Research Article, Int J Cardiovasc Res Vol: 8 Issue: 1
Cardiovascular Effects of Cyclical Dietary Vitamin C Withdrawal in Mice Deficient in Internal Synthesis Vitamin C and producing human lipoprotein (a): Gulo(-/-); Lp(a)+
Lei Shi, Aleksandra Niedzwiecki*, Vadim Ivanov and Matthias Rath
Dr Rath Research Institute, Santa Clara, CA 95050, USA
*Corresponding Author : Aleksandra Niedzwiecki
Dr Rath Research Institute, Santa Clara, CA 95050, USA
Tel: (408)5675050
E-mail: a.niedz@drrath.com
Received: November 13, 2018 Accepted: November 27, 2018 Published: January 08, 2019
Citation: Shi L, Niedzwiecki A, Ivanov V, Rath M (2019) Cardiovascular Effects of Cyclical Dietary Vitamin C Withdrawal in Mice Deficient in Internal Synthesis Vitamin C and producing human lipoprotein (a): Gulo(-/-); Lp(a)+. Int J Cardiovasc Res 8:1. doi: 10.4172/2324-8602.1000369
Abstract
Background: Scientific knowledge of the impact of periodic dietary vitamin C intake and withdrawal on the development of cardiovascular disease has been limited. Our earlier study using a transgenic Gulo(-/-); Lp(a)+ mouse model mimicking human metabolism in respect to a lack of internal synthesis of vitamin C and expression of human lipoprotein (a) [Lp(a)], a recognized risk factor for cardiovascular disease, showed that vitamin C deficiency triggers vascular deposition of Lp(a) and atherosclerosis. In this study we used this mouse model to investigate the effect of cyclical vitamin C withdrawal and its continuous supplementation on metabolic factors related to cardiovascular health, such as lipid profile and vascular plaque development.
Methods: Gulo (-/-); Lp (a)+ mice were subjected to 4 weeks of dietary vitamin C withdrawals followed by 4 weeks of a resupplementation pattern for 20 weeks in total. Mice supplemented with vitamin C continuously for 20 weeks served as reference control. Mice were harvested after 4, 8, 12, 16, and 20 weeks and analyzed. Serum ascorbic acid, lipid profile, lipoproteins, vascular lesion, and Lp(a) deposition were evaluated.
Results: We observed that periodic vitamin C withdrawals resulted in a less healthy blood lipid profile and showed indications of metabolic adaptation to vitamin C withdrawals. In addition, mice experiencing recurring dietary vitamin C withdrawals were prone to the development of early atherosclerotic lesions with Lp(a) deposition in the structurally compromised areas of the vascular wall, compared to mice continuously supplemented with vitamin C.
Conclusion: This study further supports the importance of consistent and sufficient intake of vitamin C in assuring optimum profile of blood risk factors, and maintaining vascular wall integrity and optimal cardiovascular health.
Keywords: Vitamin C; Lipoprotein(a); Gulo(-/-); Vascular plaques; Cardiovascular health; Vitamin C withdrawal; Cyclical diet
Introduction
Vitamin C is essential for maintaining normal body function and optimal health. Most animals, except a few species (i.e., guinea pigs and some primates), can produce it internally according to their physiological needs. Humans lost the ability to synthesize vitamin C as a result of the inactivating mutation of the gene encoding for a key enzyme in the pathway of ascorbic acid biosynthesis - gluconolactone oxidase (Gulo). As a result, humans must acquire vitamin C through dietary sources.
Vitamin C is a potent antioxidant and an essential catalyst in collagen synthesis. In addition, it is responsible for proper structure and stability of collagen helixes as a cofactor of prolyland lysyl-hydroxylases. Vitamin C deficiency is manifested by the impairment of connective tissue, delayed wound healing, lost integrity and weakening of the blood vessels (hemorrhages), which are all characteristics of scurvy. Although scurvy has become rare in modern society, vitamin C dietary intake varies due to seasons, location of residency, diet, and climate, etc., all of which may result in its periodic deficiency or suboptimal levels in the body [1-3]. In particular, vitamin C intake and plasma vitamin C levels fluctuate with the seasons. For example, a study conducted in northern India in people over 60 showed that the dietary vitamin C intake ranged from 15.7mg/day during the rainy season to 30.6mg/day in the winter when the intake of vegetables was higher [2] in 2011. In rural Gambia, seasonal variations in vitamin C levels were extremely wide in pregnant and lactating women, and ranged from 0.2mg/dl in the rainy season to 1.2mg/dl in the harvest season [4]. Plasma vitamin C concentrations also fluctuated in people in Northwestern Russia from marginal vitamin C deficiency in the spring to its optimal level in the fall [5]. Moreover, clinical studies have demonstrated that seasonal dietary vitamin C deficiency is related to increased blood cholesterol levels and has been associated with increased risk of cardiovascular disease (CVD) [6]. Thus, it was recommended that vascular disease patients take vitamin C supplements throughout the year to keep a consistent normal cholesterol level in winter [6-8]. An epidemiologic study among 11,348 Americans showed a significant decrease in cardiovascular mortality with higher vitamin C intake [9].
In order to better understand the impact of vitamin C on the body’s metabolism and the development of CVD, various animal models have been developed that allow for dietary manipulation of vitamin C intake. Of particular, the Gulo gene knockout mouse model [Gulo(-/-)], which lacks the key enzyme in the ascorbic acid synthesis pathway, makes it like humans, entirely dependent on dietary vitamin C. Using this model Maeda et al. showed that low intake of vitamin C triggered vascular wall damage and elevated blood cholesterol levels [10]. Another unique aspect of human metabolism is the production of lipoprotein (a) [Lp(a)+], which is considered as one of the independent risk factors for atherosclerosis [11,12]. Lp(a) consists of a highly polymorphic glycoprotein apolipoprotein a [apo(a)] linked by a covalent disulfide bridge to a protein in cholesterol-rich LDL particle – apolipoprotein B-100 (apo B) [13]. Elevated plasma Lp(a) levels were associated with increased incidence of CVD [14]. Due to the similar structure to plasminogen, the component of the Lp(a) - apo(a) protein - competes with plasminogen in inhibiting fibrinolysis [15,16]. Rath and Pauling suggested that the appearance of the Lp(a) molecule in human metabolism at the time of GULO mutation and its many other characteristics, predisposes Lp(a) as a physiological surrogate for vitamin C in protecting the integrity of the vascular wall compromised by vitamin C deficiency [17]. To address these important aspects of human metabolism, we developed a new mouse model that is not capable of endogenous ascorbic acid production [Gulo(-/-)] and at the same time it synthesizes human Lp(a) (Lp(a)+). An earlier study in Gulo(-/-); Lp(a)+ mice has confirmed that Lp(a) deposits in structurally compromised areas of the vascular wall weakened by vitamin C-deficient conditions [18].
While vitamin C has been widely researched in various aspects of cardiovascular health, there is a lack of information on the physiological impact of irregular dietary intake of vitamin C. Therefore, we investigated the effects of periodic vitamin C withdrawal followed by its supplementation on key metabolic aspects relevant to cardiovascular health, such as lipid levels and the artery structure in Gulo(-/-); Lp(a)+ mice [19].
Materials and Methods
Mice and genotyping
Human Gulo(-/-); Lp(a)+ mice were generated as described [18]. Briefly, homozygous Gulo(-/-) mice were bred from heterozygous Gulo(+/-) breeders BALB/cBy-Gulosfx/J (Jackson Laboratory, Sacramento, CA). Human apo(a) [h-apo(a)] transgenic mice (Mutant Mouse Regional Resource Center, Columbia, MO) and human apoB- 100(h-apoB-100) transgenic mice (Taconic Farms Inc., Hudson, NY) were cross-bred with homozygous Gulo(-/-) mice separately to produce Gulo(-/-); h-apo(a)+ mice and Gulo(-/-); h-apoB-100+ mice. These two transgenic mice were then bred to generate the Gulo(-/- );Lp(a)+ strain.
Genotyping for ascertaining the homozygosity of the Gulo locus knockout and the presence of h-apo (a) and h-apo B genes was performed by Taqman FAM probe Real Time-PCR at Transnetyx (Cordova, TN) by using mouse tail clips.
All animal experiments were conducted with humane and customary care, and followed a protocol approved by the internal Institutional Animal Safety Review Committee. All mice were housed in a barrier facility with a 12-hour light/12-hour dark cycle.
Experimental design
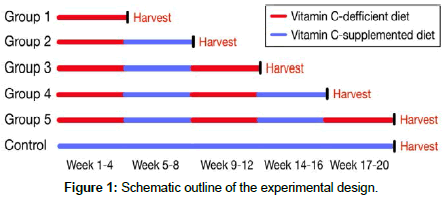
To mimic variation in vitamin C intake in comparison to its consistent supply, the experimental design simulated two conditions: one in which vitamin C was periodically withdrawn from the diet, and one in which vitamin C was maintained at a constant level during the entire duration of the experiment. Two diets were used in the study: a vitamin C deprivation diet (LabDiet® Laboratory Rodent Diet 5001) and a vitamin C-supplemented diet (a modified LabDiet® Laboratory Rodent Diet 5001 with 0.1% 1000 PPM vitamin C). Assuming that a mouse consumes about 4 g of food per day, the vitamin C-supplemented diet provided approximately 4 mg of ascorbic acid daily and the vitamin C-deprivation diet provided 0 mg of ascorbic acid. All mice were maintained on the vitamin C-supplemented diet and distilled water before the experiment started.
Since our earlier study indicated gender-related metabolic differences in Gulo(-/-); Lp(a)+ mice with an impact on the development of atherosclerosis [18], only females were used in the experiments. As such, 15- to 17-week-old female mice were separated into five experimental groups and one control group according to their vitamin C deprivation and supplementation cycles. All experimental groups were given a diet alternating between vitamin C deprivation for 4 weeks and vitamin C supplementation for 4 weeks (Figure 1). Animals were harvested after 4, 8, 12, 16, and 20 weeks and analyzed. The control group was given a vitamin C-supplemented diet for the full 20-week duration of the experiment.
Ascorbic Acid determination
Serum samples were collected from blood drawn via cardiac puncture at the time of harvest of each group. Mice livers were collected, fast frozen in liquid nitrogen, and stored in - 80°C. Before analysis, liver samples were homogenized in double distilled water. Serum and liver ascorbic acid levels were measured using the BioVision Ferric Reducing Ascorbate Assay (FRASC) Kit (Milpitas, CA), and expressed as nmole/mL or nmole/mg tissue protein, respectively.
Lipoprotein and Apo lipoprotein determination
Total cholesterol (Total-C), high density cholesterol (HDL-C), low density cholesterol (LDL-C), and triglyceride (TG) levels were determined by homogeneous enzymatic colorimetric assay performed at the Comparative Pathology Laboratory (CPL) at the University of California, Davis (Davis, CA).
Serum h-apo(a) levels were determined by using the IBL International GmbH Lp(a) Enzyme Immunoassay (Hamburg, Germany). Serum h-apoB-100 was determined by using the Assaypro AssayMax Human Apolipoprotein B Enzyme Kit (St. Charles, MO).
Histology
Mouse aortas were collected, trimmed in ice cold PBS and fixed in 10% neutral buffered formalin. The aortas were then embedded in paraffin, sectioned and stained for Hematoxylin and Eosin, Elastic Van Gieson stain, and immunostained for h-apo(a), h-apoB-100, and fibrinogen at Histotox Labs, Inc. (Boulder, CO).
Atherosclerotic plaques developed in the aortic arch and aortic root were counted in all experimental groups and the control group. Plaque numbers in each group were compared by t-test at a significance level of 0.05.
Quantitation of h-apo(a) stain was conducted using Image J (National Institutes of Health). The colocation of h-apo(a) and h-apoB-100 indicate the deposition of Lp(a). Thus, plaques in the aortic arch and root with colocation of h- apo(a) and h-apoB-100 stain were selected as the regions of interest. The positively h-apo(a) stained area on each plaque was measured and expressed as a percentage of the total plaque area.
Statistical analysis
All data are presented as means ± standard deviation. Significant differences between means were determined by Student’s t-tests at a significance level of 0.05 with Microsoft Excel.
Results
Ascorbic acid levels
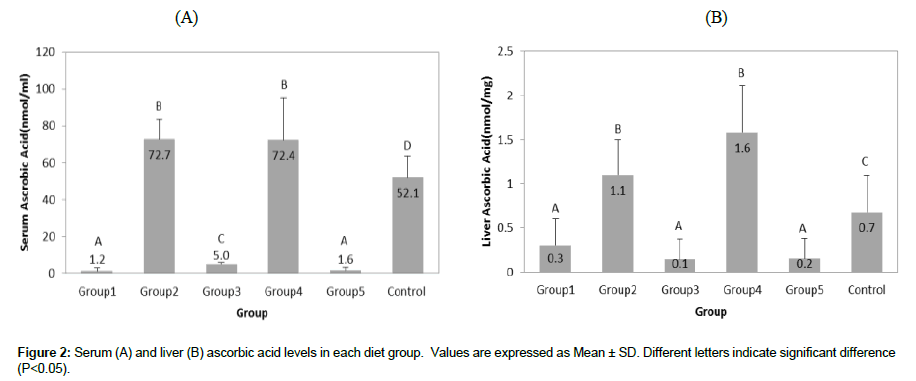
Ascorbic acid levels in the serum and liver of Gulo(-/-);Lp(a)+ mice during each 4-week period of vitamin C supplementation and deprivation are shown in Figure 2 A and B, respectively. Serum ascorbic acid levels closely followed the dietary pattern of vitamin C intake (Figure 2A). They were significantly lower during vitamin C deprivation periods, such as in Groups 1, 3, and 5 (1.2 ± 1.7, 5.0 ± 1.0, and 1. 6 ± 1.9 nmol/mL, respectively; P<0.01) and were significantly higher in Groups 2 and 4 (72.7 ± 10.9 and 72.4 ± 22.9 nmol/mL, respectively; P<0.01) with resumed vitamin C supplementation. Also, serum ascorbic acid levels in Groups 2 and 4 were significantly higher (by 40% and 39%, respectively) compared to the Control Group (52.1 ± 11.6 nmol/mL; P< 0.05).
Ascorbic acid levels in the liver also fluctuated with the vitamin C supplementation cycle (Figure 2B). As such, ascorbic acid levels were significantly lower in Groups 1, 3 and 5 (0.3 ± 0.3, 0.1 ± 0.2, and 0.2 ± 0.2 nmol/mg, respectively; P<0.05) during each of the 4 weeks of vitamin C deprivation and were significantly higher after vitamin C resumption, such as in Groups 2 and 4 (1.1 ± 0.4 and 1.6 ± 0.5 nmol/mg, respectively; P<0.01). Mice in the Control Group which had uninterrupted intake of vitamin C in the diet had lower vitamin C content in the liver ( (0.7 ± 0.4 nmol/mg; P<0.05) compared to Groups 2 and 4, with liver ascorbate levels higher by 62% and 133%, respectively.
Serum human apo(a) (h-apo(a)) and human apo B (h-apoB) levels
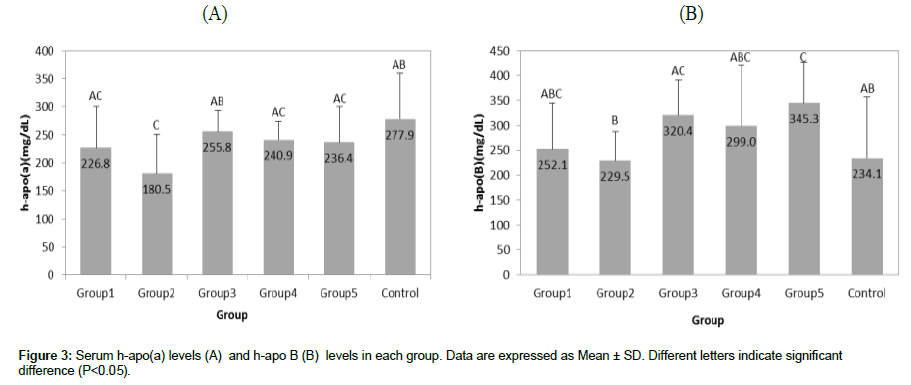
As presented in Figure 3A, the serum h-apo(a) level did not differ significantly between all groups, except it was 20% lower in Group 2 (180.5 ± 70.8 mg/dL) compared to Group 1 ( 226.8 ± 73.9 mg/dL ) . Serum h-apo(a) level in Group 2 was significantly lower than in the Control Group (277.9 ± 81.5 mg/dL; P<0.05). Serum h-apo(a) levels did not significantly differ in Groups 3, 4, and 5 (255.8 ± 37.4, 240.9 ± 33.3, and 236.4 ± 64.1 mg/dL, respectively; P>0.05).
Figure 3B presents serum h-apoB levels in mice upon 4 weeks of vitamin C withdrawal and supplementation cycles. The first vitamin C re-supplementation cycle followed by 4 weeks of withdrawal (Group 2) resulted in a significant decrease of h-apoB from 252.1 ± 92.1 mg/ dL (Group 1) to 229.5 ± 58.4 mg/dL (Group 2). Subsequent vitamin C deprivation periods resulted in higher h-apo B serum levels: 320.4 ± 70.0 mg/dL (Group 3) and 345.3 ± 80.8 (Group 5). The steadily vitamin C supplemented Control Group had serum h-apo B level at 234.1 ± 122.7 mg/dL, which was lower than in the vitamin C deprived Groups 1, 3 and 5.
Lipid profile
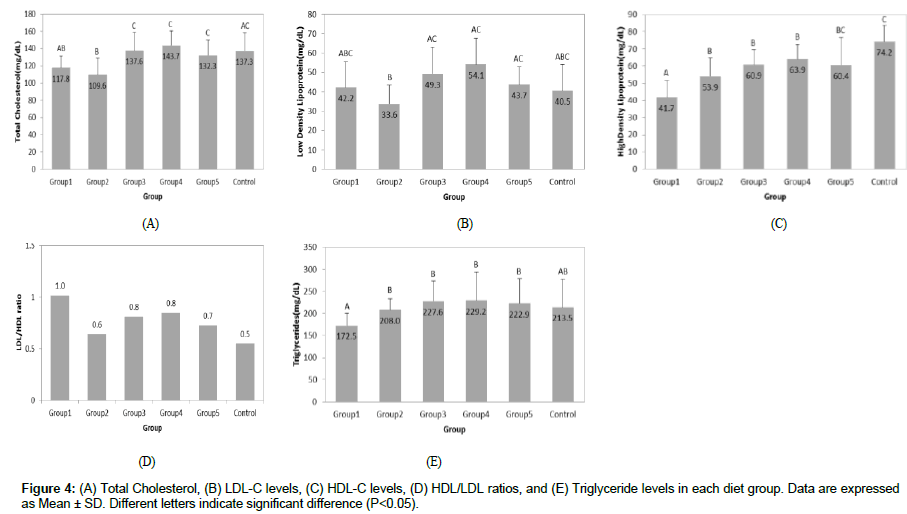
Serum Total Cholesterol (Total-C) and LDL cholesterol (LDL-C) levels: As presented in Figure 4A, the serum total cholesterol (Total-C) reached the lowest level after the first vitamin C resumption period (Group 2, 109.6 ± 19.4 mg/dL). Total-C levels were higher and at similar levels in Groups 3, 4, and 5 (137.6 ± 21.5, 143.7 ± 17.0, and 132.3 ± 17.6 mg/dL, respectively) and they were not significantly different from the Control Group (137.3 ± 21.3; P>0.05).
We observed the same pattern for LDL-C values (Figure 4B), with the lowest level (33.6 ± 9.8 mg/dL) in Group 2 after the first vitamin C re-supplementation. Groups 3, 4, and 5 had significantly higher LDL-C serum levels than Group 2; however, there was no statistical difference between them. The LDL-C level in the Control Group receiving the continuous supply of vitamin C was lower than in Groups 1, 3, 4 and 5, but the differences did not reach statistical significance.
High Density Lipoprotein-Cholesterol (HDL-C): Figure 4C shows that the serum high density lipoprotein-cholesterol (HDL-C) levels were the lowest in Group 1 after the first 4 weeks of vitamin C withdrawal (41.7 ± 9.9 mg/ dL). The highest HDL-C level was observed in the Control Group (74.2 ± 9.8 mg/dL; P<0.05), which was 44% higher when compared to Group 1. With the following 4 weeks of vitamin C resumption, HDL-C levels significantly increased in Group 2 (53.9 ± 11.1 mg/dL; P<0.05) and remained basically at the same level in Groups 3, 4, and 5 (60.9 ± 8.6, 63.9 ± 8.6, and 60.4 ± 16.3 mg/dL, respectively; P>0.05). However, HDL-C levels in Groups 3 and 4 were still significantly lower than the Control Group (P<0.05). HDL-C levels in Group 5 were also 19% lower than the Control Group, but not significantly (P=0.56).
Figure 4D shows that the LDL/HDL ratio was highest in Group 1 compared to all other groups and it dramatically decreased to 0.6 in Group 2 after vitamin C re-supplementation, which was comparable to the level observed in the Control Group (0.5). Further cycles of vitamin C deprivation and supplementation did not show significant changes in this ratio.
Serum Triglycerides (TG): Serum triglycerides (TG) levels remained fairly consistent throughout the experiment (Figure 4E). Statistical significance was only found in triglyceride levels in Group 1 after the first 4 weeks of vitamin C deprivation compared to the Control Group (172.5 ± 27.8 and 213.5 ± 64.4 mg/dL, respectively; P<0.05). Triglyceride levels in all experimental groups were not significantly different from the Control Group (P>0.05).
Vascular lesions development
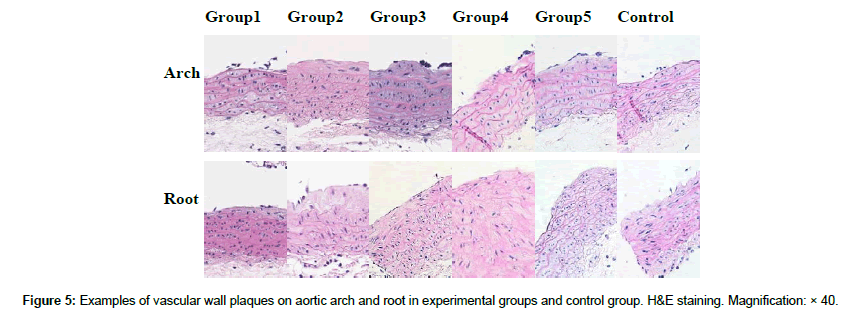
Altered vitamin C deprivation and re-supplementation cycles resulted in the development of structural perturbations in the aortic wall and the appearance of vascular lesions. Vascular plaques were detected mainly at vessel areas subjected to elevated hemodynamic stress from blood flow, such as the sites of the aortic root, arch, and adjacent branching regions. Due to the missing branches during aorta collection, only the aortic root and arch were used for histological analysis. Figure 5 shows the representative images of H&E staining sections of the aortic arch and root at various time points/groups. Already after 4 weeks of vitamin C deprivation, we found mild intima thickening and elastic fiber disruptions. Only one large plaque was found on the left subclavian artery in Group 4 (Picture not shown). No other large plaques were found in the other groups.
Table 1 shows the incidence of lesions detected in the aortic arch and root, the total plaque number, and the average plaque number per mouse in the experimental groups and the Control Group. In all groups, six aorta samples were randomly selected for the calculation of the total plaque number. The average plaque number per mouse reflects only mice that developed plaques in the aortas, not a total number of mice in the experimental group. The results show, that one third of the mice in Group 1 (exposed to the first 4 weeks of vitamin C deprivation) developed vascular plaques with an average of 1 plaque per animal. In Group 2 (first vitamin C re-supplementation) and Group 3 (second cycle of vitamin C withdrawal), about 67% of the animals developed plaques with an average 1.3 and 1.2 plaque per animal, respectively. All mice in Groups 4 and 5 developed vascular plaques, however, vitamin C supplemented Group 4 had 1.2 plaques per animal and Group 5 had developed 2.3 plaques per animal. The Control Group, which was continuously consuming vitamin C, had 67% of mice with plaques with an average 1.3 plaques per animal, similar to the animals in Groups 2 and 3. The average plaque number per animal in Group 5 was significantly higher than the Control Group (P<0.05; Table 1).
| Mice with plaques | |||||
|---|---|---|---|---|---|
| Group | Mice No. | No. | % | Total plaques No. | Avg. plaques No. per mouse+ |
| 1 | 6 | 2 | 33 | 2 | 1.0 |
| 2 | 6 | 4 | 67 | 4 | 1.0 |
| 3 | 6 | 4 | 67 | 5 | 1.3 ± 0.5 |
| 4 | 6 | 6 | 100 | 7 | 1.2 ± 0.4 |
| 5 | 6 | 6 | 100 | 14 | 2.3 ± 0.5* |
| Control | 6 | 4 | 67 | 5 | 1.3 ± 0.5 |
Table 1: The incidence of lesions, total plaques number, and average plaques number per mouse in experimental groups and the control group.
Lp(a) deposition in the vascular wall
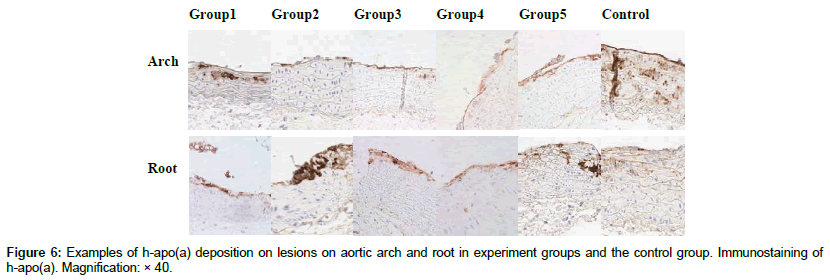
Figure 6 shows the examples of the h-apo(a) deposition in the aortic arch and root in all experimental groups based on immunostaining analysis. The h-apo(a) deposits were found in the intima and media layers in the experimental groups and the Control group. In the Control group there was a negligent h- apo(a) deposition in the aortic root, however, more and scattered h-apo(a) deposits were present in the arch area.
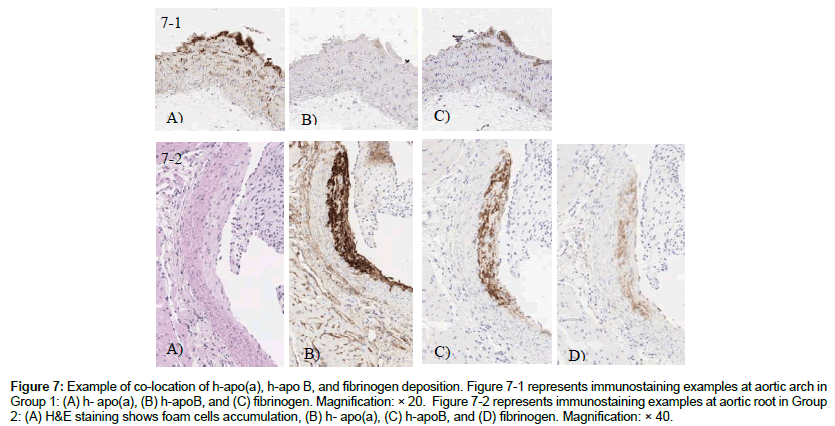
Immunohistological analysis for the co-location of h-apo(a), h-apoB, and fibrinogen showed overlapping immunostaining only in the intimal layer and plaques, but none in the media layer of the aortic wall. Figure 7-1 shows an example of this analysis in animals in Group 1. The co-location of h-apo(a) and h-apo B-100 , which indicates the presence of Lp(a), was only found in lesions and the damaged areas of the aortic wall, as indicated in Figure 7-2.
Figure 7: Example of co-location of h-apo(a), h-apo B, and fibrinogen deposition. Figure 7-1 represents immunostaining examples at aortic arch in Group 1: (A) h- apo(a), (B) h-apoB, and (C) fibrinogen. Magnification: × 20. Figure 7-2 represents immunostaining examples at aortic root in Group 2: (A) H&E staining shows foam cells accumulation, (B) h- apo(a), (C) h-apoB, and (D) fibrinogen. Magnification: × 40.
The frequency of Lp(a) deposition indicated by the colocation of h-apo(a) and h-apo B is shown in Table 2. The number of mice showing Lp(a) deposits in the plaques was the highest in Groups 1, 3 and 5, all of which were deprived of vitamin C in the diet. The percentage of mice with Lp(a) deposits was lower in Groups 2 and 4, which were resumed with vitamin C supplementation. Only 1 out 4 mice in the Control Group was found with vascular Lp(a) deposition. Also, very little h-apo B was collocated with h-apo(a) on the aortic wall in the Control Group. This may indicate that it is a rare event for the Lp(a) deposits on the aortic wall when a high dose of vitamin C was supplied regularly.
| Mice with Lp(a) deposition | ||||
|---|---|---|---|---|
| Group | Mice No. | No. | % | % of h-apo(a) stained area on plaques |
| 1 | 6 | 5 | 83 | 48.1 ± 10.7a |
| 2 | 6 | 3 | 50 | 27.9 ± 23.8ab |
| 3 | 6 | 4 | 67 | 34.3 ± 13.4a |
| 4 | 6 | 2 | 33 | 15.8 ± 2.5b |
| 5 | 11 | 6 | 55 | 38.7 ± 13.1a |
| Control | 4 | 1 | 25 | 26.9 ± 0ab |
Table 2: Incidence of Lp(a) deposition and percentage of h-apo(a) stained area in Lp(a) containing plaques. Different letters indicate significant difference (P<0.05).
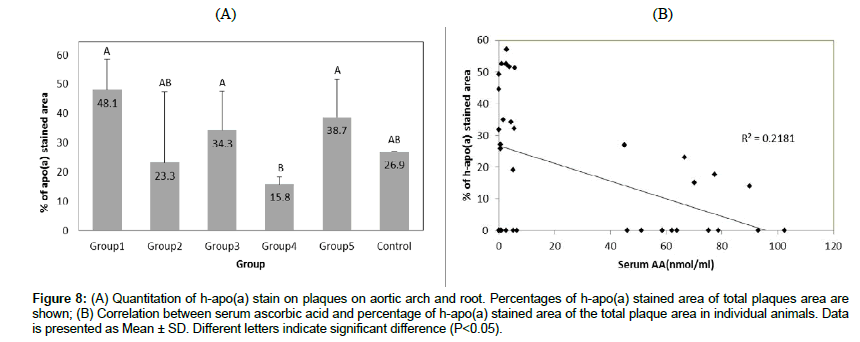
The percentage of h-apo(a) stained area was measured in lesions with Lp(a) deposition as described in Materials and Methods. The percentage of h-apo(a) stained area in plaques was lower in mice with resumed vitamin C intake (Groups 2 and 4) and higher in Groups 1, 3, and 5 which had the vitamin C deficient diet. The percentage of h-apo(a) stained plaques was significantly lower in Group 4 than Groups 3 and 5 (P<0.05; Table 2; Figure 8A). We found a reversed relationship between the percentage of h-apo(a) stained area and serum ascorbic acid levels (Figure 8B). The correlation was significant with serum ascorbic acid levels explaining about 22% of the variance in the percentage of the h-apo(a) deposition on plaques (P<0.05).
Figure 8: (A) Quantitation of h-apo(a) stain on plaques on aortic arch and root. Percentages of h-apo(a) stained area of total plaques area are shown; (B) Correlation between serum ascorbic acid and percentage of h-apo(a) stained area of the total plaque area in individual animals. Data is presented as Mean ± SD. Different letters indicate significant difference (P<0.05).
Discussion
Lipoprotein (a), which appeared in human metabolism about 40 million years ago at the time of the genetic change in humans resulting in a loss of internal vitamin C production, is considered a repair molecule that would counteract potentially fatal consequences of ascorbate deficiency on the extracellular matrix and vascular wall [17,19]. Previously we have shown that hypoascorbemia and vitamin C depletion (scurvy) in Gulo(-/-);Lp(a)+ mice resulted in increased serum levels of Lp(a) and the accumulation of intact Lp(a) molecules in the vascular wall, paralleling the development of atherosclerosis [18].
Since vitamin C intake is rarely constant over longer periods of time, we investigated the effect of periodic changes in vitamin C dietary intake levels on selected parameters relevant to cardiovascular health. Gulo(-/-); Lp(a)+ mice were divided into separate groups and were subjected to 4-week cycles of dietary vitamin C withdrawal followed by its supplementation during the 20-week period in parallel to a continuous vitamin C supplementation for the duration of the study.
We observed that during the vitamin C withdrawal periods both serum and liver ascorbic acid fell to very low levels and then significantly increased during the vitamin C resumption periods. This corroborates the data showing that in Gulo(-/-)mice vitamin C was rapidly depleted in the serum, liver, kidney, and heart already after 1 week of ascorbate withdrawal [20]. Because of a lack of buffering ability in storing ascorbic acid during dietary vitamin C deficiency, the authors suggested a constant intake of vitamin C in order to maintain its optimal tissue concentration and metabolism [20]. We found an interesting phenomenon that during vitamin C re-supplementation periods (Groups 2 and 4), both the serum and liver ascorbic acid concentrations were reaching significantly higher levels than in the mice continuously supplemented with vitamin C for 20 weeks (Control Group) (Figure 2A and 2B). The second cycle of re-supplementation especially resulted in ascorbate liver levels over twice as high as in mice continuously supplied with ascorbate. It appears that these animals tended to respond to this dietary challenge through metabolic adaptation, such as by increased retention capacity of the critical nutrient. This would support earlier suggestions that change in availability of an essential micronutrient may lead to the adaptation of its absorption in order to maintain normal body functions [21,22]. Further support of the metabolic adaptation also comes from the analysis of lipid profile parameters which were more dramatically affected during the first cycle of vitamin C withdrawal and resumption, but not after the second and third cycle of vitamin C deprivation. This was especially seen in the changes of the serum HDL-C levels (Figure 4C).
Our data show that 4-week alterations in the dietary vitamin C intake in Gulo(-/-); Lp (a) + mice also had an effect on the vascular wall structure as reflected by the number of plaques developed in the aortic root and arch. This confirms our earlier findings showing that dietary vitamin C deficiency is an underlying factor promoting the deposition of Lp(a) and the development of early atherosclerosis [18]. It is well known that vitamin C is essential for the synthesis and proper collagen structure as a cofactor of proline and lysine hydroxylases. Also another important structural protein component in the aorta, elastin, contains a significant amount of hydroxyproline. It has been shown that vitamin C deficiency in Gulo(-/-) mice can generate structural perturbations in the vascular wall resulting from impaired hydroxylation of lysine and proline as indicated by Maeda et al. [10].In in vivo studies conducted in guinea pigs, which similar to humans are unable to produce vitamin C internally, indicated that the hydroxyproline content in elastin in the aortas was ascorbic acid dose dependent and it decreased with the severity of vitamin C deficiency [23]. The impaired biosynthesis of collagen and elastin compromises integrity of the aortic wall and enhances early lesion development [24].
Our findings showed that consistent supplementation of vitamin C can protect against the development of atherosclerotic lesions despite the genetic trait of these mice making them prone to atherosclerosis (production of Lp(a) and a lack of internal vitamin C synthesis). Similar results were also found in other animal models [10,19]. In this study we did not observe a decrease in the number of vascular plaques after the resumption of vitamin C intake for 4 weeks. The daily vitamin C dose applied in this study (4 mg) for 4 weeks allowed to achieve serum ascorbate levels close to the values reported in wild type mice (about 63 mcM). In our previous study the intake of 2.75 mg vitamin C for 6 weeks resulted in a slightly lower serum ascorbate level (50 mcM) and a minor deposition of Lp(a) inside the vascular wall. It is possible that more vitamin C is needed for a complete vascular wall repair during the 4-week period, or its intake should continue for a longer time in order to restore optimal structure of the aortic wall. In this respect it was suggested that about 13 mg of ascorbate a day is needed to obtain tissue saturation level in the Gulo(-/-)mice model [25]. However, the pattern of Lp(a) deposition and the percentage of plaques with h-apo(a) would suggest that 4 mg of vitamin C intake for 4 weeks can initiate structural changes in the vascular wall and consequently lower the requirements for biological “repair” by the Lp(a).
Our results show that Lp(a) was present inside the damaged vascular wall and plaques. This confirms the earlier findings in human studies indicating Lp(a), not LDL, as the main component of vascular plaques [26]. It also supports our previous study results in guinea pigs and Gulo(-/-); Lp(a)+ mice models that Lp(a) accumulates in the arterial wall structurally weakened by ascorbate deficiency [18,19]. It has been suggested that apo(a) is pro-atherogenic by modulating the function and permeability of vascular endothelial cells [27-29], and Lp(a) entering the arterial intima can be taken up by macrophages thereby contributing to foam cell formation [30]. However, this and our earlier study point to the Lp(a) role as a vascular repair molecule, not a damaging factor itself and that vascular plaque development is the consequence of this overshooting “repair” process [18,19]. As such, low ascorbic acid levels resulting in a decreased ratio of hydroxylysine to lysine and increased vascular endothelial gaps, can enhance the influx and binding of Lp(a) [19,23]. Our previous study also found that the deposition of Lp(a) inside the vascular wall of Gulo(-/-);Lp(a)+ mice is reversely correlated with vitamin C intake, and a complete dietary vitamin C absence results in a significant Lp(a) deposition in the vascular wall [18]. Here we found an inverse relationship between the percentage of h-apo(a) stained area in plaques and the serum ascorbic acid concentration. This result further supports the role of Lp(a) functioning as a mobile repair molecule for the vascular wall during ascorbic acid depletion.
The reversed relationship between HDL-C and vitamin C levels has been found in Gulo(-/-)mice and observed in many human studies [10,31-32]. Our findings showed that periodic vitamin C deprivation was accompanied by a significantly lower level of HDL-C, thus a less healthy cardiovascular profile. This would imply that variation in vitamin C intake levels related to, for example, changing dietary patterns or its inconsistent supplementation, could negatively affect cardiovascular health. Epidemiological studies have shown that the seasonal variations in vitamin C intake correlate with serum cholesterol levels and CVD events, which in many countries tend to rise in winter and decrease in summer [6,33]. Thus, our results provide further support for the importance of consistent vitamin C supplementation for optimum cardiovascular health.
In conclusion, our study shows that periodic vitamin C deprivation compared to its continuous intake can facilitate metabolic changes and the Lp(a) accumulation in the vascular wall leading to the development of early atherosclerotic lesions. Therefore, regular optimum intake of vitamin C is necessary for maintaining optimum profile of blood risk factors, preserving vascular wall integrity and assuring optimal cardiovascular health.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
None.
Founding Statement
The research study was funded by Dr. Rath Health Foundation (Santa Clara, CA, USA), a non-profit organization.
Acknowledgments
We thank IDEXX BioResearch for providing histology slides, and the Comparative Pathology Laboratory (CPL) at the University of California, Davis, for serum lipid cholesterol analysis. We also thank Taufeeq Ahmed for mouse colony management and Cathy Flowers for editing the manuscript.
References
- Torheim LE, Ferguson EL, Penrose K, Arimond M (2010) Women in resource-poor settings are at risk of inadequate intakes of multiple micronutrients. J Nutr 140: 2051-2058.
- Ravindran RD, Vashist P, Gupta SK, Young IS, Maraini G, et al. (2011) Prevalence and risk factors for vitamin C deficiency in north and south India: a two centre population based study in people aged 60 years and over. PLoS ONE 6: e28588.
- Chiplonkar SA, Agte VV, Mengale SS, Tarwadi KV (2002) Are lifestyle factors good predictors of retinol and vitamin C deficiency in apparently healthy adults? Eur J Clin Nutr 56: 96-104.
- Bates CJ, Prentice AM, Paul AA (1994) Seasonal variations in vitamins A, C, riboflavin and folate intakes and status of pregnant and lactating women in a rural Gambian community: Some possible implications. Eur J Clin Nutr 48: 660-668.
- Paalanen L, Prättälä R, Alfthan G, Salminen I, Laatikainen T (2013) Seasonal variation in plasma vitamin C concentration in Pitkäranta, Northwestern Russia. Eur J Clin Nutr 67: 1115.
- Dobson HM, Muir MM, Hume R (1984) The effect of ascorbic acid on the seasonal variations in serum cholesterol levels. Scott Med J 29: 176-182.
- Ginter E, Kajaba I, Nizner O (1970) The Effect of Ascorbic Acid on Cholesterolemia in Healthy Subjects with Seasonal Deficit of Vitamin C. Nutr Metab 12: 76–86.
- MacRury SM, Muir M, Hume R (1992) Seasonal and climatic variation in cholesterol and vitamin C: Effect of vitamin C supplementation. Scott Med J 37: 49-52.
- Enstrom JE, Kanim LE, Klein MA (1992) Vitamin C intake and mortality among a sample of the United States population. Epidemiology 3: 194-202.
- Maeda N, Hagihara H, Nakata Y, Hiller S, Wilder, et al. (2000) Aortic wall damage in mice unable to synthesize ascorbic acid. Proc Natl Acad Sci USA 97: 841-846.
- Genest JJ Jr, Martin-Munley SS, McNamara JR, Ordovas JM, Jenner J, et al. (1992) Familial lipoprotein disorders in patients with premature coronary artery disease. Circulation 85: 2025-2033.
- Schreiner PJ, Morrisett JD, Sharrett AR, Patsch W, Tyroler HA, et al. (1993) Lipoprotein [a] as a risk factor for preclinical atherosclerosis. Arterioscler Thromb 13: 826-833.
- Lawn RM (1992) Lipoprotein (a) in heart disease. Sci Am 266: 54-60.
- Nordestgaard BG, Chapman MJ, Ray K, Boren J, Andreotti F, et al. (2010) Lipoprotein(a) as a cardiovascular risk factor: current status. European Heart Journal 31: 2844-2853.
- McLean JW, Tomlinson JE, Kuang WJ, Eaton DL, Chen EY, et al. (1987) cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature 330: 132-137.
- Loscalzo J, Weinfeld M, Fless GM, Scanu AM (1990) Lipoprotein (a), fibrin binding, and plasminogen activation. Arteriosclerosis 10: 240-245.
- Rath M, Pauling L (1990a) Hypothesis: Lipoprotein (a) is a surrogate for ascorbate. Proc Natl Acad Sci USA 87: 6204-6207.
- Cha, J, Niedzwiecki A, Rath M (2015) Hypoascorbemia induces atherosclerosis and vascular deposition of lipoprotein (a) in transgenic mice. Am J Cardiovasc Dis 5: 53-62.
- Rath M, Pauling L (1990) Immunological evidence for the accumulation of lipoprotein (a) in the atherosclerotic lesion of the hypoascorbemic guinea pig. Proc Nadl Acad Sci USA 87: 9388-9390.
- Vissers MC, Bozonet SM, Pearson JF, Braithwaite LJ (2011) Dietary ascorbate intake affects steady state tissue concentrations in vitamin C-deficient mice: Tissue deficiency after suboptimal intake and superior bioavailability from a food source (kiwifruit). Am J Clin Nutr 93: 292-301.
- Storey KB, Storey JM (2005) Biochemical Adaptation to Extreme Environments. In: Walz W (ed) Integrative Physiology in the Proteomics and Post-Genomics Age. pp.169-200.
- Hall KD (2009) Predicting metabolic adaptation, body weight change, and energy intake in humans. Am J Physiol Endocrinol Metab 298: 449-466.
- Barnes MJ, Constable BJ, Kodicek E (1969) Studies in vivo on the biosynthesis of collagen and elastin in ascorbic acid-deficient guinea pigs. Biochemical J 113: 387-397.
- Berillis P (2013) The Role of Collagen in the Aorta’s Structure. Open Circ Vasc J 6: 1-8
- Kim H, Bae S, Yu Y, Kim Y, Kim HR, et al. (2012) The analysis of vitamin C concentration in organs of gulo(-/-) mice upon vitamin C withdrawal. Immune Netw 12: 18-26.
- Rath M, Niendorf A, Reblin T, Dietel M, Krebber HJ, et al. (1989) Detection and quantification of lipoprotein (a) in the arterial wall of 107 coronary bypass patients. Arteriosclerosis 9: 579-592.
- Pellegrino M, Furmaniak-Kazmierczak E, LeBlanc JC, Cho T, Cao K, et al. (2004) The apolipoprotein(a) component of lipoprotein(a) stimulates actin stress fiber formation and loss of cell-cell contact in cultured endothelial cells. J Biol Chem 279: 6526-6533.
- Liu L, Craig AW, Meldrum HD, Marcovina SM, Elliott BE, et al. (2009) Apolipoprotein(a) stimulates vascular endothelial cell growth and migration and signals through integrin alpha beta3. Biochem J 418: 325-336.
- Cho T, Romagnuolo R, Scipione C, Boffa MB, Koschinsky ML (2012) Apolipoprotein(a) stimulates nuclear translocation of β-catenin: A novel pathogenic mechanism for lipoprotein(a). Mol Biol Cell 24: 210-221.
- Nielsen LB, Nordestgaard BG, Stender S, Niendorf A, Kjeldsen K (1995) Transfer of lipoprotein(a) and LDL into aortic intima in normal and in cholesterol-fed rabbits. Arterioscler Thromb Vasc Biol 15: 1492-1502.
- Ness AR, Khaw KT, Bingham S, Day NE (1996) Vitamin C status and serum lipids. Eur J Clin Nutr 50: 724-729.
- Itoh R, Yamada K, Oka J, Echizen H, Suyama Y, Murakami K (1990) Serum ascorbic acid and HDL cholesterol in a healthy elderly Japanese population. Int J Vitam Nutr Res 60: 360-365.
- Fares A (2013) Winter Cardiovascular Diseases Phenomenon. N Am J Med Sci 5: 266-279.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi