Research Article, J Biochem Eng Bioprocess Vol: 6 Issue: 3
Biokinetic Characterization of Ferroplasma acidiphilum
Jamal Daoud and Dimitre Karamanev*
Department of Chemical and Biochemical Engineering, Western University, London, Ontario, N6G 5B9, Canada
*Corresponding Author: Dimitre Karamanev
Department of Chemical and Biochemical Engineering, Western University, London, Ontario, N6G 5B9, Canada
Tel: +1 (519) 661-2111
E-mail: dkaraman@uwo.ca
Received date: 01 November, 2022, Manuscript No. JBEBT-22-78839; Editor assigned date: 04, November, 2022, PreQC No. JBEBT-22-78839 (PQ); Reviewed date: 18, November, 2022, QC No. JBEBT-22-78839; Revised date: 18, January, 2023, Manuscript No. JBEBT-22-78839 (R); Published date: 25, January, 2023, DOI: 10.36648/JBEBT.1000063
Citation: Daoud J, Karamanev D (2023) Biokinetic Characterization of Ferroplasma acidiphilum. J Biochem Eng Bioprocess 6:1
Abstract
Recently a mixed culture dominated by the iron-oxidizing microorganisms Leptospirillum and Ferroplasma has been used in a large-scale microbial fuel cell for electrical power generation. There are many factors that affect the kinetics of iron oxidation by the mixotroph Ferroplasma acidiphilum. This study investigated the effects of pH, temperature, and substrate and yeast extract concentrations in order to arrive at kinetically favorable operating conditions with minimal jarosite precipitation. Furthermore, F. acidiphilum was cultured with Leptospirillum sp. in order to determine its viability as a species capable of limiting the organic by-products of chemolithotrophic microbial growth. Bacterial characterization in the culture was accomplished using Fluorescent In Situ Hybridization (FISH). It was found that the ferrous iron oxidation was most favorable at yeast extract concentration of 0.02% (w/v), pH of 1.6, temperature of 35°C and an initial ferrous iron concentration of 1 g/L yielding a maximum specific growth rate of 0.0351-0.042 h-1. Moreover, F. acidiphilum displayed a symbiotic relationship with its chemolithotrophic counterpart, Leptospirillum sp. in that they were able to utilize the metabolic organic products of the chemolithotroph and limit the organic concentration to ~20 ppm Total Organic Carbon (TOC), well below the threshold concentration of 250 ppm for chemolithotroph activity.
Keywords: Ferroplasma acidiphilum; Bioelectrochemical system; Mixotroph; Ferrous iron oxidation; Biokinetics; Total organic carbon
Introduction
Iron-oxidizing microorganisms are very important in bio-metallurgy [1] and in the formation of Acid Mine Drainage (AMD) [2]. In these processes metal sulfides are solubilized by oxidative dissolution, producing soluble metal ions and acid. Another important application of iron-oxidizing microorganisms is for the electrical power generation in a microbial fuel cell named BioGenerator [3], where a symbiotic culture of Leptospirillum ferriphilum and Ferroplasma is used. In the BioGenerator, there is an internal electrochemical regeneration of ferrous iron and therefore, the system is a closed loop in terms of liquid medium. No microorganisms leave the BioGenerator for a periods of up to 4 years [4]. After a brief exponential growth phase, part of the Leptospirillum population starts dying due to an overgrowth, releasing organic substances. At that time, Ferroplasma starts consuming the organic products of death of Leptospirillum, thus keeping the concentration of organics in the bio generator below the toxicity level for the living Leptospirillum. In addition, it is advisable to reduce as much as possible the amount of jarosite produced during the ferrous iron bio oxidation. Therefore, it is of great importance for each of the three above mentioned processes (bio-metallurgy, AMD formation and electrical power generation) to know the kinetics of ferrous iron oxidation, organics consumption, jarosite formation and microbial growth individually and in combination of Leptospirillum and Ferroplasma.
Among the most important of the iron-oxidizing microorganisms are Acidithiobacillus ferrooxidans, Leptospirillum spp. and archaea of the genus Ferroplasma. Only one species of the genus Ferroplasma has a validated name so far: The mixotroph F. acidiphilum [5] in addition to non-validated species such as F. acidarmanus and F. thermophilum. One of the Ferroplasma strains, Ferroplasma acidiphilum YT, was isolated from gold-bearing aresenopyrite-pyrite concentrate bioleaching pilot plant [6]. Ferroplasma acidiphilum BRL-115 was studied more recently [7]. F. acidiphilum, like A. ferrooxidans and Leptospirillum, can obtain energy from the oxidation of ferrous ion. The overall biochemical reaction of the oxidation of ferrous ions is;
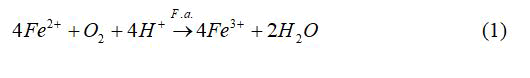
The rate of iron oxidation of F. acidiphilum has not been widely investigated. Past studies have emphasized on measuring protein concentration as a measure of cell growth [8]. However, it is important to obtain the effect of different parameters on the cell growth and substrates (organics and iron) consumption of F. acidiphilum in a reactor setting. The most important factors affecting the growth of F. acidiphilum include pH, temperature, organic and inorganic substrate concentration.
The effect of temperature on the rate of iron oxidation by F. acidiphilum was studied by employing the Ratkowsky equation [9];
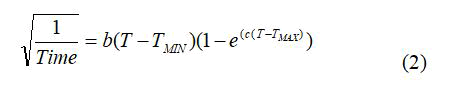
Where, T is the temperature, “Time” is generally the generation time or the time taken to reach a specific condition, TMIN is the theoretical extrapolated minimum temperature for growth, TMAX is the theoretical extrapolated maximal temperature for growth, and “b” and “c” are fitting parameters. For F. acidiphilum using Fe2+ as a substrate, the following constants were obtained: TMIN=12.7°C; T=39.6°C; TMAX=47.2°C; b=0.00431; c=0.345; Ea=79 kJ/mol.
F. acidiphilum is commonly grown on modified 9 K medium originally developed by Silverman and Lundgren. The ferrous iron oxidation occurs via reaction 1. As ferric ions accumulate, protons are consumed which result in an increase in pH. However, there is a reaction in competition with the hydrolysis reaction giving products of basic ferric hydroxysulphates with the formula MFe3(SO4)2(OH)6, where M=K+, Na+, NH4+, Ag+, or H3O+.
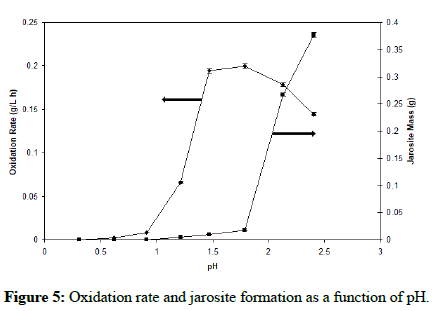
A study of A. ferrooxidans, a bacterium that shares the same environment niche as F. acidiphilum, revealed that the main parameter affecting the jarosite formation was pH. Several experiments yielded results showing oxidation rates as high as 0.181-0.194 g/Lh, with low jarosite precipitation of 0.0125-0.0209 g at pH of 1.6-1.7 with an operating temperature of 35°C [10].
Several studies have indicated the requirement of an organic nutrient (yeast extract or vitamins) as a growth factor for F. acidiphilum growth, Thiobacillus sp. [11] or Thermophiles [12]. This requirement raises questions as to how the bacteria satisfy this nutritional requirement in acid mine solutions. A requirement for oligopeptides has been reported for T. acidiphilum, but individual fractions of oligopeptides from yeast extract would not support growth [13]. It was speculated that yeast extract may: Contain or scavenge a micronutrient, protect the organism from the high H+ gradient, or participate with ion transporters.
It is well documented that F. acidiphilum is able to grow in AMD environments shared by A. ferrooxidans and Leptospirillum, for example in Iron Mountain, California [14]. In the absence of the latter two organisms which produce organic metabolites, other organics such as yeast extract are needed as an organic source for F. acidiphilum growth [15].
Organics can arise as autotrophic bacterial cells excrete intermediates of their metabolic pathway into the medium [16]. With time, these organics accumulate and limit the bacterial growth rate of the chemolithotrophic bacteria, Leptospirillum sp. and A. ferrooxidans. It is reported that organic concentrations in excess of 250 ppm of organic carbon inhibit iron-oxidizing by the aforementioned chemolithotrophs [17].
The main goal of this study was to perform a thorough kinetic characterization of the mixotroph Ferroplasma acidiphilum. Firstly, it is important to find the effect of yeast extract concentration on the kinetics of organic and inorganic substrates consumption. Using this information, the next step was to find the conditions that minimize jarosite precipitation while not comprising iron oxidation. It was also important to analyze the organic dependence of the organism and its growth kinetics. The next and final step involved the characterization of Ferroplasma growth and organic dependence in a mixed culture with the chemolithotrophic Leptospirillum ferriphilum microorganism, resembling the organisms’ natural environment. This will give valuable insight into the capability of Ferroplasma to control the organic levels in conjunction with chemolithotrophic iron oxidizing microorganisms.
Materials and Methods
Equipment
The pH of each trial was adjusted using sulfuric acid and monitored using a pH meter (Orion Model No. 420A). For the shake flask experiments, the bacteria were allowed to grow in a rotary flask shaker with speed and temperature adjustment (New Brunswick Scientific Model No. G-25 R). For the ferric and total iron analyses, we used a spectrophotometer (Varian Cary 50) and sulfosalicylic acid as an indicator [18]. For the of jarosite weight measurement, filter paper with a pore size of 25 μm was used to collect the precipitates produced in each flask, followed by the procedure described by Daoud and Karamanev.
For the Total Organic Carbon (TOC) measurement, we used a tekmar dohrmann apollo 9000 TOC analyzer, which uses a combustion based analyzer of organic carbon. Moreover, to measure microbial concentration in liquid, we used a Zeiss Axioskop 40 upright microscope. A 5 μl sample deposed on a glass slide was covered by a cover glass and viewed at 1000-fold magnification, which are calibrated according to the viewing area in relation to the pixel area.
Chemicals and microorganisms
All the chemicals used in this study were of analytical grade, including 100% H2SO4, components of the 9 K medium, sulfosalicylic acid, ammonium hydroxide (30%) and the chemical constituents of the FISH buffers. The strain Ferroplasma acidiphilum was obtained from the Deutsche Sammlung von Microorganismen (DSM12658). Leptospirillum ferriphilum was obtained from acid mine drainage of the Richmond Mine in Iron Mountain, California.
Effects of yeasts extract, pH, temperature and organics
In each experiment 100 mL Erlenmeyer flasks were used. For the study of the yeast extract, six 250 mL erlenmeyer flasks were used, one for each yeast extract concentration in the range of 0%-2% (w/v) in ten-fold intervals. 125 mL of modified 9 K medium along with the varying yeast extract concentrations (by adding the appropriate volume of 10% (w/v) yeast extract solution) were added to each flask. The desired pH of 1.7 was obtained by adding H2SO4 drop-wise, with continuous agitation and pH measurement. Further, 20 mL of F. acidiphilum inoculum was added to each flask. The inoculum contained an average of 109 cells per milliliter. After the flasks were plugged with glass wool, they were placed in a rotary shaker at 35°C with a rotational speed of 260 rpm. The Fe3+ and Fetotal concentrations in the flasks were measured initially and at appropriate time intervals throughout the course of oxidation. Furthermore, 30 mL samples from each flask were also withdrawn periodically for TOC measurement in order to analyze the organic accumulation/consumption.
In the study of the pH effect, several 100 mL flasks were used, one for each pH range of 0.3-2.5 in intervals of 0.3 pH. 100 mL of modified 9 K medium and the appropriate yeast extract concentration were added into each of the eight flasks. When the iron oxidation was near completion, the flasks were removed from the shaker in order to measure jarosite precipitation. Firstly, the liquid in each flask was filtered under vacuum using filter paper (pore size 0.45 μm). The solids on the filter paper were then returned back to the corresponding empty flask by washing them with distilled water. This combined the filtered jarosite with that deposited on the flask walls. Ten mL of 50% H2SO4 were then added to each flask in order to dissolve the jarosite and resuspend the wall deposits. Finally, the total iron concentration was measured using the sulfosalycilic method and the volume of mixture of each flask was measured using graduated cylinders in order to obtain the total mass of jarosite present.
For the study of the temperature effect, an appropriate number of 100 mL flasks was selected in order to have pH intervals of 0.1. We then followed the same procedure as described above, except we set the flask shaker temperature consecutively to 25°C, 35°C, 40°C and 50°C.
The mean ferrous iron oxidation rate was determined by calculating the concentration of iron oxidized and dividing it by the time period in which the oxidation occurred. This calculation was performed during the exponential growth phase.
The study of organics effect involved inoculating three different 100 mL flasks with 5 mL of 109 bacteria per milliliter. The first flask contained 100 mL of modified 9 K solution containing ferrous iron plus the optimal yeast extract concentration. Flask two contained 100 mL of modified 9 K solution with no yeast extract added. Finally, the third flask contained 100 mL of Fe free modified 9 K solution containing the appropriate amount of yeast extract. In order to measure the cell concentration, a 5 μL sample was analyzed and cells were counted using a phase contrast microscope. As in the first experiment, TOC was measured. This experiment allowed us to further analyze the extent of the bacterium’s organotrophic and mixotrophic capabilities.
Effect of substrate (ferrous iron) concentration
The microbial growth rate was studied. In classic Monod kinetics, this is accomplished by evaluating the growth curve of the organism through variation of the substrate concentration, in this case, ferrous iron.
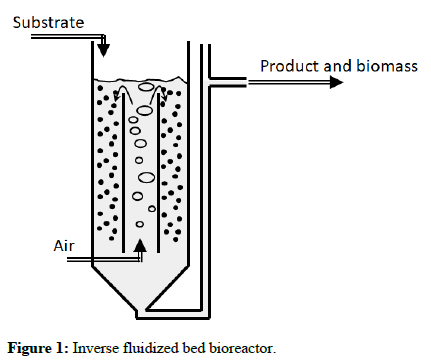
In this experiment, using the optimal conditions obtained earlier, we examined the substrate effect at 5 g/L, 10 g/L, 20 g/L and 40 g/L. One of the most important conditions during the study of microbial growth kinetics of suspended culture is the elimination of immobilized microorganisms, which usually appear as a biofilm on the inner reactor walls. For elimination of the biofilm, an inverse fluidized bed bioreactor was used. The inverse fluidized bed reactor was a 1.5 L vessel which contained 2 mm-3 mm low-density polyethylene spheres with smooth surface. The continuous shearing of the spheres with the bioreactor walls and with each other resulted in the complete elimination of biofilm from the bioreactor. It has already been shown that smooth polyethylene surface does not support biofilm growth under high shear conditions. Nevertheless, the spheres were exchanged with new ones every 3 days. The reactor was constructed using pelxiglass and contained an inner draft tube that was aerated using a perforated sparger at 0.5 VVM. It has already been shown that the rate of gas-liquid oxygen mass transfer in this type of bioreactor is high enough to maintain high oxygen concentration in the liquid medium. The outer diameters of the reactor and the draft tube were 6 cm and 3 cm, respectively. Also, the height of the reactor was 55 cm, while the height of the draft tube was 45 cm. The reactor configuration is shown. This is in order to improve the mixing and aeration in order to provide a more thorough study of the bacteria in a batch regime. The reactors were filled with 1.2 L of modified 9 K medium (Fe2+ concentration modified accordingly) with the appropriate yeast extract concentration, then inoculated with 45 mL of 109 bacteria per milliliter. To measure iron oxidation, Fe3+ and Fetotal measurements were performed using the sulfosalycilic acid method. Also, biomass concentration was also measured using cell counts under a phase contrast microscope.
Mixed culture growth
This final part of the experiment was based on the compilation of the results produced in the earlier experiments. Bearing in mind the optimal conditions uncovered earlier, we inoculated 1.2 L of 9 K medium (not containing yeast extract) with 20 mL of the chemolithotrophic iron-oxidizer Leptospirillum ferriphilum and the mixotroph F. acidiphilum in a 1.5 L inverse fluidized bed reactor Figure 1. Iron oxidation, biomass concentration and TOC were measured periodically using the sulfosalycilic acid, microscope count method, and TOC analyzer, respectively. Also, a 10 mL sample was taken at each reading for the purpose of performing fluorescent in-situ hybridization (FISH) in order to quantify and qualitatively identify each strain with time.
Analytical procedures
The analysis of ferrous and ferric iron concentrations at different times in the bacterial samples was done using a precise quantitative method, which is not affected by the presence of microorganisms in solution. The method utilizes a spectrophotometer for the colorimetric measurement of red-colored ferric-sulfosalicylate complex, which corresponds to the concentration of Fe3+ in solution. Ammonia is then added, causing the 5-Sulfosalicylic Acid (SSA) to form a yellow complex with all the iron ions, which corresponds to the concentration of total iron in solution [18].
Fluorescent In Situ Hybridization (FISH)
For FISH analysis, 10 mL samples were centrifuged and resuspended with freshly prepared 4% paraformaldehyde/phosphate buffered saline (PBS; 130 mM sodium chloride, 10 mM sodium phosphate buffer, pH 7.2) solution. The fixed samples were stored at 4°C for 1-3 hours. Following fixation, the samples were centrifuged and washed with PBS buffer to remove the fixative. The cells were then resuspended in a PBS/96% ethanol solution (1:1, v/v), and stored at -20°C prior to hybridization.
Prior to hybridization, the fixed samples or control cells were partially homogenized by vortexing. Ten microliter aliquots of each sample were spotted to each of three wells on a clear cell slide ER-203B-2 (Erie scientific, Portsmouth, NH). The samples on the slides were dried at room temperature, then dehydrated and permeabilized by passing through a 50%, 80% and 96% ethanol series (3 min each). Samples were air dried again at room temperature. For each slide, a master mix resuspended in 1.7 mL Eppendorf centrifuge tubes was made by adding 100 μL of hybridization buffer, 0.9 M NaCl, 20 mM Tris/HCl (pH 7.4), 0.01% SDS (w/v), containing 100 ng of labeled probe, in the presence of formamide. The mixture was gently mixed. A 25 μL aliquot of master mix containing the probe was applied to each well on a slide. A cover glass (22 mm × 50 mm, FISHER finest) was placed on top of each slide. Slides were incubated for 90 min at 46°C in a rotary shaker, but without shaking.
After hybridization, washes were done in diffuse light to avoid bleaching of fluorescence. Cover glasses were removed from slides by dipping them in a Coplin staining jar filled with pre-warmed wash buffer. Then, slides were immediately placed in a wash buffer band incubated at 48°C for 15 minutes. The buffer was composed of 20 mM Tris/HCl (pH 7.4), 0.01% SDS (w/v), 5 mM EDTA. The Leptospirillum probe (LF655) corresponded to nucleotide sequences (5’-3’) CGCTTCCCTCTCCCAGCCT; the formamide/NaCl concentrations were 35/70 mM. The Ferroplasma probe (FER656) corresponded to nucleotide sequences (5’-3’) CGTTTAACCTCACCCGATC; the formamide/NaCl concentrations were 25/149 mM. Slides were rinsed in distilled water to remove salts and SDS, and air-dried in a dark room at room temperature. The slides were then counterstained with 4’,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) working solution (200 μg/mL) for 10 minutes and then rinsed in sterile distilled water before being dried in the dark at room temperature. Each slide was mounted with the anti-fading agent vectashield (vector laboratories, Burlingame, CA), covered with a cover glass, and observed after storage at -20°C.
For viewing the slides, we used a Zeiss Axioskop 40 upright microscope with an HBO 50W UV light source. Probes LF655 and FER656 were labeled at the 5’ end with Texas Red and Cy5 fluorescent dyes, respectively. To view the different fluorescently labeled probes and the nucleic acid specific DAPI dye, various filter sets were used. Chroma filter set C-73347, Zeiss filter sets 00 (488000-0000) and 50 (488050-0000) were used for viewing DAPI, Texas Red and Cy5, respectively.
Results and Discussion
As mentioned above, this study involved various parts with the overall goal of kinetically characterizing Ferroplasma acidiphilum in order to arrive at appropriate conditions of operation which promote organic consumption and minimize jarosite precipitation while not compromising iron-oxidation. Furthermore, this will lead us to attain the final goal of growing a mixed culture of the mixotroph with a chemolithotrophic iron oxidizer, Leptospirillum ferriphilum, with the aim of controlling the metabolite organic accumulation produced by the chemolithotroph.
Yeast extract concentration
To study the effect of yeast extract concentration, initially 0.02% (w/v) yeast extract concentration, pH of 1.7 and temperature of 35°C were used since they were reported to give fast bacterial growth. This allowed us to test for both the appropriate and limiting organic concentrations along with a better understand of the organic dependence of the organism with respect to organic accumulation/ consumption.
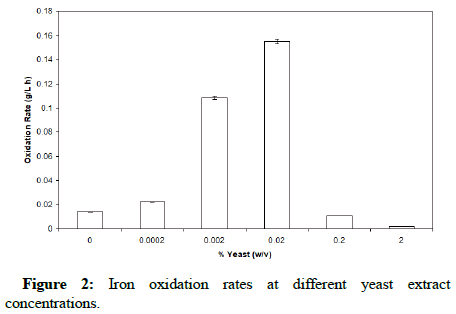
Several experimental runs were performed in order to obtain the optimal yeast extract concentration that yielded the optimal combination of iron oxidation and organic consumption. This was performed at a pH of 1.7 and temperature of 35°C. The yeast extract concentrations examined were 0%, 0.0002%, 0.002%, 0.02%, 0.2% and 2% (w/v). Figure 2 shows the iron oxidation rate for the various concentrations.
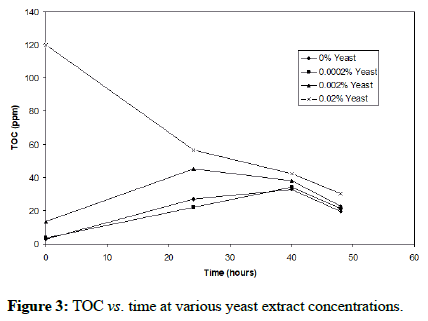
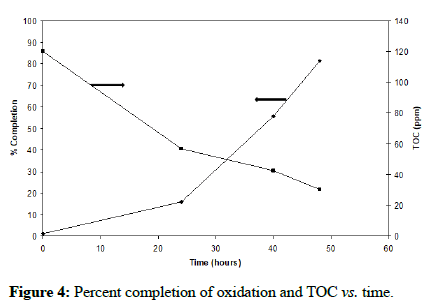
It is clear that the iron oxidation is greatly enhanced when the yeast extract concentration is 0.02% (w/v) and inhibited at concentrations of 0.2% (w/v) and above. This result supports the previous finding. Figure 3 shows that the organic carbon rises to a level of approximately 45-50 ppm before being consumed by the bacteria until a plateau is reached around 20 ppm. Moreover, it is apparent that at 0.02% (w/v) yeast extract, the bacteria utilize the available organics, reaching a plateau at approximately 30 ppm toward the end of the oxidation; this is more clearly shown in Figure 4. These results point to the organic dependence of F. acidiphilum as a mixotrophic organism. That confirms the previous findings. We will later examine in this study the organotrophic nature of the organism as well as its ability to control organic carbon alone and a mixed chemolithotrophic culture.
PH
Using the optimal yeast extract concentration of 0.02% (w/v) and the suggested temperature of 35°C, the effect of pH was studied. The jarosite mass produced was measured at the end of the oxidation period in which the bacteria oxidized most of Fe2+ into Fe3+. Figure 5 clearly indicates that at a pH range of 1.4-1.6, low amount of jarosite was produced while still maintaining high iron oxidation rates.
Temperature
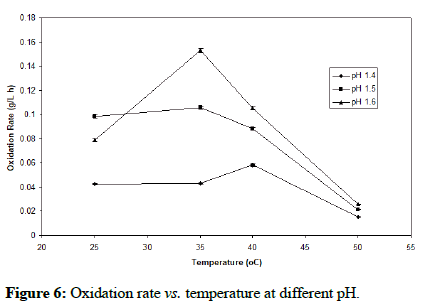
For this experiment, the appropriate pH range and yeast extract concentration from earlier findings, were selected in order to test for the optimum temperature. Figure 6 clearly shows that at pH of 1.6 and 35°C, the oxidation rate is maximal. Furthermore, thermal deactivation was observed at 50°C under the entire studied pH range. The optimal pH and temperature ranges support previous findings.
Organics
As mentioned before, the optimal yeast extract concentration was found to be 0.02% (w/v). It was also shown from that at this concentration, the amount of organic carbon decreases with time as the iron oxidation goes to completion until a plateau is reached. This indicates the mixotrophic behaviour of the organism. However, in order to further study the organic dependence and organotrophic characteristics of the organism, we analyzed F. acidiphilum growth under optimal conditions (pH 1.6, temperature of 35°C and yeast extract concentration of 0.02% w/v), without yeast extract, and with only yeast extract.
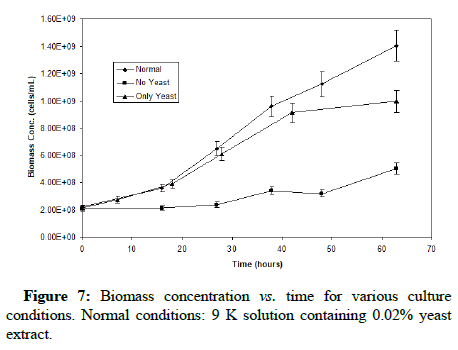
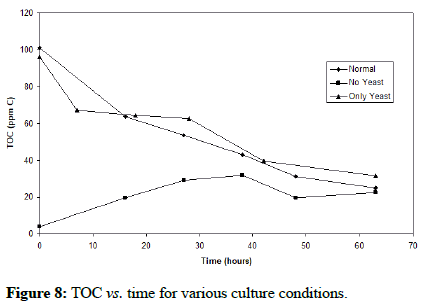
Figure 7 shows that the greatest biomass concentration increase was observed in the normal culture (Fe+ yeast extract), followed by the culture only provided with yeast extract. Cell growth was not fast in the absence of yeast extract. These biomass trends for the normal and only yeast extract cultures had both the opposite trend to the one for TOC shown in Figure 8. The organic carbon concentration as well as the cell growth both displayed very similar trends for the normal and only yeast extract cultures, with increasing biomass concentrations with time, and decreasing TOC that plateaus at approximately 20 ppm. This confirms the occurrence and similarity in trends of mixotrophic and organotrophic growth of the organism.
Fe2+ concentration
The specific growth rate of F. acidiphilum was studied by culturing the microorganism in different substrate (ferrous iron) concentrations. The specific growth rate was obtained from equation 3 under exponential phase growth;

To determine the specific growth rate, equation 4 was used;
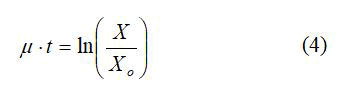
The specific growth rate for different substrate concentrations was then used in a μ vs. S plot in order to estimate the organism’s maximum specific growth rate and the concentration at which substrate inhibition occurs. This relationship followed the Monod kinetics;
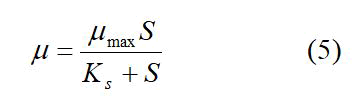
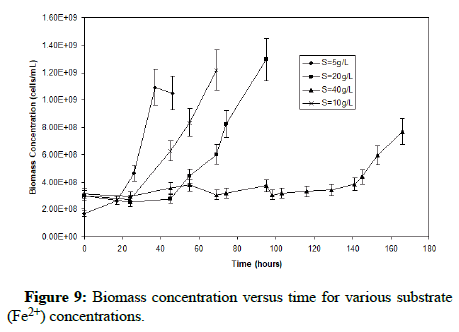
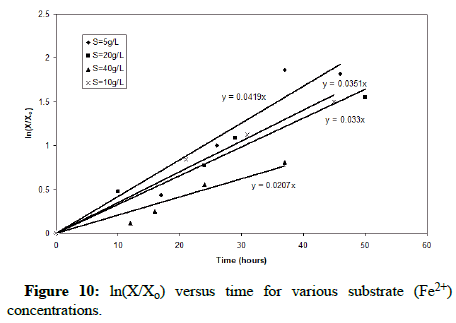
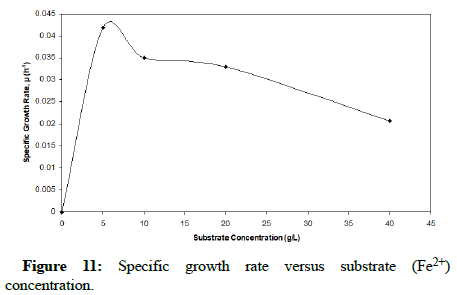
Figure 9 shows that the lag time for bacterial growth increases with the increase of the substrate concentration. Using the data from the exponential growth phase of the microorganisms, Figure 10 provides a plot of ln(X/Xo) versus time in which the slope gives the growth rate constant. It is clear that as the substrate concentration increases above 5 g/L ferrous iron, the specific growth rate decreases. Figure 11 shows the trend of specific growth rate versus substrate concentration, giving a maximum specific growth rate of 0.035-0.042 h-1, while displaying the trend in substrate inhibition above 20 g/L ferrous iron.
Mixed culture growth
Following the characterization of F. acidiphilum, it was important to analyze the strain’s capability to grow in a mixed culture with its chemolithotrophic counterpart, Leptospirillum ferriphilum, with the goal of utilizing the organic metabolite accumulation as its organic source for growth. This will in turn limit the organic accumulation and control its concentration below the threshold lethal level of 100 mg/L TOC.
The mixed culture grown parameters were close to the optimal conditions of both iron-oxidizing strains. Since the optimal growth for L. ferriphilum occurs at 40°C and pH of 1.1, and that for F. acidiphilum was observed at 35°C at pH of 1.6, it was decided to culture both in yeast extract-free 9 K media at pH of 1.3 and 38°C.
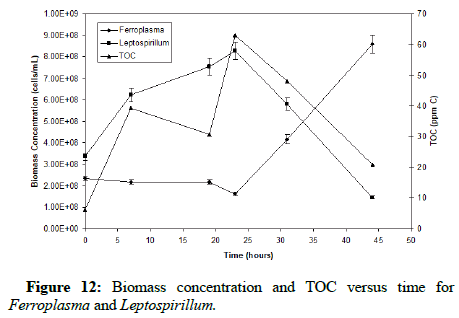
Figure 12 shows the relationship between the biomass concentration of each strain and TOC of the solution versus time. At the beginning of the oxidation, it is clear that the chemolithotroph Leptospirillum ferriphilum is dominant. However, as the oxidation progresses and both pH and organic levels rise, the Ferroplasma becomes more active and displays its mixotrophic capability by consuming the organics and lowering the TOC to the 20 ppm level, analogous. As the oxidation goes to completion, the mixotroph becomes the dominant organism.
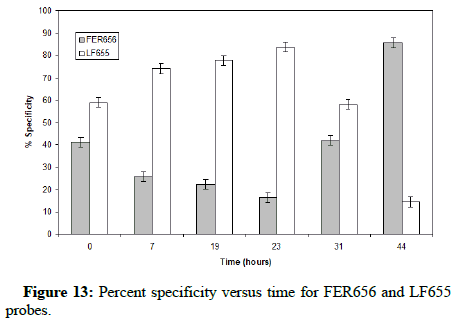
Figure 13 displays the results yielded from the FISH analysis by showing the percentage of each fluorescent probe that bound its respective bacteria.
Conclusion
In this study, we were able to successfully characterize optimal conditions for F. acidiphilum growth and iron oxidizing capabilities while limiting the jarosite precipitation and enhancing the organic consumption. F. acidiphilum was found to function optimally at a pH of 1.6 and a temperature of 35°, provided 0.02% (w/v) yeast extract concentration. Under these conditions, the maximum specific growth rate was found to be between 0.035 and 0.042 h-1. Furthermore, F. acidiphilum was shown to be able to consume organics mixotrophically and organotrophically, whereby decreasing the organics concentration and maintaining the total organic carbon level at approximately 20 ppm.
F. acidiphilum was also shown to be able to utilize the organic metabolite accumulation of the chemolithotroph Leptospirillum in a mixed culture. The organic buildup as a result of the initial domination of chemolithotrophic growth was effectively counteracted by the growth of F. acidiphilum in order to again bring the total organic carbon to a low of 20 ppm.
Therefore, F. acidiphilum is a very promising organism for use in conjunction with chemolithotrophs such as Leptospirillum for bioleaching and also for electrical power generation. The issue of organic accumulation is effectively addressed by the mixotrophic growth of F. acidiphilum.
Acknowledgements
This work was supported by the natural sciences and engineering research council of Canada under grant number R2815A19.
References
- Ilankoon IMSK, Tang Y, Ghorbani Y, Northey S, Yellishetty M, et al (2018) The current state and future directions of percolation leaching in the Chinese mining industry: Challenges and opportunities. Miner Eng 125:206-222.
- Mohapatra BR, Douglas Gould W, Dinardo O, Koren DW (2011) Tracking the prokaryotic diversity in acid mine drainage-contaminated environments: A review of molecular methods. Miner Eng 24:709–718.
- Karamanev D, Pupkevich V, Penev K, Glibin V, Gohil J, et al (2017) Biological conversion of hydrogen to electricity for energy storage. Energy 129:237-245.
- Pupkevich V, Karamanev D (2018) Long-term study of a new bioelectrochemical technology the biogenerator. Int J Hydrogen Energy 43:7731-7737.
- Castelle CJ, Roger M, Bauzan M (2015) The aerobic respiratory chain of the acidophilic archaeon Ferroplasma acidiphilum: A membrane-bound complex oxidizing ferrous iron. Biochim Biophys Acta Bioenerg 1847:717–728.
- Golyshina OV, Pivovarova TA, Karavaiko GI, Kondrateva TF, Moore ER, et al. (2000) Ferroplasma acidiphilum gen. nov., sp. nov., an acidophilic, autotrophic, ferrous-iron-oxidizing, cell-wall-lacking, mesophilic member of the Ferroplasmaceae fam. nov., comprising a distinct lineage of the archaea. Int J Syst Evol Microbiol 50:997–1006.
[Crossref] [Google Scholar] [PubMed]
- Merino MP, Andrews BA, Parada P, Asenjo JA (2016) Characterization of Ferroplasma acidiphilum growing in pure and mixed culture with Leptospirillum ferriphilum. Biotechnol Prog 32:1390–1396.
[Crossref] [Google Scholar] [PubMed]
- Franzmann PD, Haddad CM, Hawkes RB, Robertson WJ, Plumb JJ (2005) Effects of temperature on the rates of iron and sulfur oxidation by selected bioleaching bacteria and archaea: Application of the ratkowsky equation. Minerals Engineering 18:1304–1314.
- Daoud J, Karamanev D (2006) Formation of jarosite during Fe2+ oxidation by Acidithiobacillus ferrooxidans. Miner Eng 19:960–967.
- Brierley JA, Norris PR, Kelly DP, le Roux NW (1978) Characteristics of a moderately thermophilic and acidophilic iron-oxidizing Thiobacillus. European J Appl Microbiol Biotechnol 5:291–299.
- Norris PR, Brierley JA, Kelly DP (1980) Physiological characteristics of two facultatively thermophilic mineral-oxidising bacteria. FEMS Microbiol Lett 7:119–122.
- Smith PF, Langworthy TA, Smith MR (1975) Polypeptide nature of growth requirement in yeast extract for Thermoplasma acidophilum. J Bacteriol 124:884–892.
[Crossref] [Google Scholar] [PubMed]
- Edwards KJ, Bond PL, Gihring TM, Banfield JF (2000) An Archaeal iron-oxidizing extreme acidophile important in acid mine drainage. Science 287:1796–1799.
[Crossref] [Google Scholar] [PubMed]
- Castelle CJ, Roger M, Bauzan M, et al (2015) The aerobic respiratory chain of the acidophilic archaeon Ferroplasma acidiphilum: A membrane-bound complex oxidizing ferrous iron. Biochim Biophys Acta Bioenerg 1847:717–728.
- Nancucheo I, Johnson DB (2010) Production of glycolic acid by chemolithotrophic Iron and sulfur-oxidizing bacteria and its role in delineating and sustaining acidophilic sulfide mineral-oxidizing consortia. Appl Environ Microbiol 76:461–467.
[Crossref] [Google Scholar] [PubMed]
- Drogui P, Mercier G, Blais J-F (2005) Bioproduction of ferric sulfate used during heavy metals removal from sewage sludge. J Environ Qual 34:816–824.
[Crossref] [Google Scholar] [PubMed]
- Karamanev DG, Nikolov LN, Mamatarkova V (2002) Rapid simultaneous quantitative determination of ferric and ferrous ions in drainage waters and similar solutions. Miner Eng 15:341–346.
- Karamanev DG, Nikolov LN (1988) Influence of some physicochemical parameters on bacterial activity of biofilm: Ferrous iron oxidation by Thiobacillus ferrooxidans. Biotechnol Bioeng 31:295-299.
[Crossref] [Google Scholar] [PubMed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi