
Should CHA2DS2-VASc Score be Used for Atrial Fibrillation in TAVR Patients?
The question as to whether the CHA2DS2-VASc score should be used in TAVR patients is one that requires further clinical investigations via a randomized-controlled trial with appropriate risk stratification. The CHA2DS2-VASc score is a well-validated tool to estimate the risk of thromboembolism in patients with AF, and it is widely used to guide decision-making regarding anticoagulation. However, the literature is scare regarding its benefit when a patient undergoes a TAVR and has concomitant AF. Currently, at CHA2DS2-VASc scores of 2, there appears to be a higher risk of bleeding compared to benefit from anticoagulation. However, the protective effects of anticoagulation were observed with a greatest impact on these with CHA2DS2-VASc score of 5 [31]. Most patients undergoing TAVR with either new-onset AF or pre-existing AF have a higher risk of stroke and thrombosis compared to their peers, and it is likely that these patients require a lower threshold for beginning anticoagulation. However, this traditional tool of estimating risks versus benefits of anticoagulation in the setting of AF have not been validated in patients undergoing TAVR.
Current Clinical Practices for Post-TAVR with Concomitant Atrial Fibrillation
Previous study showed that patients who develops AF after their TAVR procedure have a higher risk of cardiovascular death, stroke or myocardial infarction (MI) when they are compared to patient previously with stroke prior the TAVR [32]. Nonetheless, currently there exist no clear practice or guidelines regarding the optimal treatment strategy for patients who develop new-onset AF after TAVR. Neither the European Society of Cardiology (ESC) nor the American Heart Association/American College of Cardiology (AHA/ACC) have a recommendation or a clinical practice guideline regarding this matter. NOAC studies are ongoing to assess benefit in this patient population. Hence according to the current literature, warfarin has been suggested as anticoagulant strategy of choice in the setting of bioprosthetic aortic valve placement with AF. At this time, according to the experts, there is no specific data to support a clear strategy. The management currently should be done on a case basis, with evaluation of risk factors and shared decision between patient and physician discussion and it is either warfarin only, NOAC only or NOAC + aspirin (ASA) . There is a need of carefully designed clinical trials to study and elaborate furthermore on the relationship between AF and TAVR (Table 4) [33].
| Trials | Regimen | Target population | Study characteristics | Outcomes |
|---|---|---|---|---|
| Apixaban in patients with atrial fibrillation after transfemoral aortic valve replacement (Seeger et al. 2017) |
Apixaban (dose adjusted per its instructions for use vs. Vitamin K Antagonist (warfarin) | Patients who underwent transfemoral TAVR (n=617), 272 of which had atrial fibrillation/new-onset AF | Antiplatelet therapy was given for 4 weeks, and restart oral anticoagulation (either vitamin K antagonist or apixaban) 48 hours post-TAVR (Seeger et al. 2017) | The early safety endpoint in patients with AF on apixaban was significantly less frequent compared to patients receiving vitamin K antagonists |
| continued versus interrupted oral anticoagulation during transfemoral transcatheter aortic valve implantation and impact of postoperative anticoagulant management on outcome in patients with atrial fibrillation (Vora et al. 2018) |
iVKA: bridged with low-molecular weight heparin or unfractionated heparin. VKA restarted 24-48 hours after TAVR cVKA: remained during periprocedural phase on VKA with goal INR: 2-3 DOAC: remained on DOAC during periprocedural phase, last dose day before and restarted morning after procedure |
Patients with pre-existing AF and on OAC at admission (n=598) who received Trans-Femoral-Transcatheter Aortic Valve Replacement for severe aortic stenosis or bioprosthetic valve failure | Retrospective study, patients stratified according to interrupted Vitamin K antagonist (iVKA) versus continued (cVKA) versus continued direct oral anticoagulants (DOAC) after TAVR from 1/2011-3/2016 | Primary outcome measure (rate of early safety events), cardiovascular mortality at 30 days, all cause one-year mortality, was significantly lowest in DOAC compared to iVKA and cVKA. |
| ** To be completed November 2020 Edoxaban Versus Standard of Care and their effects on clinical outcomes in patients having undergone transcatheter aortic valve implantation in atrial fibrillation -ENVISAGE-TAVI AF (Van Mieghem et al. 2018) |
Edoxaban 60mg/day vs. VKA-based therapy (target INR: 2-3) | 1,400 patients with an indication for chronic oral anticoagulation after successful transfemoral TAVR | Eligible patients randomized in 1:1 to experimental arm (edoxaban) or control arm (approved vitamin K antagonist), with antiplatelet therapy at discretion of physician | Net adverse clinical events (composite of all-cause death, myocardial infarction, ischemic stroke, systemic thrombo-embolism, valve thrombosis, and major bleeding events) |
Table 4: Trials assessing anticoagulation for TAVR with concomitant AF.
Suggested Algorithm for Management of TAVR Patients with Atrial Fibrillation
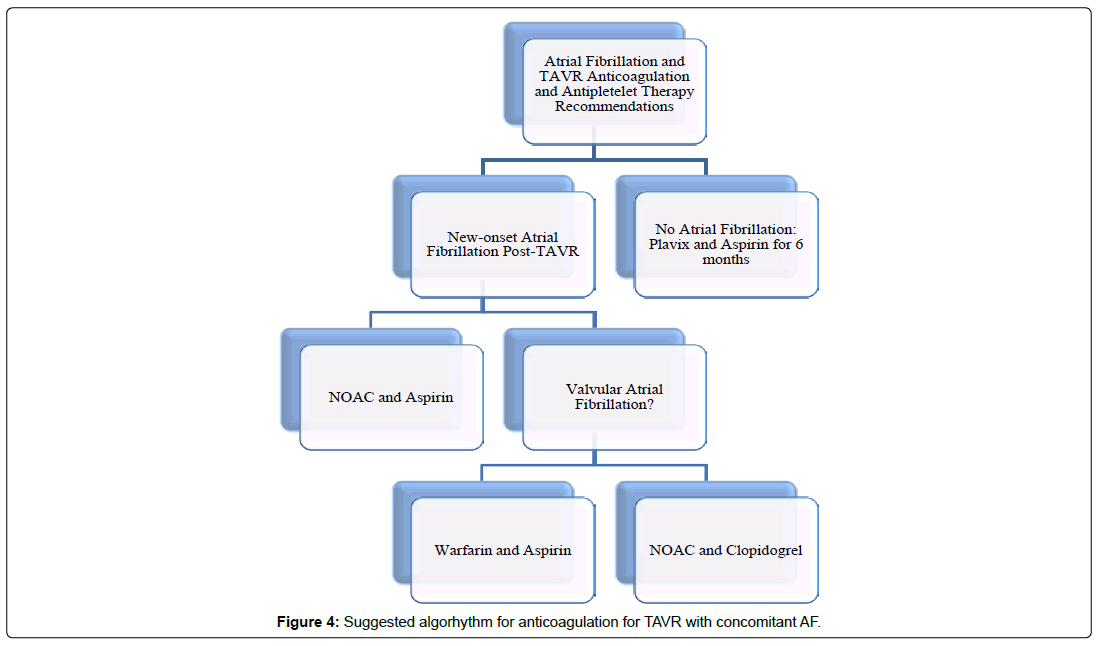
Based on some recent randomized controlled trials, transcatheter aortic valve replacement (TAVR) has become the treatment of choice for management of severe aortic valve stenosis regardless of patient surgical risk. Atrial fibrillation is a common morbidity frequently found concomitantly in patients undergoing TAVR both as a pre-existing condition and newonset post-procedure. The choice of anticoagulation in patients who have undergone TAVR and have AF is lacking in robust evidence to lead to clinical guidelines. Although there is limited data, this current review proposes an algorithm (Figure 3) and suggests that if a patient has preexisting nonvalvular AF prior to TAVR, we recommend continuing NOAC without concomitant use of antiplatelet. If a patient has new onset AF after TAVR, we recommend stopping previous antiplatelet agent and initiate a NOAC. If patient has valvular AF prior or after TAVR, we recommend vitamin K antagonist therapy (International Normalized Ratio (INR) 2-3) (Figure 4). However, further randomized trials need to better evaluate the optimal antiplatelet and anticoagulant therapy in these patients.
References
- Nombela-Franco L, Webb JG, de Jaegere PP, Toggweiler S, Nuis RJ, Mangner. (2012) Timing,predictive factors, and prognostic value of cerebrovascular events in a large cohort of patients undergoing transcatheter aortic valve implantation. Circulation 126: 3041-3053.
- Nijenhuis VJ, Bennaghmouch N, van Kuijk JP (2015) Antithrombotic treatment in patients undergoing transcatheter aortic valve implan- tation (TAVI). Thromb Haemost 113: 6746-6785.
- Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, et al. (2019) Trans-catheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med 380: 1706-1715.
- Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, et al. (2019) Transcatheter aortic-Valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 380: 1695-1705.
- Pasic M, Unbehaun A, Buz S, Drews T, Hetzer R, et al. (2015) Annular rupture during transcatheter aortic valve replacement: classification, pathophysiology, diagnostics, treatment approaches, and prevention. JACC Cardiovasc Interv 8(1 Pt A): 1-9.
- Maan A, Heist EK, Passeri J, Inglessis I, Baker J, et al. (2015) Impact of atrial fibrillation on outcomes in patients who underwent transcatheter aortic valve replace- ment. Am J Cardiol 115: 220-226.
- Mok M, Urena M, Nombela-Franco L, Ribeiro HB, Allende R, et al. (2013) Clinical and prognostic implications of existing and new-onset atrial fibrillation in patients undergoing transcatheter aortic valve implantation. J Thromb Thrombolysis 35: 450-455.
- Cribier A (2012) Development of transcatheter aortic valve implantation (TAVI): a 20-year odyssey. Arch Cardiovasc Dis 105: 146-152.
- Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, et al. (2016) Trans- catheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 374:1609-1620.
- Tang GH (2018) TAVR with the largest balloon-expandable vs self-expanding valve in very large annuli: a multicenter study. Structural Heart Disease Summit, Chicago, IL.
- Vlastra W, Chandrasekhar J, Munoz-Garcia AJ, Tchetche D, de Brito FS, et al. (2019) Comparison of balloon-expandable vs. self-expandable valves in patients undergoing transfemoral transcatheter aortic valve implantation: from the CENTER-Collaboration. Eur Heart J. 40: 456-465.
- Rogers T, Steinvil A, Gai J, Torguson R, Koifman E, et al. (2017) Choice of balloon-expandable versus self-expanding transcatheter aortic valve impacts hemodynamics differently according to aortic annular size. Am J Cardiol 119: 900-904.
- Tarantini G, Mojoli M, Urena M, Vahanian A (2017) Atrial fibrillation in patients undergoing transcatheter aortic valve implantation: epidemiology, timing, predictors, and outcome. Eur Heart J 38: 1285-1293.
- Abdel-Wahab M, Mehilli J, Frerker C, Neumann FJ, Kurz T, et al. (2014) Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcatheter aortic valve replacement: The choice randomized clinical trial. JAMA 311: 1503-1514.
- Nuis RJ, Van Mieghem NM, Schultz CJ, Moelker A, van der Boon RM, et al. (2012) Frequency and causes of stroke during or after transcatheter aortic valve implanta- tion. Am J Cardiol 109: 1637-1643.
- Hamandi M, Farber AJ, Tatum JK, Brinkman WT, Brown DL, Acute stroke intervention after transcatheter aortic valve replacement. Proc (Bayl Univ Med Cent) 31: 490–492.
- Holmes DR, Mack MJ, Kaul S, Agnihotri A, Alexander KP, et al. (2012) ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol 59: 1200-1254.
- Saxena A, Shi WY, Bappayya S, Dinh DT, Smith JA, et al. (2013) Postoperative atrial fibrillation after isolated aortic valve replacement: a cause for concern? Ann Thorac Surg 95: 133-140.
- Kalich BA. Allender JE. Hollis IB ( 2018 ) Medication management of patients undergoing transcatheter aortic valve replacement. Pharmacotherapy 38: 122-138.
- Appel CF, Hultkvist H, Nylander E, Ahn H, Nielsen NE, et al. (2012) Transcatheter versus surgical treatment for aortic stenosis: patient selection and early outcome. Scand Cardiovasc J 46: 301-307.
- Ludhwani D, Wieters JS (2019) Paroxysmal atrial fibrillation. StatPearls, Treasure Island (FL).
- Harb S, Hussein AAH, Saliba WIS, Wu YU, Xu BX, et al. (2018) Effect of anticoagulation on mortality by CHADSVASC score in patients with atrial fibrillation: Comparison to patients without atrial fibrillation. European Heart Journal 39: 1075.
- Chen LY, Chung MK, Allen LA, Ezekowitz M, Furie KL, et al. (2018) Atrial fibrillation burden: moving beyond atrial fibrillation as a binary entity: a scientific statement from the american heart association. Circulation 137: e623-e644.
- Glenn K (2018) Patients with new-onset afib after tavr at highest risk for complications. American college of cardiology.
- Vavuranakis M, Kolokathis AM, Vrachatis DA, Kalogeras K, Magkoutis NA, et al. (2016) Atrial fibrillation during or after tavi: incidence, implications and therapeutical considerations. Curr Pharm Des 22: 1896-1903.
- Mojoli M, Gersh BJ, Barioli A, Masiero G, Tellaroli P, et al. (2017) Impact of atrial fibrillation on outcomes of patients treated by transcatheter aortic valve implantation: A systematic review and meta-analysis. Am Heart J 192: 64-75.
- Leon MB, Smith CR, Mack M, Miller DC, Moses JW, et al. (2010) Trans-catheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 363: 1597-1607.
- Vora AN, Dai D, Matsuoka R, Harrison JK, Hughes GC, et al. (2018) Incidence, management, and associated clinical outcomes of new-onset atrial fibrillation following transcatheter aortic valve replacement: An analysis from the STS/ACC TVT Registry. JACC: Cardiovascular Interventions 11: 1746-1756.
- Van Mieghem NM, Unverdorben M, Valgimigli M, Mehran R, Boersma E, et al. (2018) Edoxaban versus standard of care and their effects on clinical outcomes in patients having undergone transcatheter aortic valve implantation in atrial fibrillation-rationale and design of the ENVISAGE-TAVI AF trial. Am Heart J 205: 63-69.
- January CT, Wann LS, Calkins H, Chen Y, Cigarroa JE, et al. (2019) 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart rhythm society. J Am Coll Cardiol 140: 126-130.
- Seeger J, Gonska B, Rodewald C, Rottbauer W, Wöhrle J, et al. (2017). "Apixaban in patients with Atrial fibrillation after transfemoral aortic valve replacement." JACC Cardiovasc Interv 10: 66-74.
- Yankelson L, Steinvil A, Gershovitz L, Leshem-Rubinow E2, Furer A, et al. (2014) Atrial fibrillation, stroke, and mortality rates after transcatheter aortic valve implantation. Am J Cardiol 114: 1861–1866.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi