Research Article, Int J Cardiovasc Res Vol: 6 Issue: 6
Cerebral Micro bleeds in Coronary Artery Disease Patients during Antiplatelet Therapy
Yoshiko Iwamoto1, Hisashi Kai2*, Kenji Fukuda1,3, Hiroki Uchiwa1, Takahiro Anegawa1, Yuji Aoki1, Hidemi Kajimoto1,4, Yusuke Uchiyama5, Toshi Abe5, Tsutomu Imaizumi6 and Yoshihiro Fukumoto1
1Department of Internal Medicine, Division of Cardio-Vascular Medicine, Kurume University School of Medicine, Kurume, Japan
2Department of Cardiology, Kurume University Medical Center, Kurume, Japan
3Department of Cerebrovascular Medicine, St. Mary’s Hospital, Kurume, Japan
4Department of Cardiology, University of Washington, Seattle, WA, USA
5Department of Radiology, Kurume University School of Medicine, Kurume, Japan
6Fukuoka International College of Health and Welfare, Fukuoka, Japan
*Corresponding Author : Hisashi Kai
Department of Cardiology, Kurume University Medical Center 155-1 Kokubu-machi, Kurume, Fukuoka 839-0863, Japan
Tel: 81-942-22-6530
E-mail: naikai@med.kurume-u.ac.jp
Received: November 15, 2017 Accepted: November 25, 2017 Published: December 04, 2017
Citation: Iwamoto Y, Kai H, Fukuda K, Uchiwa H, Anegawa T, et al. (2017) Cerebral Micro bleeds in Coronary Artery Disease Patients during Antiplatelet Therapy. Int J Cardiovasc Res 6:6. doi: 10.4172/2324-8602.1000329
Abstract
Background: Brain hemorrhage is a serious complication of antiplatelet therapy, particularly dual antiplatelet therapy (DAPT), in patients with coronary artery disease (CAD) who undergo percutaneous coronary intervention (PCI). It has been suggested that cerebral micro bleeds (CMBs) detected on magnetic resonance imaging (MRI) are a risk factor for future cerebral haemorrhage. However, little is known about CMBs in CAD patients during antiplatelet therapy. We investigated the temporal changes of CMBs and determined the risk factors for CMBs in patients with CAD on antiplatelet therapy.
Methods: This study prospectively enrolled 14 CAD patients who underwent antiplatelet therapy (DAPT in 13 patients) and had no history of symptomatic stroke. Brain MRI was performed at baseline and after 8-month follow-up.
Results: Baseline MRI revealed CMBs in two patients (14%). New CMBs were detected by follow-up MRI in two other patients (14%). CMB-positive patients had a greater number of coronary artery lesions (p=0.04) and a tendency to have a higher SYNTAX score at baseline (p=0.06) than CMB-negative patients. Although blood pressure (BP) at baseline did not differ between the CMB-positive and CMB-negative patients, BP after 8 months was significantly higher in CMB-positive than in CMB-negative patients (systolic BP: p=0.03, diastolic BP: p=0.02).
Conclusions: CAD patients with severe coronary artery lesions and poor BP control appear to be at higher risk for CMBs during antiplatelet therapy. Accordingly, strict coronary risk control, especially BP control, is necessary to prevent new CMBs in CAD patients receiving long-term antiplatelet therapy
Keywords: Antiplatelet therapy; Coronary artery disease; Hypertension; MRI; Cerebral hemorrhage
Introduction
It is recommended that patients with coronary artery disease (CAD) receiving antiplatelet therapy including dual antiplatelet therapy (DAPT) for the secondary prevention or prevention of stent thrombosis after percutaneous coronary intervention (PCI). Cerebral hemorrhage is a serious complication of antiplatelet therapy, especially DAPT. CAD patients have several atherosclerosis risk factors, such as hypertension, diabetes, and hyperlipidemia, and they are prone to have systemic atherosclerosis including cerebrovascular disease [1]. Therefore, CAD patients on antiplatelet therapy are considered at a very high risk for cerebral hemorrhage.
Cerebral micro bleeds (CMBs) are small, round, boundary clear, hypo intensity lesions seen on T2*-weighted magnetic resonance imaging (MRI) that reflect histological changes around the small vessels, such as hemosiderin deposition in macrophages, hyaline degeneration, and amyloid deposition [2]. It is considered that CMBs are a new marker of cerebral small vessel disease, which provides evidence of blood leakage from pathologically fragile small vessels mainly affected by hypertensive arteriolopathy (i.e. deep CMBs) or cerebral amyloid angiopathy (i.e. lobar CMBs) [2-4]. A largescale prospective observational study has shown that the presence of CMBs is associated with a subsequent cerebral hemorrhage and ischemic stroke in 2,102 healthy elderly individuals [5]. Further, a systematic review and meta-analysis of prospective cohort studies has demonstrated that patients with CMBs have an increased risk for recurrent stroke, particularly for cerebral hemorrhage after ischemic stroke or transient ischemic attack [6]. Therefore, this suggests that the presence of CMBs is a potential risk factor for future cerebral hemorrhage. In this regard, there are concerns over the safety of antithrombotic therapy using antiplatelet and/or anticoagulant agents for the primary and secondary prevention of cerebrovascular disease in patients with CMBs. Among patients on oral anticoagulant therapy, it has been shown that the presence and number of CMBs, particularly lobar CMBs, are associated with a higher risk of future cerebral hemorrhage [7-9]. However, it remains controversial whether antiplatelet therapy using aspirin or thienopyridines would increase the risk for CMBs formation in patients with cerebrovascular disease or the risk for future cerebral hemorrhage in patients with CMBs [10-16].
At present, little is known about the prevalence of CMBs in CAD patients on antiplatelet therapy. Moreover, whether antiplatelet therapy would be a risk for CMBs remains unknown in CAD patients. Therefore, the temporal changes of CMBs and the risk factors for CMBs were investigated in CAD patients on antiplatelet therapy who have a history of symptomatic stroke.
Methods
Study population
This prospective observational study enrolled CAD patients for whom coronary angiography (CAG) and elective PCI were planned between March 2012 and October 2012 in Kurume University Hospital. Patients with a history of symptomatic stroke and those on warfarin or direct oral anticoagulants were not included. This study was performed in accordance with the Declaration of Helsinki and was approved by the Kurume University Ethics Committee. All patients gave their written informed consent. [UMIN Registration ID: UMIN000007367]
Protocol
At the time of registration (baseline), medical history, smoking habits, and medications were evaluated. The baseline MRI was performed within 3-7 days after the index CAG/PCI (except 1 case with CAG alone). Eight patients had received DAPT at least one month before the index CAG/PCI, whereas DAPT was started within one week before the index PCI in five patients. Blood pressure (BP) was measured using a sphygmomanometer in a quiet, appropriate environment at room temperature after 5-min rest in the sitting position in a chair, according to the Japanese Society of Hypertension guidelines for the management of hypertension 2009 [17]. Measurements were performed twice with a 2-minute interval, and the two measurements were averaged. Eight months later, patients underwent the follow-up MRI.
Hypertension was defined as systolic BP (SBP) ≥140 mmHg and/ or diastolic BP (DBP) ≥90 mmHg or receiving antihypertensive drugs. Diabetes mellitus was diagnosed when HbA1c exceeded 6.5% or oral antidiabetic drugs and/or insulin were prescribed. Dyslipidemia was defined as low-density lipoprotein cholesterol ≥140 mg/dL, highdensity lipoprotein cholesterol ≤ 40 mg/dL and/or triglycerides ≥150 mg/dL.
Brain MRI and definition of CMBs
Brain MRI was performed using a 3.0-T MR system (Signa HDxt, GE Healthcare, Little Chalfont, England). MRI sequences were obtained in the axial plane with the following parameters: slice thickness = 5 mm, slice gap = 1 mm, TR/TE = 11 ms/5 ms for T1, TR/TE = 6000 ms/76 ms for diffusion weighted imaging, TR/TE = 9002 ms/140 ms for fluid-attenuated inversion recovery, and TR/ TE = 600 ms/20 ms and flip angle = 30° for T2*-weighted gradient echo. The imaging protocol consisted of T2*-weighted gradient echo, T1-weighted spin echo, T2-weighted fast spin echo, fluid-attenuated inversion recovery, and cerebral magnetic resonance angiography. The MRI examinations were evaluated visually for the presence of CMBs, cerebral infarction, white matter lesions, and arterial stenosis on the basis of the agreement of two cerebrovascular physicians specializing in brain MRI blinded to other clinical information. MRI imaging was performed under the same conditions, such as slice thickness and inter slice gap, throughout the present study. Special attention was given to match MRI slices of the same patient at baseline and at follow-up by using the intracranial anatomical components as the guide marks. CMBs were defined as small in diameter (2-10 mm), homogeneous, boundary clear, round foci of low signal intensities, which did not appear on more than two thinsliced images, on T2*-weighted MRI in accordance with the Micro bleed Anatomical Rating Scale (MARS) [18]. CMBs mimics were carefully excluded; particularly, linear and curvilinear hypoinstensity lesions in the subcortical area and the juxtacortical subarachnoid space were eliminated as the cortical vessels, according to the MARS [18]. A lacunar infarct was defined as a small, deep infarct of diameter less than 15 mm, with low signal intensity on T1-weighted images. White matter lesions were evaluated using a semi quantitative method proposed by Fazekas [19]: periventricular hyper intensity (PVH) was graded as 0=absent, 1=“caps” or pencil-thin lining, 2=smooth “halo”, or 3=irregular PVH extending into the deep white matter. Deep white matter hyper intensity (DWMH) was graded as 0= absent, 1=punctate foci, 2=beginning confluence of foci, and 3=large confluent areas.
Severity of coronary lesions
CAG was evaluated at the time of the index PCI for the severity of coronary lesions at baseline. The number of coronary artery lesions with ≥75% stenosis according to the AHA classification and the SYNTAX score were assessed based on the agreement of two blinded PCI specialists [20].
Statistics
Data are described as means ± SD. Statistical analysis was performed using commercially available software (JMP 11, SAS Institute Japan, Tokyo, Japan). Unpaired Student’s t-test and the χ2 test were used for comparisons of baseline characteristics and demographics and the MRI findings between the CMB-positive and CMB-negative groups. Mann-Whitney’s U test was used to compare the number of coronary lesions, SYNTAX score, SBP level, and SBP change between the two groups. A value of p<0.05 was considered significant.
Results
This study enrolled 14 CAD patients with no history of symptomatic stroke (Table 1). Their mean age was 70.4 ± 5.8 years, and 85.7% were males. At baseline, systolic BP was 120.3 ± 17.1 mm Hg and diastolic BP was 68.3 ± 13.6 mm Hg. Among the 14 patients, two and seven had a history of effort angina and old myocardial infarction, respectively. Seven patients had undergone PCI or coronary artery bypass grafting. Symptomatic peripheral artery disease was found in one patient. On the index admission, 10 and one patients were diagnosed as having effort angina and silent myocardial ischemia, respectively, and three had acute coronary syndromes.
| Total | CMB-positive | CMB-negative | P | |
|---|---|---|---|---|
| Patient number, N | 14 | 4 | 10 | – |
| Age, years | 70.4 ± 5.8 | 72.0 ± 5.3 | 69.7 ± 5.3 | 0.526 |
| Male, N (%) | 12 (85.7) | 4 (100.0) | 8 (80.0) | 0.334 |
| Systolic blood pressure, mmHg | 120.3 ± 17.1 | 113.5 ± 19.3 | 123.0 ± 19.3 | 0.370 |
| Diastolic blood pressure, mmHg | 68.3 ± 13.6 | 63.0 ± 14.2 | 70.4 ± 14.2 | 0.378 |
| CAD Diagnosis on Index Admission | ||||
| Effort angina, N (%) | 10 (71.1) | 1 (25.0) | 8 (80.0) | 0.157 |
| Silent myocardial ischemia, N (%) | 1 (7.1) | 1 (25.0) | 1 (10.0) | 0.469 |
| Acute coronary syndrome, N (%) | 3 (21.4) | 2 (50.0) | 1 (10.0) | 0.099 |
| Past History of CAD and PAD | ||||
| Effort angina, N (%) | 2 (14.2) | 0 (0.0) | 2 (20.0) | 0.334 |
| Old myocardial infarction, N (%) | 7 (50.0) | 1 (24.0) | 6 (60.0) | 0.237 |
| Prior PCI/CABG, N (%) | 7 (50.0) | 0 (0.0) | 7 (70.0) | 0.017 |
| Peripheral artery disease, N (%) | 1 (7.1) | 0 (0.0) | 1 (10.0) | 0.512 |
| Risk Factors and Comorbidities | ||||
| Hypertension, N (%) | 9 (64.3) | 4 (100.0) | 5 (50.0) | 0.078 |
| Diabetes, N (%) | 7 (50.0) | 3 (75.0) | 4 (40.0) | 0.237 |
| Smoking, N (%) | 9 (64.3) | 4 (100.0) | 5 (50.0) | 0.078 |
| Dyslipidemia, N (%) | 13 (92.9) | 3 (75.0) | 10 (100.0) | 0.101 |
| Dual Antiplatelet Therapy | ||||
| Baseline, N (%) | 8 (57.1) | 1 (25.0) | 7 (70.0) | 0.124 |
| Follow-up, N (%) | 13 (92.9) | 3 (75.0) | 10 (100.0) | 0.101 |
Table 1: Baseline characteristics and demographics.
MRI findings
On baseline MRI, two (14.3%) of 14 patients had CMBs; one lobar CMB was detected in each subject (Table 2). Follow-up MRI showed new CMBs in two other patients (14.3%): one subject showed three lobar CMBs in the bilateral cerebral cortices and another had one deep CMB in the left putamen. The four patients who had CMBs on the follow-up MRI were classified as the CMB-positive group, and the remaining 10 patients were classified as the CMB-negative group.
| Total | CMBs-positive | CMBs-negative | P | |
|---|---|---|---|---|
| Patient number, N | 14 | 4 | 10 | – |
| CMBs | ||||
| Baseline, N (%) | 2 (14.3) | 2 (50) | 0 (0) | – |
| Follow-up, N (%) | 4 (28.6) | 4 (100.0) | 0 (0) | – |
| Other MRI Findings at Baseline | ||||
| Arterial stenosis, N (%) | 1 (7.1) | 0 (0) | 1 (10.0) | 0.512 |
| Old infarction, N (%) | ||||
| Lacunar | 3 (21.4) | 1 (25.0) | 2 (20.0) | 0.836 |
| Atherothrombotic | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| Cardiogenic | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| White matter lesions | ||||
| Periventricular hyper intensity | ||||
| Grade 0 or 1, N (%) | 14 (100.0) | 4(100.0) | 10(100.0) | |
| Grade 2, N (%) | 0 (0.0) | 0(0.0) | 0(0.0) | |
| Grade 3, N (%) | 0 (0.0) | 0(0.0) | 0(0.0) | |
| Deep white matter hyper intensity | ||||
| Grade 0 or 1, N (%) | 7 (50.0) | 2(50.0) | 5(50.0) | |
| Grade 2, N (%) | 5 (35.7) | 1(25.0) | 4(40.0) | |
| Grade 3, N (%) | 2 (14.3) | 1(25.0) | 1(10.0) | |
Table 2: MRI Findings.
At baseline, most patients had mild white matter lesions, and the PVH and DWMH grades did not differ between the CMB-positive and CMB-negative groups (Table 2). Lacunar infarction was shown in one and two patients of the CMB-positive and CMB-negative groups, respectively. Right vertebral artery stenosis was found in one patient of the CMB-negative group. Except for CMBs, the MRI findings did not change during the 8-month follow-up in each patient. Furthermore, the appearance of new CMBs was not associated with the presence of small artery disease and large artery disease at baseline.
Baseline characteristics and demographics
Table 1 shows the baseline characteristics and demographics of the studied patients. Age, the prevalence of males, and baseline BP levels did not differ between the CMB-positive and CMB-negative groups. There were no differences in the CAD diagnosis at the index admission, past history of CAD, coronary risk factors, comorbidities, and DAPT use between the two groups.
Severity of coronary artery lesions
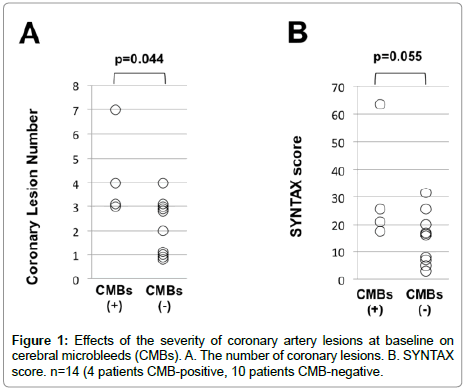
Baseline CAG showed that the number of coronary artery lesions with ≥75% stenosis according to the AHA classification was greater in the CMB-positive group than the CMB-negative group (p=0.044, Figure 1A). On the baseline CAG, the CMB-positive group tended to have a higher SYNTAX score than the CMB-negative group (p=0.055, Figure 1B).
BP changes
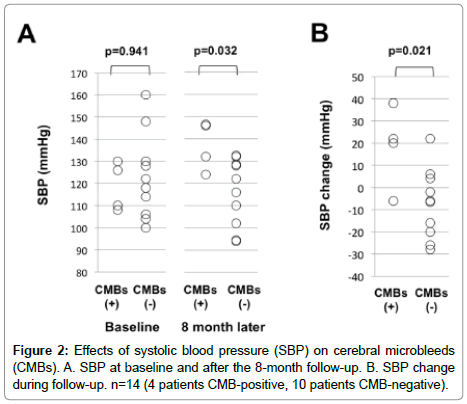
At baseline, systolic BP did not differ between the CMB-positive and CMB-negative groups (Figure 2A). After the 8-month follow-up, the CMB-positive group had higher BP than the CMB-negative group (p=0.032, Figure 2A). Moreover, the BP change during the 8-month follow-up was significantly greater in the CMB-positive group than the CMB-negative group (p=0.021, Figure 2B).
Discussion
CAD patients on antiplatelet therapy are considered to be at high risk for cerebral hemorrhage. However, the prevalence of CMBs, a possible risk factor for future cerebral hemorrhage, remains unknown. The present pilot study demonstrated for the first time that, in CAD patients on antiplatelet therapy, the prevalence of CMBs was 14.3% at baseline and then doubled during follow-up of only 8 months (Table 2). It has been shown that CMBs are detected in 4.4% of healthy Japanese elderly subjects [5]. A systematic review demonstrated that the prevalence of CMBs was 5% in healthy adults, 34% in patients with ischemic stroke, and 60% in patients with cerebral hemorrhage [21]. Therefore, the risk of CMBs appears to be relatively higher in CAD patients on antiplatelet therapy than in the general healthy population but lower than in patients with cerebrovascular disease. The Rotterdam Scan Study and the AGESReykjavik Study showed that the prevalence of CMBs increased by 3.6% during 3.4-year follow-up and 18.4% during 5.2-year follow up, respectively, in healthy elderly populations [22,23]. Therefore, it is noteworthy that the occurrence of new CMBs was markedly higher in CAD patients on antiplatelet therapy than in the healthy elderly population. In addition to high comorbidity with CAD and cerebrovascular disease [1,24], antiplatelet therapy might aggravate the CMB risk in CAD patients, although it remains controversial whether antiplatelet therapy increases the risk of CMB formation in patients with cerebrovascular disease [4,10-16].
The most important finding of this study was that the CMBpositive group had more severe coronary artery lesions and more poorly controlled hypertension compared to the CMB-negative group in patients with CAD on antiplatelet therapy (Figures 1 and 2). It has been shown that the severity of coronary artery lesions is associated with the comorbidity and severity of other vascular diseases in CAD patients [24]. Thus, it is plausible that patients with severe CAD may be susceptible to CMBs. After the 8-month follow-up, the CMBpositive group had higher BP than the CMB-negative group, which is in line with the previous studies showing that hypertension increased the risk of CMBs by 2- to 4-fold in the healthy general population [5,14].
Limitation of this study
The number of patients studied was small and the observation period was short. However, this prospective pilot study would provide the rationale and valuable information for conducting and designing a future large-scale prospective observational study to clarify the incidence of CMBs and the long-term outcomes, particularly cerebral hemorrhage, in CAD patients with CMBs. Such a future study would address whether the presence of CMBs can be a predictor of cerebral hemorrhage in CAD patients on antiplatelet therapy.
Conclusions
The present study suggests that CAD patients on antiplatelet therapy have at a high risk for CMBs. Poor BP control and severe coronary artery lesions are considered the risks for CMBs in CAD patients on antiplatelet therapy. Accordingly, strict coronary risk control, especially BP control, is necessary in CAD patients on antiplatelet therapy, especially DAPT, to prevent new CMBs and the possible associated complications. A future large-scale prospective study is needed to determine whether the prevention of CMBs would reduce the risk of future cerebral hemorrhage in CAD patients on antiplatelet therapy.
Acknowledgements
This study was supported partly by JSPS KAKENHI Grant (26460756, 15K4567). The authors would like to thank Katsue Shiramizu, Miyuki Nishikata, Miho Kogure, and Makiko Kiyohiro for their secretarial assistance.
References
- Yamazaki T, Goto S, Shigematsu H, Uchiyama S, Nagai R, et al. (2007) Prevalence, awareness and treatment of cardiovascular risk factors in patients at high risk of arteriothrombosis in Japan – Results from domestic baseline data of the Reduction of Atherothrombosis for Continued Health (REACH) registry. Circ J 71: 995-1003.
- Fazekas F, Kleinert R, Roob G, Kleinert G, Kapeller P, et al. (1999) Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: Evidence of microangiopathy-related microbleeds. AJNR 20: 637-642.
- Shoamanesh A, Kwok CS, Benavente O (2011) Cerebral microbleeds: histopathological correlation of neuroimaging. Cerebrovasc Dis 32: 528-534.
- Wu Y, Chen T (2016) An up-to-date review on cerebral microbleeds. J Stroke Cerebrovasc Dis 25: 1301-1306.
- Bokura H, Saika R, Yamaguchi T, Nagai A, Oguro H, et al (2011) Microbleeds are associated with subsequent hemorrhagic and ischemic stroke in healthy elderly individuals. Stroke 42: 1867-1871.
- Charidimou A, Kakar P, Fox Z, Werring DJ (2013) Cerebral microbleeds and recurrent stroke risk: Systematic review and meta-analysis of prospective ischemic stroke and transient ischemic attack cohorts. Stroke 44: 995-1001.
- Greenberg SM, Eng JA, Ning M, Smith EE, Rosand J (2004) Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke 35: 1415-1420.
- Lovelock CE, Cordonnier C, Naka H, Al-Shahi Salman R, Sudlow CL, et al. (2010) Antithrombotic drug use, cerebral microbleeds, and intracerebral hemorrhage a systematic review of published and unpublished studies. Stroke 41: 1222-1228.
- DeSimone CV, Graff-Radford J, El-Harasis, MA, Rabinstein AA, Holmes DR Jr, et al. (2017) Cerebral amyloid angiopathy. Diagnosis, clinical implications, and management strategies in atrial fibrillation. J Am Coll Cardiol 70: 1173-1182.
- Nishikawa T, Ueba T, Kajiwara M, Miyamatsu N, Yamashita K (2009) Cerebral microbleeds in patients with intracerebral hemorrhage are associated with previous cerebrovascular diseases and white matter hyperintensity, but not with regular use of antiplatelet agents. Neurol Med Chir (Tokyo) 49: 333-338.
- Naka H, Nomura E, Kitamura J, Imamura E, Wakabayashi S, et al. (2013) Antiplatelet therapy as a risk factor for microbleeds in intracerebral hemorrhage patients: Analysis using specific antiplatelet agents. J Stroke Cerebrovasc Dis 22: 834-840.
- Imaizumi T, Inamura S, Kohama I, Yoshifuji K, Nomura T, et al. (2013) Antithrombotic drug uses and deep intracerebral hemorrhages in stroke patients with deep cerebral microbleeds. J Stroke Cerebrovasc Dis 22: 869-875.
- Darweesh SK, Leening MJ, Akoudad S, Loth DW, Hofman A, et al. (2013) Clopidogrel use is associated with an increased prevalence of cerebral microbleeds in a stroke-free population: the Rotterdam study. J Am Heart Assoc 2: e000359.
- Yamashiro K, Tanaka R, Okuma Y, Ueno Y, Tanaka Y, et al. (2014) Associations of durations of antiplatelet use and vascular risk factors with the presence of cerebral microbleeds. J Stroke Cerebrovasc Dis 23: 433-240.
- Liu S, Li C (2015) Antiplatelet drug use and cerebral microbleeds: A meta-analysis of published studies. J Stroke Cerebrovasc Dis 24: 2236-2244.
- Wang DN, Hou XW, Yang BW, Lin Y, Shi JP, et al. (2015) Quantity of cerebral microbleeds, antiplatelet therapy, and intracerebral hemorrhage outcomes: A systematic review and meta-analysis. J Stroke Cerebrovasc Dis 24: 2728-2737.
- Ogihara T, Kikuchi K, Matsuoka H, Fujita T, Higaki J, et al. (2009) The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2009). Hypertens Res 32: 3-107.
- Gregoire SM, Chaudhary UJ, Brown MM, Yousry TA, Kallis C, et al. (2009) The Microbleed Anatomical Rating Scale (MARS): Reliability of a tool to map brain microbleeds. Neurology 73: 1759-1766.
- Fazekas F, Chawiuk JB, Alavi A, Hurtig HI, Zimmerman RA, et al. (1987) MR signal abnormalities at 1.5T in Alzheimer’s dementia and normal aging. AJR 149: 351-356.
- Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, et al. (2009) Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 360: 961-972.
- Cordonnier C, Al-Shahi Salman R, Wardlaw J (2007) Spontaneous brain microbleeds: Systematic review, subgroup analyses and standards for study design and reporting. Brain 130: 1988-2003.
- Poels MM, Ikram MA, van der Lugt A, Hofman A, Krestin GP, et al. (2011) Incidence of cerebral microbleeds in the general population (The Rotterdam Scan Study). Stroke 42: 656-661.
- Ding J, Mitchiel GF, Bots ML, Sigurdsson S, Harris TB, et al. (2015) Carotid arterial stiffness and risk of incident cerebral microbleeds in order people. The Age, Gene/Environment Susceptibility (AGES) –Reykjavik Study. Arterioscler Thromb Vasc Biol 35: 1889-1895.
- Imori Y, Akasaka T, Ochiai T, Oyama K, Tobita K, et al. (2014) Co-existence of carotid artery disease, renal artery stenosis, and lower extremity peripheral arterial disease in patients with coronary artery disease. Am J Cardiol 113: 30-35.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi