Research Article, Int J Cardiol Res Vol: 12 Issue: 6
Assessment of Left Ventricular Systolic Function in Heart Failure Patients with Preserved Ejection Fraction by 2D Speckle Tracking Echocardiography
Mohamed Elhoshy1, Samir Rafla2*, Tarek Elzawawy2, Nesma Mahmoud Morsi2 and Gehan Magdy2
1Department of Cardiology, Alexandria University, Prague, Czech Republic Egypt
2Department of Cardiology and Angiology, Alexandria University, Prague, Czech Republic Egypt
*Corresponding Author:
Samir Rafla
Department of Cardiology and Angiology,
Alexandria University,
Prague,
Czech Republic Egypt;
E-mail: smrafla@yahoo.com
Received date: 05 July, 2023, Manuscript No. ICRJ-23-104923;
Editor assigned date: 10 July, 2023, PreQC No. ICRJ-23-104923 (PQ);
Reviewed date: 24 July, 2023, QC No. ICRJ-23-104923;
Revised date: 05 December, 2023, Manuscript No. ICRJ-23-104923 (R);
Published date: 12 December, 2023, DOI: 10.4172/2324-8602.1000527
Citation: Elhoshy M, Rafla S, Elzawawy T, Morsi NM, Magdy G (2023) Assessment of Left Ventricular Systolic Function in Heart Failure Patients with Preserved Ejection Fraction by 2D Speckle Tracking Echocardiography. Int J Cardiol Res 12:6.
Abstract
Heart failure is still a chief clinical syndrome impacting health with growing prevalence and incidence rates. Almost half of the patients still have preserved Ejection Fraction (EF). However, they show the same morbidity and mortality as those of reduced EF. EF measurement is counted till now, the reliable estimate for precise assessment of the Left Ventricular (LV) systolic function. However, using strain and strain rate by Speckle Tracking Echocardiography (STE) to estimate Global Longitudinal Strain (GLS) is more accurate for measuring myocardial contractility. Therefore, it has a diagnostic and predictive morbidity and mortality benefit in studying Heart Failure patients with preserved Ejection Fraction (HFpEF) superior to EF estimation alone.
Objectives: To assess the value of 2D STE in evaluating LV systolic function in HFpEF.
Methods: We enrolled in 45 subjects. Fifteen patients with Diastolic Dysfunction (DD) and signs and symptoms of HF (HFpEF group), 15 patients with DD and no signs or symptoms of HF (asymptomatic DD group), and 15 normal subjects matched for age and gender (control group). We screened patients by conventional echocardiography and obtained echocardiography of 4, 2, and 3 chambers views. In HFpEF patients, we correlated clinical, echocardiographic, and biomarker parameters with global longitudinal strain.
Results: HFpEF patients showed significantly higher LVMI than both DD and control groups (P value<0.001 for both). GLS led to significantly lower values in the HFpEF group than in the DD and control groups (p value<0.001 for both). The cutoff point to diagnose HF in DD cases according to GLS was ≤ -16.24 with sensitivity and specificity of 80.0% and 93.33%, respectively, compared to the asymptomatic DD group. The cutoff point in GLS b etween D D c ases a nd c ontrols w as ≤ -19.05 with sensitivity and specificity of 80.0% and 73.33%, respectively. According to univariate analysis, GLS in HF patients was affected by hypertension, DM, and lateral S'.
Keywords: Global longitudinal strain; Heart failure; Preserved ejection fraction; Speckle tracking echocardiography
Abbreviations:
DD: Diastolic Dysfunction; LV: Left Ventricular; STE: Speckle Tracking Echocardiography; GLS: Global Longitudinal Strain; EF: Ejection Friction; TDI: Tissue Doppler Imaging; ROC: Receiver Operating Characteristic curve; IVST: The Interventricular Septal Thickness; LVEDD: Left Ventricular End-Diastolic Dimensions; LVESD: Left Ventricular End- Systolic Dimensions; LVPWT: Left Ventricular Posterior Wall Thickness; LVMI: Left Ventricular Mass Index; LAVI: Left Atrium Volume Index
Introduction
Heart Failure (HF) is a complex clinical syndrome that results from any structural or functional impairment of ventricular filling or ejection of blood HF may be associated with a broad spectrum of LV functional abnormalities, ranging from patients with standard LV size and preserved Ejection Fraction (HFpEF) to those with severe dilatation and markedly reduced EF (HFrEF) [1].
HFpEF currently accounts for ~50% of the general heart failure population, and its 1 year and 5 years survival rates are similar to or only slightly better than those of patients with reduced ejection fraction [2,3]. Diagnosis of HFpEF should include the following three obligatory characteristics:
• Presence of signs or symptoms of heart failure.
• Preserved systolic function.
• Evidence of diastolic dysfunction.
LV systolic function is commonly reported as an Ejection Fraction (EF). However, the measurement of EF is a simplistic approach, closely correlated to the radial component of myocardial deformation. It does not account for the complex three-dimensional deformation with the important longitudinal and circumferential plane. Subtle changes in myocardial deformation occur before the more extensive impairment of the LV that is detectable by changes in EF [4,5]..
Two-dimensional echocardiography, currently the most frequently used technique for the structural analysis of the heart, has limitations regarding observing the cardiac anatomy. It is often performed subjectively with visual interpretation (eye-bawling), which depends on the eye of an experienced observer.
Tissue-Doppler Imaging (TDI) is a technique that measures highamplitude signals of myocardial tissue motion. Echocardiographic techniques utilizing tissue-doppler imaging are more sensitive than conventional echocardiography in detecting regional changes in left ventricular contractile function. However, assessing apical myocardial segments, for instance, is known to be suboptimal with tissue-doppler imaging. Furthermore, due to its doppler-based properties, tissuedoppler imaging velocity cannot discriminate active deformation of the left ventricle (due to myocardial fibers shortening and lengthening) from passive deformation.
Strain and strain rate imaging has been demonstrated to be a more reliable method to quantify myocardial function. Essentially, myocardial strain represents the rate of myocardial deformation or stretch reflected by;
• Longitudinal and circumferential strain (shortening of the
myocardium) and
• Radial strain (lengthening of the myocardium) [6].
Indeed, prior studies suggest LV longitudinal function assessed by tissue doppler imaging may be impaired in HFpEF. However, the myocardial strain measured with tissue-Doppler imaging depends on the angle of incidence between the ultrasound beam and myocardial motion due to its Doppler-based properties. At gradients more significant than 20°, Doppler-derived strain and strain rate are significantly underestimated. TDI also assesses only mitral annular motion [7].
Speckle Tracking Echocardiography (STE) is an imaging modality for quantifying left ventricular volumes and function, which is angleindependent. Speckle tracking is a technique that tracks the movement of natural acoustic markers, or 'speckles,' present on standard grey scale ultrasound tissue images. These speckles appear due to scattering, reflection, and interference of the ultrasound beam in myocardial tissue, but they do not exist as natural structures. With wall motion tracking software, speckle movement (and, therefore, myocardial tissue movement) can be visualized from frame to frame during the cardiac cycle. STE allows the determination of the magnitude of deformation of all myocardial segments along all three orthogonal axes of the heart (radial, circumferential, and longitudinal). It can assess myocardial strain or active thickening, independent of the Doppler angle of incidence used in tissue-Doppler strain methods. STE's accuracy, validity, and clinical applications have rapidly accumulated since its introduction [8].
Materials and Methods
This study included a total of 30 patients and 15 healthy controls. All patients had diastolic dysfunction and were divided into two groups. Group I included 15 patients diagnosed as HFpEF based on clinical diagnosis of heart failure and the presence of diastolic dysfunction based on conventional echocardiography and tissue Doppler imaging. Group II included another 15 patients diagnosed with diastolic dysfunction based on traditional echocardiography and tissue Doppler imaging without signs or symptoms of heart failure. All patients were collected from the Alexandria main university hospital and medical research institute hospital and gave written informed consent. They were evaluated by history taking, clinical examination, and 12-lead ECG. Patients were examined by conventional M-mode and 2D transthoracic echocardiographic analysis using standard parasternal and apical views to assess left ventricular chamber dimensions and function and left atrium volumes. Conventional echodoppler assessed left ventricular diastolic function via transmitral inflow velocities. Pulsed wave tissue Doppler echocardiography was acquired at the septal and lateral mitral annuli. Currently performed offline, images for speckle-tracking echocardiographic analysis were obtained and recorded using conventional 2-dimensional scale echocardiography of 4, 2, and 3-chamber views during breath holding with stable electrocardiographic tracing. Standard Echocardiography and STE were done using PHILIPS, IE, 33, and QLAB software [9].
Statistical analysis
Data were fed to the computer and analyzed using IBM SPSS software package version 20.0. (Armonk, NY: IBM corp) [10]. Qualitative data were described using numbers and percentages. Quantitative data were described using range (minimum and maximum), mean, standard deviation, and median. The significance of the obtained results was judged at the 5% level. The Chi-square test was used for categorical variables to compare different groups. Monte Carlo correction was used for chi-square when more than 20% of the cells have an expected count less than 5, F-test (ANOVA) for normally distributed quantitative variables to compare between more than two groups, and post hoc test (Tukey) for pairwise comparisons. The Receiver Operating Characteristic curve (ROC) was generated by plotting sensitivity (TP) on the Y axis versus 1-specificity (FP) on the X axis at different cutoff values. The area under the ROC curve denotes the diagnostic performance of the test. A location of more than 50% gives an acceptable performance, and an area of about 100% is the best performance for the test. Sensitivity: The capacity of the test to correctly identify diseased individuals in a population "true positives." The greater the sensitivity, the more minor the number of unidentified cases of "false negatives." Specificity: The capacity of the test to correctly exclude individuals who are free of the disease "true negatives." The greater the specificity, the fewer "false positives" will be included. Regression: To detect the most independent/affecting factor for GLS.
Results
Table 1 shows the study population's demographic data, risk factors, and clinical characteristics cross-matched for age and gender. The groups were compared regarding age, gender, body mass index, risk factors for diastolic dysfunction, symptoms and signs, arterial blood pressure measurements, and lab. Investigations. Group I (HFpEF) comprised 15 patients with diastolic dysfunction having signs and symptoms of H.F. and included seven males and eight females. Their ages had a mean of 55.67 ± 4.62 years, and their Body Mass Index (BMI) had a standard of 30.90 ± 5.23 kg/m2, the highest of all groups. Group II (D.D.) comprised 15 patients with diastolic dysfunction and no signs or symptoms of H.F., including ten males and five females. Their ages had a mean of 51.80 ± 6.38 years, and their BMI had a standard of 29.61 ± 2.64) kg/m2. Group III (control) included nine males and six females. Their ages had a mean of 53.0 ± 5.83 years, and their BMI had a standard of 27.78 ± 2.14 kg/m2. Regarding the risk factors for diastolic dysfunction, hypertension was significantly higher in group I and group II than in the control. There were ten hypertensive patients out of 15 (66.7%) and 9 out of 15 (66.0%) in group I and II, respectively, compared to nonhypertensive patients in the control group. There was no significant difference between the three groups regarding D.M. and smoking. There were 4 (26.6%) diabetic patients out of 15 in both group I and group II, and no diabetic subjects in group III. Regarding smoking, there were four smokers (26.7%) and one ex-smoker (6.7%) in group I compared to 4 smokers (26.7%), four ex-smokers (26.7%) in group II, and three smokers (20.0%) in the control group. Systolic B.P. was significantly higher in group I than in the control group (p=0.011), with no significant difference between group I and group II. Diastolic B.P. was significantly higher in group I than in the control group (p=0.035), and no significant difference between group I and group II. Heart rate showed no significant difference between group I and group ii and control (p=0.692).
| Group I (n=15) | Group II (n=15) | Group III (n=15) | P | |
|---|---|---|---|---|
| Age (years) | 55.67 ± 4.62 | 51.80 ± 6.38 | 53.0 ± 5.83 | 0.172 |
| Body mass index (BMI) (kg/m2) | 30.90 ± 5.23 | 29.61 ± 2.64 | 27.78 ± 2.14 | 0.069 |
| HTN | 66.70% | 60.00% | 0 | <0.001* |
| DM | 26.70% | 26.70% | 0 | 0.099 |
| Dyspnea | 100.00% | 0 | 0 | <0.001* |
| PND | 60.00% | 0 | 0 | <0.001* |
| Systolic blood pressure (mmHg) | 129.33 ± 12.80 | 124.0 ± 10.56 | 117.33 ± 8.84 | 0.016* |
| Diastolic blood pressure (mmHg) | 80.0 ± 7.56 | 75.33 ± 9.90 | 72.0 ± 7.75 | 0.044* |
| Heart rate (beat/min) | 73.13 ± 8.04 | 72.0 ± 4.88 | 74.0 ± 5.76 | 0.692 |
| INR | 1.01 ± 0.08 | 0.98 ± 0.08 | 0.97 ± 0.07 | 0.453 |
| FBG | 103.29 ± 13.69 | 98.47 ± 15.51 | 90.47 ± 9.42 | 0.032* |
| Cholesterol | 197.67 ± 12.48 | 195.13 ± 17.51 | 194.07 ± 9.7 | 0.76 |
| Triglycerides | 127.07 ± 14.13 | 122.0 ± 14.30 | 125.80 ± 11.40 | 0.561 |
Table 1: Shows the study group's demographic data, risk factors, and clinical characteristics.
Conventional echocardiographic data are shown in Table 2. The Inter Ventricular Septal Thickness (IVST) of group I was significantly higher than that of group II (p=0.012) and also considerably more than that of the control group (p=<0.001). Left Ventricular End-Diastolic Dimensions (LVEDD) were significantly higher in group I than in group II (p=0.005). Left Ventricular End-Systolic Dimensions (LVESD) showed no significant difference between the studied groups (p=0.191). Left Ventricular Posterior Wall Thickness (LVPWT) in group I was significantly higher than that of the control (p=<0.001). There was no significant difference in EF between the three groups. Left Ventricular Mass Index (LVMI) was significantly higher in group I than in group II and group III (p<0.001) and <0.001), respectively. The Left Atrium Volume Index (LAVI) was higher in group I than in group II and group III (p<0.001). There was a statistically nonsignificant difference between the groups. Left atrium dimensions in 4 chamber views and two-chamber view (area and length) showed no significant difference between the three groups. The Left Atrium Volume Index (LAVI) showed a higher index for group I than in group II and the control group (p=0.057). The E/A ratio of conventional echo in group I and group II showed a significant difference from the control group: This ratio was above 1 in all control cases (p=<0.001).
| M-mode | Group I (n=15) | Group II (n=15) | Group III (n=15) | P |
|---|---|---|---|---|
| IVSd (cm) | 1.26 ± 0.24 | 1.06 ± 0.18 | 0.98 ± 0.09 | <0.001* |
| LVEDD (cm) | 5.27 ± 0.36 | 4.78 ± 0.47 | 4.93 ± 0.34 | 0.005* |
| LVEDs (cm) | 3.15 ± 0.35 | 2.93 ± 0.44 | 2.93 ± 0.33 | 0.191 |
| LVPWt (cm) | 1.13 ± 0.17 | 1.02 ± 0.11 | 0.93 ± 0.07 | <0.001* |
| EF % | 70.0 ± 4.75 | 68.93 ± 6.36 | 71.13 ± 3.76 | 0.5 |
| LVMI (g/m2) | 131.13 ± 36.06 | 91.67 ± 18.23 | 87.93 ± 8.48 | <0.001* |
| Sig. bet. grps | p1< 0.001*, p2< 0.001*, p3=0.904 | |||
| LA dimensions LA 4 ch area | 16.81 ± 3.15 | 15.27 ± 3.47 | 14.59 ± 2.68 | 0.148 |
| Length | 5.28 ± 0.52 | 5.29 ± 0.76 | 5.04 ± 0.72 | 0.516 |
| LA 2 ch area | 19.0 ± 5.34 | 16.19 ± 3.71 | 14.82 ± 2.24 | 0.020* |
| Length | 5.32 ± 0.66 | 5.11 ± 0.64 | 5.01 ± 0.51 | 0.369 |
| LAVI | 27.07 ± 9.01 | 22.40 ± 8.72 | 20.33 ± 4.08 | 0.057 |
| E/A | 0.75 ± 0.20 | 0.88 ± 0.29 | 1.22 ± 0.14 | <0.001* |
Note: IVST: The Interventricular Septal Thickness; LVEDD: Left Ventricular End-Diastolic Dimensions; LVESD: Left Ventricular End-Systolic Dimensions; LVPWT: Left Ventricular Posterior Wall Thickness EF: Ejection Fraction; LVMI: Left Ventricular Mass Index; LA 4ch: Left Atrium Dimensions In 4 Chamber View; LA 2ch: Left Atrium Dimensions In 2 Chamber View; LAVI: Left Atrium Volume; E/A: The E/A Ratio of the conventional echo.
p1: p-value for comparing heart failure and diastolic dysfunction.
p2: p-value for comparing heart failure and control.
p3: p-value for comparing between diastolic dysfunction and control.
*: Statistically significant at p ≤ 0.05.
Table 2: Comparison between studied groups according to conventional M-mode echocardiography, left atrium area and volume, and trans mitral doppler flow.
Table 3 displays tissue doppler imaging data which showed an e' wave at the septal mitral annulus in group I and group II significantly lower than that in the control group (p=<0.001). The a' wave at the septal mitral annulus was considerably lower in the control group than that in group I (p=<0.001*) and also significantly lower than that of group II (p=<0.001). Septal S wave was lower in group I and group II compared to group III. The septal E/e ratio in group I showed the highest value, significantly different from the control group (p=0.001). The percentage was also significantly higher in group II compared to the control group (p=0.042).
The e' wave at the lateral mitral annulus in group I and group II was significantly lower than that in the control group (p=0.001). The Lateral a' wave was lower in the control group than that in group I and group II (p=0.061). The lateral S wave was significantly lower in group I and group II than in group III (p=0.002). Lateral E/e' was significantly higher in group I and group II than in the control group (p=0.033). The mean of e' (mean septal and lateral e') was significantly lower in group I and group II than in group III (p=<0.001). The mean of E/e' was significantly higher in group I and group II than in group III (p=0.004).
| Tissue doppler | Group I (n=15) | Group II (n=15) | Group III (n=15) | P |
|---|---|---|---|---|
| septal mitral annulus E.' | 5.01 ± 0.84 | 5.73 ± 0.84 | 8.45 ± 0.59 | <0.001* |
| A' | 9.49 ± 2.25 | . 10.22 ± 1.48 | 6.73 ± 1.47 | <0.001* |
| S' | 7.14 ± 1.53 | 7.49 ± 1.44 | 8.03 ± 0.79 | 0.183 |
| E/e' | 12.06 ± 2.65 | 10.99 ± 2.77 | 8.87 ± 1.15 | 0.002* |
| lateral mitral annulus E' | 7.64 ± 1.30 | 8.02 ± 1.22 | 11.75 ± 0.83 | <0.001* |
| A' | 11.55 ± 3.0 | 11.06 ± 1.50 | 9.58 ± 2.16 | 0.061 |
| S' | 7.54 ± 1.37 | 8.82 ± 2.88 | 10.14 ± 0.87 | 0.002* |
| E/e' | 8.06 ± 2.52 | 7.74 ± 1.56 | 6.37 ± 0.81 | 0.030* |
| Mean (septal and lateral es) | 6.32 ± 0.92 | 6.87 ± 0.88 | 10.10 ± 0.58 | <0.001* |
| Mean E/e' | 10.06 ± 2.47 | 9.36 ± 2.04 | 7.62 ± 0.92 | 0.004* |
Note: p1: p-value for comparing heart failure and diastolic dysfunction.
p2: p-value for comparing heart failure and control.
p3: p-value for comparing diastolic dysfunction and control.
*: Statistically significant at p ≤ 0.05.
Table 3: Comparison between the three studied groups according to tissue Doppler imaging.
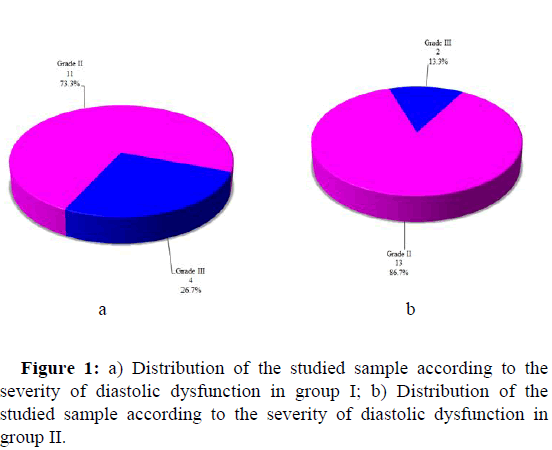
The severity of diastolic dysfunction is shown in Table 4. 11 patients (73.3%) in group I showed grade II DD, and four patients (26.7%) had grade III DD. 13 patients in group II (86.7%) showed grade II DD and two patients (13.3%) had grade III DD (Figure 1).
| Group I (n=15) | Group II (n=15) | χ | p | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| Grade I | 0 | 0 | 0 | 0 | 0.833 | MCp=0.36 |
| Grade II | 11 | 73.3 | 13 | 86.6 | ||
| Grade III | 4 | 26.7 | 2 | 13.3 | ||
Note: χ2, p: χ2 and p values for Chi-square test for comparing between the two groups.
MC: Monte Carlo test.
*: Statistically significant at p ≤ 0.05.
Group I: Patients with signs and symptoms of heart failure.
Group II: Patients without signs and symptoms of heart failure.
Table 4: Comparison between the two studied groups according to the severity of diastolic dysfunction.
Two-dimensional speckle tracking echocardiography is shown in Table 5. Average peak systolic strain in basal, mid, and apical segments of the anterior wall showed lower values in group I and group II than the control group (only significantly in the basal part (p=<0.032). There was also a significant difference between group II and the control only in basal segments' average peak systolic strain (p=0.013). The average strain of the three segments was significantly lower in group I than in group II (p=0.006) and also lower than control (p=<0.001). The mean values for the peak longitudinal strain in the basal, mid, and apical-lateral segments of group I was lower than that of group II, and the control (only statistically significant for the basal and apical-lateral segments (p=0.021 and 0.004, respectively). The same segments were statistically significantly lower in group I than control (P=<0.001 for both segments). The average strain of the three segments was significantly lower in group I than in group II and the control (p=<0.001 in both groups). The average systolic longitudinal strain in the basal, mid, and apical-septal segments was significantly lower in group I than in group II and the control (p=0.008 and <0.001, respectively). The mean value for the peak systolic longitudinal strain in the basal segment was statistically lower in group I than in group II and the control (p=<0.001 and 0.004, respectively). The mean value for the peak systolic longitudinal strain in the mid-inferior segment was statistically lower in group I than in group II and the control (p=<0.021 and 0.004, respectively). The mean value for the peak systolic longitudinal strain in the apical-inferior segment was statistically lower in group I only than in the control (p=0.009). The average strain of the three segments was significantly lower in group I than in group II and the control (p=0.016 and <0.001, respectively). The mean values for the peak systolic longitudinal strain in the basal and mid-Antero-septal segments of group I was lower than that of group II and the control. (Only statistically significant in the basal septal segment (P=<0.001 for both groups). The average strain of the three segments was significantly lower in group I than in group II and the control (p=0.009 and <0.001, respectively). The mean values for the peak systolic longitudinal strain in the basal and mid posterior segments were lower in group I than group II and the control (only significantly in the basal segment) (p=0.001 and 0.014, respectively). The average strain of the three segments was significantly lower in group I than the control (p=<0.001) and also in group II than the control (p=0.035). The mean values for the peak systolic longitudinal strain in the apex segment were significantly lower in group I than in group II and the control (p=0.006 and 0.005, respectively). The mean values for Global Longitudinal Strain (GLS) for the previously mentioned 17 segments were significantly lower in group I than in group II and the control (p-<0.001 and <0.001, respectively).
| Group I (n=15) | Group II (n=15) | Group III (n=15) | P | |
|---|---|---|---|---|
| PSLS of anterior wall: Basal | -16.87 ± 5.67 | -22.27 ± 6.18 | -23.73 ± 4.98 | 0.004* |
| Mid | -14.20 ± 5.51 | -16.93 ± 7.32 | -19.93 ± 5.95 | 0.055 |
| Apical | -10.67 ± 3.77 | -11.80 ± 3.97 | -11.93 ± 2.76 | 0.565 |
| Average | -13.91 ± 3.01 | -17.0 ± 2.48 | -18.53 ± 2.21 | <0.001* |
| PSLS of lateral wall: Basal | -15.47 ± 7.85 | -21.53 ± 5.79 | -22.87 ± 3.40 | 0.003* |
| Mid | -15.0 ± 7.32 | -17.93 ± 7.82 | -20.20 ± 3.05 | 0.097 |
| Apical | -8.60 ± 3.0 | -15.73 ± 3.47 | -16.07 ± 1.98 | <0.001* |
| Average | -13.02 ± 3.49 | -18.40 ± 3.37 | -19.71 ± 1.61 | <0.001* |
| PSLS of septal wall: Basal | -12.47 ± 6.63 | -15.07 ± 5.15 | -15.33 ± 4.61 | 0.302 |
| Mid | -18.40 ± 7.38 | -20.80 ± 6.50 | -20.7 ± 2.79 | 0.454 |
| Apical | -15.80 ± 6.87 | -18.67 ± 3.99 | -20.07 ± 1.87 | 0.051 |
| Average | -15.56 ± 2.95 | -18.18 ± 2.28 | -18.71 ± 1.17 | 0.001* |
| PSLS of inferior wall: Basal | -12.80 ± 3.82 | -14.93 ± 5.09 | -20.47 ± 4.29 | <0.001* |
| Mid | -15.87 ± 4.70 | -20.13 ± 4.88 | -21.07 ± 2.55 | 0.003* |
| Apical | -16.60 ± 5.22 | -18.33 ± 4.84 | -21.93 ± 3.92 | 0.011* |
| Average | -15.09 ± 3.32 | -17.80 ± 2.03 | -21.16 ± 2.15 | <0.001 |
| PSLS of antero-septal wall basal | -9.93 ± 5.70 | -18.93 ± 6.81 | -19.33 ± 2.02 | <0.001* |
| Mid | -18.47 ± 7.91 | -19.27 ± 7.78 | -23.80 ± 2.96 | 0.071 |
| Average | -14.20 ± 4.96 | -19.10 ± 5.22 | -21.57 ± 1.96 | <0.001* |
| PSLS of posterior wall basal | -13.07 ± 6.96 | -14.87 ± 4.97 | -20.47 ± 2.77 | 0.001* |
| Mid | -16.27 ± 6.33 | -20.13 ± 7.91 | -21.60 ± 2.06 | 0.051 |
| Average | -14.67 ± 4.67 | -17.50 ± 4.32 | 21.03 ± 1.33 | <0.001* |
| PSLS of the apex | -11.80 ± 2.83 | -15.80 ± 4.39 | -15.87 ± 2.53 | 0.002* |
| Global longitudinal strain | -14.25 ± 1.91 | -17.83 ± 1.39 | -19.7 ± 0.86 | <0.001* |
Note: PSLS: Peak Systolic Longitudinal Strain.
Table 5: Comparison between the three studied groups according to two-dimensional speckle tracking echocardiography.
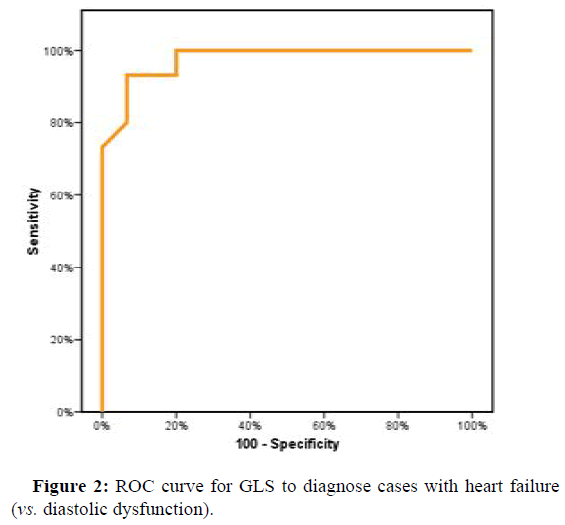
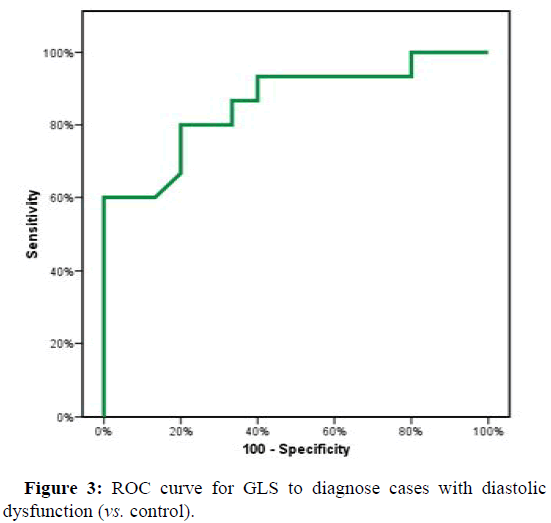
ROC curve showed a cutoff point to diagnose H.F. in D.D. cases according to GLS of (≤ -15.94) with Sensitivity and specificity (86.67% and 93.33%, respectively). The cutoff point in GLS between D.D. cases and control were (≤ -19.05) with Sensitivity and specificity of (80.0% and 73.33%, respectively) (Figures 2 and 3).
Univariate analysis for the parameters affecting GLS in group I (HF patients) showed that hypertension, D.M., and lateral S' are affecting GLS in HF patients, but with no significance of any of them over the other (Table 6).
| AUC | P | 95% C I | Cut off | Sensitivity | Specificity | PPV | NPV | ||
|---|---|---|---|---|---|---|---|---|---|
| L.L | U.L | ||||||||
| GLS | 0.976* | <0.001* | 0.932 | 1.02 | ≤ -16.24 | 93.33 | 80 | 82.4 | 92.3 |
Note: AUC: Area Under a Curve.
P value: Probability value.
CI: Confidence Intervals.
*: Statistically significant at p ≤ 0.05.
Table 6: Comparison between the three studied groups according to sensitivity and specificity for GLS to diagnose cases with heart failure (vs. diastolic dysfunction).
Discussion
HFpEF includes patients with left ventricular diastolic HF and preserved EF the term diastolic heart failure refers specifically to the pathogenesis of the disease. Asymptomatic DD by some denoted as preclinical Heart Failure (HF) is expected in the community. Patients often develop reduced QOL and show increased cardiovascular risk. Follow-up studies revealed frequent progression to HFrEF. Diastolic dysfunction can develop due to cardiac and cardiac-related diseases, such as myocardial ischemia, amyloidosis, and other infiltrative diseases, thyroid disorders (Table 7).
| AUC | p | 95% CI | Cut off | Sensitivity | Specificity | PPV | NPV | ||
|---|---|---|---|---|---|---|---|---|---|
| L.L | U.L | ||||||||
| GLS | 0.860* | 0.001* | 0.725 | 0.995 | ≤ -19.05 | 80 | 73.33 | 75 | 78.6 |
Note: AUC: Area under a Curve.
P value: Probability value.
CI: Confidence Intervals.
*: Statistically significant at p ≤ 0.05.
Table 7: Agreement (sensitivity, specificity) for GLS to diagnose cases with diastolic dysfunction (vs. control).
Although D.D. is a dominant feature of HFpEF, experts view it as a complex syndrome in which multiple cardiac, vascular, and noncardiac determinants impair cardiovascular reserve. The combination of arterial hypertension and diabetes mellitus worsens Doppler indices of left ventricular diastolic function [11,12].
The primary measurements of systolic function are measures of contractility that include EF. Although EF may not consistently be a valid or reliable estimate of proper myocardial contractility, it is the most commonly used method for assessing LV function. Another marker of LV contractility is strain rate imaging; specifically, Global Longitudinal Strain (GLS) proved to have diagnostic and predictive morbidity and mortality benefits in studying HFpEF superior to measurement of EF. GLS has been utilized for evaluating the diastolic function, acute decompensated HFpEF, acute MI, and reduced exercise capacity in HFpEF patients [13].
Morris, et al. did a meta-analysis to confirm if the global longitudinal systolic function of the Left Ventricle (LV) is affected in patients with Heart Failure with preserved Ejection Fraction (HFpEF) [14]. Patients with HFpEF had significantly lower GLS than control subjects (mean -15.7% (range -12 to -18.9) vs. mean -19.9% (range -17.1 to -21.5), (p<0.001). Also, GLS was abnormal in asymptomatic patients with HFpEF (mean -15.5% vs. mean -18.3% (Table 8).
| GLS (group I) | Univariate | #Multivariate | ||
|---|---|---|---|---|
| P | B (95% C.I) | p | B (95% C.I) | |
| Females | 0.091 | -1.67 (-3.64-0.305) | ||
| Age (years) | 0.412 | 0.095 (-0.146-0.336) | ||
| BMI (kg/m2) | 0.366 | -0.092 (-0.30-0.120) | ||
| HTN | 0.031* | 2.17* (0.227-4.12) | 0.442 | 0.821 (-1.44-3.087 |
| DM | 0.031 | -0.074 (-0.149-0.0) | 0.17 | 0.050 (-0.125-0.025) |
| Smoking | 0.376 | 0.965 (-1.30-3.23) | ||
| Systolic blood pressure | 0.258 | 0.046 (-0.038-0.131) | ||
| Diastolic blood pressure | 0.063 | 0.124 (-0.008-0.256) | ||
| Heart rate (beat/min) | 0.925 | 0.006 (-0.136-0.149) | ||
| FBG | 0.168 | -1.56 (-3.88-0.752) | ||
| E.F. % | 0.601 | -0.059 (-0.297-0.179) | ||
| LVMI (g/m2) | 0.39 | -0.013 (-0.043-0.018) | ||
| LAVI | 0.135 | -0.086 (-0.202-0.031) | ||
| Mean E/e' | 0.702 | -0.083 (-0.542-0.376) | ||
| Septal S' | 0.105 | -0.544 (-1.21-0.13) | ||
| Lateral S' | 0.029* | -0.784 (-1.47- -0.094) | 0.125 | -0.553 (-1.28-0.180 |
Note: B: Constant for linear Regression, CI: Confidence Interval
#: All variables with p<0.05 was included in the multivariate.
*: Statistically significant at p ≤ 0.05.
Table 8: Univariate and multivariate analysis (linear regression) for the parameters affecting GLS (n=15) for the HF group.
In our work, GLS was significantly lower in the HF group (mean -14.25 ± 1.91) than the DD group (mean -17.83 ± 1.39) and also than the control group (mean -19.7 ± 0.86). The cutoff point of GLS between the HF and DD groups was (≤ -16.24), with a sensitivity of 93.33% and specificity of 80.0%. The cutoff point between D.D. and control groups was (≤ -19.05), with a sensitivity of 80.0% and specificity of 73.3%.
GLS was correlated only with HTN, DM, and lateral S'. GLS was inversely associated with HTN, meaning GLS was lower in patients with higher BP. Also, GLS was lower in patients with DM regarding lateral S'; it was directly related to GLS with a lower GLS in patients with lower values of lateral S.' Multivariate showed that all are affecting GLS without priority of one over the other. This correlation difference between our studies and others is due mainly to the small sample size.
Conclusions
The global longitudinal strain was significantly lower in HFpEF compared to asymptomatic diastolic dysfunction patients, suggesting that GLS can detect subclinical systolic dysfunction in patients with preserved EF.
The global longitudinal strain in HFpEF patients significantly correlates with hypertension, DM, and lateral S'.
Limitations
• The study involved a small number of patients.
• Without laboratory or invasive assessment, heart failure patients
were selected upon signs, symptoms, and echocardiographic
parameters.
• All the studied patients were on medications such as beta blockers
and ACEIs which may affect GLS.
Acknowledgements
All the nursing staff, technicians, and residents.
Declaration
Ethics approval and consent to participate: The local ethics committee: The ethical committee of the faculty of medicine Alexandria university, number 00012098, approved the study. We obtained informed consent: Written/verbal permission from the patient or the patient's parent/carer. All methods followed relevant guidelines and regulations (declaration of Helsinki).
Consent for Publication
All authors consent for publication.
Availability of Data and Materials
The datasets used or analyzed during the current study are available from the corresponding author upon request.
Competing Interests
None.
Conflict of Interest
The authors declare no conflict of interest in preparing this article.
Funding
None.
Authors' Contributions
• SR: Was involved in writing the paper and submitting it.
• MSE: Involved in the study concept, design.
• TE: Involved in the study concept, design.
• NMM: Involved in the study concept, design, data collection, and
writing.
• GM: Involved in the study concept, design, and practical methods.
All authors contributed to the writing-original draft preparation and
approved the final version.
References
- Geraci SA, Horwich T, Januzzi JL, Levy WC (2013) 2013 ACCF/AHA guideline for the management of heart failure. Circulation 128:240-327.
- Shuai XX, Chen YY, Lu YX, Su GH, Wang YH, et al. (2011) Diagnosis of heart failure with preserved ejection fraction: Which parameters and diagnostic strategies are more valuable?. Eur J Heart Fail 13:737-745.
[Crossref] [Google Scholar] [PubMed]
- Oghlakian GO, Sipahi I, Fang JC (2011) Treatment of heart failure with preserved ejection fraction: Have we been pursuing the wrong paradigm?. Mayo Clin Proc 86:531-539.
[Crossref] [Google Scholar] [PubMed]
- Petersen JW, Nazir TF, Lee L, Garvan CS, Karimi A (2013) Speckle tracking echocardiography-determined measures of global and regional left ventricular function correlate with functional capacity in patients with and without preserved ejection fraction. Cardiovasc Ultrasound 11:20.
[Crossref] [Google Scholar] [PubMed]
- Hensel KO, Jenke A, Leischik R (2014) Speckle-tracking and tissue-doppler stress echocardiography in arterial hypertension: A sensitive tool for detection of subclinical LV impairment. Biomed Res Int 2014:472562.
[Crossref] [Google Scholar] [PubMed]
- Flu WJ, van Kuijk JP, Bax JJ, Gorcsan J, Poldermans D (2009) Three-dimensional speckle tracking echocardiography: A novel approach in the assessment of left ventricular volume and function?. Eur Heart J 30:2304-2307.
[Crossref] [Google Scholar] [PubMed]
- Kraigher-Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, et al. (2014) Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol 63:1-10.
[Crossref] [Google Scholar] [PubMed]
- Uematsu M (2015) Speckle tracking echocardiography-Quo Vadis?. Circ J 79:735-741.
[Crossref] [Google Scholar] [PubMed]
- Kotz SA, Read CB, Balakrishnan N, Vidakovic BR (2006) Wiley encyclopedia of statistical sciences. Wily, New York.
- Kirkpatrick LA (2015) A simple guide to IBM SPSS Statistics-Version 23.0. Cengage Learning.
- Miller AB, Pina IL (2009) Understanding heart failure with preserved ejection fraction: Clinical importance and future outlook. Congest Heart Fail 15:186-192.
[Crossref] [Google Scholar] [PubMed]
- Lourenço AP, Leite-Moreira AF, Balligand JL, Bauersachs J, Dawson D, et al. (2018) An integrative translational approach to study heart failure with preserved ejection fraction: A position paper from the working group on myocardial function of the European society of cardiology. Eur J Heart Fail 20:216-227.
[Crossref] [Google Scholar] [PubMed]
- Lekavich CL, Barksdale DJ, Neelon V, Wu JR (2015) Heart Failure preserved Ejection Fraction (HFpEF): An integrated and strategic review. Heart Fail Rev 20:643-653.
[Crossref] [Google Scholar] [PubMed]
- Morris DA, Ma XX, Belyavskiy E, Kumar RA, Kropf M, et al. (2017) Left ventricular longitudinal systolic function analysed by 2D speckle-tracking echocardiography in heart failure with preserved ejection fraction: A meta-analysis. Open Heart 4:e000630.
[Crossref] [Google Scholar] [PubMed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi