Research Article, J Plant Physiol Patho Vol: 11 Issue: 2
Changes in Antioxidant Enzyme Activity and Viral Load in Zucchini Cultivars Infected with Papaya ringspot virus-Watermelon type
Ludiana Canton1, Eduardo Gainete Ramos1, David Marques de Almeida Spadotti2, Jorge Alberto Marques Rezende2, Tiago Camponogara Tomazetti1, Danila Souza Oliveira Coqueiro3, Robson Marcelo Di Piero1
1Universidade Federal de Santa Catarina, Centro de CIencias Agrarias, Florianopolis, SC, Brazil
2Escola Superior de Agricultura Luiz de Queiroz, Piracicaba, SP, Brazi
3Universidade Federal da Bahia, Instituto Mutidisciplinar em Saude, Vitoria da Conquista, BA, Brazil
*Corresponding Author:Robson Marcelo Di Piero, Universidade Federal de
Santa Catarina, Centro de CIencias Agrárias, Florianopolis, SC, Brazil ; Email:
robson.piero@usfc.br
Received date: 09 January, 2023, Manuscript No. Jppp-23-86391;
Editor assigned date: 12 January, 2023, Pre QC No. Jppp-23-86391 (PQ);
Reviewed date: 25 January, 2023, QC No. Jppp-23-86391;
Revised date: 30 January, 2023, Manuscript No. Jppp-23-86391 (R);
Published date: 06 February, 2023, DOI: 10.4172/2329-955X.1000324
Citation: Ludiana Canton, Eduardo Gainete Ramos, David Marques de Almeida Spadotti, Jorge Alberto Marques Rezende, Tiago Camponogara Tomazetti, et al. (2023). Changes in Antioxidant Enzyme Activity and Viral Load in Zucchini Cultivars Infected with Papaya ringspot virus-Watermelon type. J Plant Physiol Pathol 11:1
Abstract
Zucchini represents one of the most important species within the Cucurbitaceous family, but diseases appear as a limiting factor. The Papaya Ringspot Virus-Watermelon type (PRSV-W) is the virus with higher occurrence in producing regions. Viral replication and disease development may promote changes involving defense-related enzymatic components. This study evaluated alterations in the oxidative metabolism as well as viral load of two cultivars of zucchini, Adele and Caserta, infected by PRSV-W. Plants were inoculated and leaf samples were collected to determine the activity of antioxidant enzymes and viral load. The intensity of the disease was measured. In local level, there was a decrease of the viral load for both cultivars, increasing significantly again at 8 days. The viral load in the new leaves of the Caserta was at least 3 times higher than in the Adele ones. Mosaic symptoms were mild in Adele and expressive in Caserta. The activity of the APX in the Caserta increased faster than in Adele in inoculated leaves, but this increase was not related to the reduction in viral load. At the systemic level, APX, presented higher activity in both cultivars, however, over time Adele reduced, which may have resulted in a lower effect of the enzyme on hydrogen peroxide and this contribute to the reduction of viral multiplication. The rapid systemic response of Adele (tolerant) and the increase in the activity of GPX and GR in the first day after inoculation, may be one of the factors that contributed to the lower load viral and severity of the symptoms. On the other hand, in the Caserta (intolerant), pronounced increases in antioxidant enzyme activity were observed over the course of disease evolution in an attempt to contain plant cell oxidative damage, which may have favored the advance of a bio trophic pathogen such as viruses.
Keywords: Papaya Ringspot Virus-Watermelon type; Cucurbita pepo; Metabolic changes; Antioxidant enzymes; Plant-virus interactions
Introduction
Plants of the family Cucurbitacea have a wide range of fruit characteristics and are grown worldwide in different environmental conditions. Cucurbits are a source of food and income for small and large producers. Cultivation of cucurbits generates employment, helping farmers to remain in business [1]. The zucchini (Cucurbita pepo) is one of the most important cultivated species of this family [2].
Viruses of the family Potyviridae, genus Potyvirus, including Papaya Ringspot Virus– Watermelon type (PRSV-W), Watermelon Mosaic Virus (WMV) and Zucchini Yellow Mosaic Virus (ZYMV) are present worldwide and reduce the production of various cucurbits [3]. Due to the difficulty of controlling and the damage caused, these viruses are the most important objective of breeding programs for C. pepo [4].
Losses caused by PRSV-W occur independently of the stage in which the plant is infected, but are more pronounced when infections occur at initial stages of the crop, and can reach 100%. C. pepo plants infected with the virus exhibit mosaic, atrophy, distortion, blisters, and narrowing of the leaf blade, which can be reduced to the main veins. The fruits show malformation and color change. Disease control is difficult, so recommended preventive measures include pest management (mainly vectors of the family Aphididae), choice of planting location and timing (avoiding sowing next to or following other cucurbit crops), weeding, crop rotation, and use of resistant cultivars, among others [5,6].
Most cultivars are susceptible to PRSV-W. A susceptible plant allows virus infection and replication [7]. Plants may be resistant to infection, replication and/or systemic invasion of the virus. Both resistant and susceptible plants may or may not tolerate the disease. Tolerant plants may show no symptoms, or if present, symptoms are barely visible; whereas virus-intolerant plants exhibit severe symptoms [8].
Plants possess mechanisms to defend themselves, regardless of the type of relationship established between plants and the pathogens. Reactive Oxygen Species (ROS) are one of the first defense responses of plants when in stress conditions, whether from abiotic or biotic causes, but they can damage the plant cell structure. Plants infected by viruses, as well as different pathogens, also produce ROS. The first studies were done by Doke and Ohashi with the Tobacco Mosaic Virus (TMV), where infected plants showed an increase in the production of superoxide (O2) [9-11].
To prevent damage to their cells, plants activate antioxidant components of defense system, which involves enzymes such as Ascorbate Peroxidase (APX), Catalase (CAT), Glutathione Reductase (GR), Guaiacol Peroxidase (GPX) and Superoxide Dismutase (SOD), among others. APX participates in the ascorbate-glutathione cycle and plays the role of eliminating Hydrogen Peroxide (H2O2) from the cytosol and chloroplasts using ascorbic acid and Docosahexaenoic Acid (DHA) to reduce H2O2 in water (H2O). The same role developed by CAT, however this enzyme acts on the peroxisomes [12,13]. CAT has a high turnover rate and is the only antioxidant enzyme that does not require one reduction-equivalent [14].
In turn, GPX plays an important role in defense against biotic stresses using H2O2 and degrading indoleacetic acid [13]. GR is an oxide reductase flavoprotein that uses NADPH to reduce oxidized Glutathione (GSSG) and turn it into reduced Glutathione (GSH), which is used to regenerate ascorbic acid from Dehydroascorbate (DHA) and Monodehydroascorbate (MDHA), the result of this reaction is converted to its oxidized form (GSSG). GR is found mainly in chloroplasts and less in mitochondria and cytosol [13]. Under stress conditions, SOD forms the first line of defense against ROS. This enzyme catalyzes the removal of O2– by dismutation of O2– and H2O2, and has different forms, such as Mn-SOD located in the mitochondria, Fe-SOD localized in chloroplasts and Cu/Zn-SOD located in the cytosol, peroxisomes and chloroplasts [15]
Thus, the main objective of this study was to evaluate alterations in the antioxidant metabolism in two zucchini cultivars infected with the PRSV-W, which show differences in susceptibility to the infection and tolerance to the disease.
Materials and Methods
Plant and pathogen
The studies were conducted at the Phytopathology Laboratory of the Center of Agricultural Sciences of the Federal University of Santa Catarina and were repeated twice. The seedlings of C. pepo were produced from seeds of the hybrid cultivars Caserta and Adele, purchased from Horticeres seeds LTDA and Sakata Seed Sudamerica, respectively. As soon as the seedlings reached the cotyledonary stage, they were transplanted into 400 ml plastic pots containing germina planta substrate. Plants were kept in the greenhouse during the experiment.
The PRSV-W isolate from the collection at the Department of Plant Pathology, ESALQ/USP, Piracicaba, SP was used in this study. It was kept in C. pepo plants.
Mechanical transmission
PRSV-W-infected C. pepo leaves, exhibiting mosaic symptoms, were used as source of inoculum for experimental mechanical transmissions. They were macerated in porcelain mortar with 200 mM potassium sodium phosphate buffer, pH 7.0, diluted at 1:20 (w/v). The inoculum was rub inoculated on the leaves of test-plants containing the abrasive silicon carbide (carborundum).
For the first experiment, PRSV-W was mechanically inoculated on the first true leaf of newly developed test-plants, which were used in the analyzes. As control, some plants were mock inoculated with buffer, whereas others were only sprayed with distilled water. Five plants of each cultivar were inoculated for each treatment, for each sample dating. Afterwards, the inoculated plants were sprayed with distilled water to remove the abrasive excess.
For the second assay, zucchini plants of both cultivars, containing three true leaves, were mechanically inoculated at the cotyledons. Mock plants inoculated with buffer were used as controls. Five plants of each cultivar were inoculated for each treatment, for each sample dating.
Enzyme activities
For the first experiment, the first true leaves from the plants (mechanically inoculated with PRSV-W, water or carborundum) were collected at 1, 2, 4 and 8 days after inoculation.
For the second assay, where the cotyledons were mechanically inoculated with PRWV-W or carborundum, the third true leaf of each plant was collected at 8, 12, 16, 20, and 30 days after inoculation. Five plants of each cultivar were inoculated for each treatment, for each sample dating.
All leaf samples were macerated with liquid nitrogen and stored inside Eppendorf tubes kept in a freezer at -80°C. To prepare the extract for evaluations of enzymatic activity, 1.5 ml of 50 mM potassium phosphate buffer pH 7.0 was added to 50 mg of previously macerated leaf tissue. The suspension was centrifuged at 20,000 g and the supernatant (protein extract) was collected for enzymatic analysis.
Ascorbate peroxidase
The determination of Ascorbate Peroxidase (APX) activity was based on Parida, et al. and Freitas and Stadnik [16,17]. For the reaction, 20 μl of sample (protein extract) was added to 280 μl of 50 mM potassium phosphate buffer, pH 7.0, containing 0.25 mM hydrogen peroxide (H2O2), 0.5 mM ascorbic acid and 0.2 mM EDTA. The decline in absorbance was visualized for 1 minute in a microplate reader at 290 nm at 25°C. The results were expressed as units of ascorbate peroxidase per minute per mg protein (APX units mg-1 protein min-1) in which one unit of APX was considered as the amount of enzyme required to reduce 0.01 absorbance units.
Catalase
For Catalase (CAT) activity, 10 μl of sample and 280 μl of reaction buffer (50 mM potassium phosphate buffer, pH 7.0, containing 15 mM H2O2) were added into wells of the Greiner (UV) microplate, under incubation at 30°C. Absorbance was measured for 3 minutes in a microplate reader at 240 nm and 30°C (adapted from Parida, et al. The results were expressed in units of catalase per minute per mg of protein (CAT units mg-1 protein min-1) in which a CAT unit was considered the amount of enzyme required to reduce 0.01 absorbance units [17].
Glutathione reductase
The activity of Glutathione Reductase (GR) was determined according to Freitas and Stadnik, with some modifications. For the reaction, 50 mM potassium phosphate buffer, pH 7.8, containing 2 mM EDTA, 0.75 mM 5,5’-dithio-bis-(2-nitrobenzoic acid) (DTNB) and 0.1 mM Nicotinamide-Adenine Dinucleotide Phosphate (NADPH) was used[17]. In 250 μl of reaction buffer (kept at 35°C in a water bath), 30 μl of sample and 20 μl of 1 mM oxidized Glutathione (GSSG) were added. The reaction was determined for 3 minutes in a microplate reader at 412 nm and 35°C. The results were expressed in units of glutathione reductase per minute per mg protein (GR units mg-1 protein min-1) in which one unit of GR was considered the amount of enzyme required to increase 0.01 absorbance units.
Guaiacol peroxidase
Guaiacol Peroxidase (GPX) activity was determined according to Luiz et al. [18], in which 50 mM potassium phosphate buffer pH 6.0 containing 0.125% H2O2 and 0.250% guaiacol was used. For the reaction, 20 μl of extract and 280 μl of reaction buffer (kept at 30°C in a water bath) were added. Subsequently, absorbance of the samples was determined for 3 minutes in a microplate reader at 470 nm wavelength. The results were expressed as units of guaiacol peroxidase per minute per mg protein (GPX units mg-1 protein min-1) in which a GPX unit was considered as the amount of enzyme needed to increase 0.01 absorbance units.
Superoxide dismutase
Superoxide Dismutase (SOD) activity was determined using 50 μl of sample and 1.7 ml of 50 mM potassium phosphate buffer, pH 7.8 containing 13 mM L-methionine, 75 μM NBT, 100 nM EDTA and 100 μM riboflavin (added to the buffer only at the time of reaction) adapted from Negro, et al. [19]. Two test tubes were prepared for this enzyme, one as blank and one as sample. The blank tube received the sample and the buffer, and it was kept in the dark to reset the spectrophotometer. In the reaction tube, the sample and buffer were added and kept in the light to determine the inhibition rate of NBT photoreduction. A third tube containing only the buffer served as a control, which was subjected to light to determine NBT total photoreduction. After incubation of the tubes in light or dark for 10 minutes, the absorbances at 560 nm were determined. Calculations for the specific activity of SOD were made considering the sample volume, the inhibition percentage and the amount of protein present in the sample. A unit of SOD was considered as the enzyme amount required to inhibit 50% of NBT photoreduction. The results were expressed in units of SOD μg protein-1.
Total protein dosage
Protein content present in the samples was determined by Bradford method [20]. 6 μl of sample and 294 μl of diluted Bradford reagent were added to a 96-well Elisa plate, which was shaken gently, and after 15 minutes, the absorbances of the samples were determined on a microplate reader at 595 nm. The values were converted to protein concentration through the known values of standard solutions of Bovine Serum Albumin (BSA), which gave rise to a standard curve.
Quantification of PRSV-W by RT-qPCR
For RT-qPCR only 3 replicates of each cultivar were used. Samples were collected at the same times used for enzymatic analysis, that is, 1, 2, 4 and 8 days after viral inoculation in the first experiment and 8, 12, 16, 20 and 30 days in the second experiment. The RNA was extracted by using a SV Total RNA Isolation System kit (Promega, “SV Total RNA Isolation System”) according to the manufacturer’s protocol. Viral RNA concentration and quality were determined using a Nanodrop 2000 Spectrophotometer (Thermo Scientific, Waltham, MA, USA) using the absorbance ratio at 260 nm and 280 nm (A260/ A280 ratio).
The set primer used (PRSVtW CP3) for PSRV-W were designed to bind conserved regions of the viral coat protein (forward 5’- TCGCAGAATGTTTGGTATGGA-3’ and reverse 5’- ATGCACTCTCTCCTGGGTAT-3’) which amplify a 116 base pairs (bp) fragmente. These primers were previously designed by using software BioEdit and PrimerQuest (IDT).
RT-qPCR reactions were performed using 1 ng of template RNA, GoTaq mastermix (1x), primer CP3 senso (0.3 μM), primer CP3 antisenso (0.3 μM), GoScript (1x), MgCl2 (25 mM) (10 mM), CXR reference dye (30 μm) (0.03 μl), template (1 ηg) water (2.7 μl). The cDNA was obtained at 37°C for 15 minutes then at 75°C for 10 minutes. The specific target amplification was analyzed by melt-curve analysis which consisted of first melting step at 95° for 15 s, annealing at 60° for 1 min, and second melting step at 95° for 15s.
RT-qPCR was conducted for 40 cycles of denaturation at 95°C for 10 s, annealing and synthesis at 60°C for 30 s, and extension at 72°C for 30 s, using a software StepOneTM Software v2.3 (Applied Biosystems, Foster City, CA, USA). Both negative (nuclease free water) and positive (symptomatic infected plants with PRSV-W) controls were included in each RT-qPCR assay.
A standard curve was obtained from a sample with the know amount of PRSV-W RNA quantified by NanoDrop2000 spectrophotometer. Five diluitions (0.01; 0.1; 1; 10 and 100 μg.mL-1) were prepared. EF1α was used as a reference gene for the calculation of ΔΔCt.
Disease incidence and severity
All plants inoculated with PRSV-W was used to determine the disease incidence (20 plants of each cultivar, in each experiment), at the end of the experiment. Plant infection with the PRSV-W was confirmed by PTA-ELISA (Plate-Trapped Antigen Enzyme-Linked Immunosorbent Assay) with some modifications [21]. The leaf samples were collected at 30 days after inoculation, macerated in liquid nitrogen and kept inside a freezer at -80°C. The test was performed according to Bonilha, et al. using specific antiserum against the PRSV-W capsidial protein. A 405 nm filter was used to read the absorbance in ELISA reader [22]. Extracts from healthy and PRSV-W infected plants were used as controls. The sample was considered positive when its mean absorbance value was three or more times higher than the mean absorbance value of the healthy plant extract.
The severity of PRSV-W symptoms in infected plants was evaluated with a scale adapted from Freitas which ranged from 0 to 3 [23]. Three evaluations were performed every 7 days from the 14th Day after Inoculation (DAI) in the 1st experiment and from 5th day in the 2nd experiment. Asymptomatic plants received score 0; score 1 was attributed to plants with leaves presenting weak mosaic symptoms, without deformation or blisters; score 2, for plants with obvious mosaic, but leaves with little deformation and blisters; and score 3 for plants presenting severe mosaic, leaf deformation, blisters and reduced growth.
Statistical methods
The enzyme activity data over time for each cultivar were subjected to analysis of variance and when significant, the means were contrasted using the Tukey test (α=0.05). The same procedure was used to analyze treatment data within the same cultivar. To compare the cultivars, the Tukey test was used. The analyzes were carried out with the aid of the statistical software Sisvar [24].
Results
Determination of antioxidant enzyme activities of zucchini plants
Ascorbate Peroxidase (APX) activity: In assessing the local effect, for Adele plants, activity of APX on inoculated leaves increased only at 2 DAI, on the other days there were no differences between treatments (Figure 1A). The activity of APX on the PRSV-W inoculated leaf of Caserta plants was higher than in the controls at 1, 2 and 8 DAI (Figure 1B). The Adele plants presented higher activity when treated with water or carborundum and compared to those of Caserta in all days after leaf inoculation (Figures 1A and 1B). The activity of this enzyme decreased over time, regardless of virus inoculation (Figures 1A and 1B). Systemic activity of APX was higher in virus infected plants than in the control at 8, 20 and 30 DAI for Adele plants (Figure 1C) and at 8, 12 and 30 DAI for those of Caserta (Figure 1D). At the systemic level, the Adele cultivar presented higher activity of APX than those of Caserta in virus infected plants (Figures 1C and 1D).
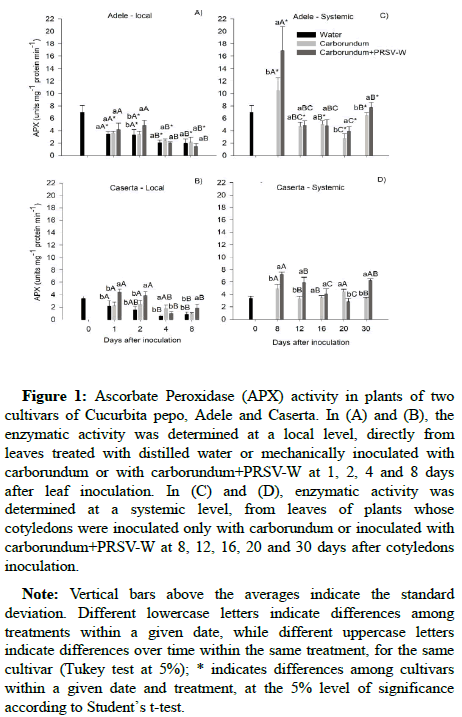
Figure 1: Ascorbate Peroxidase (APX) activity in plants of two cultivars of Cucurbita pepo, Adele and Caserta. In (A) and (B), the enzymatic activity was determined at a local level, directly from leaves treated with distilled water or mechanically inoculated with carborundum or with carborundum+PRSV-W at 1, 2, 4 and 8 days after leaf inoculation. In (C) and (D), enzymatic activity was determined at a systemic level, from leaves of plants whose cotyledons were inoculated only with carborundum or inoculated with carborundum+PRSV-W at 8, 12, 16, 20 and 30 days after cotyledons inoculation.
Note: Vertical bars above the averages indicate the standard deviation. Different lowercase letters indicate differences among treatments within a given date, while different uppercase letters indicate differences over time within the same treatment, for the same cultivar (Tukey test at 5%); * indicates differences among cultivars within a given date and treatment, at the 5% level of significance according to Student’s t-test.
Catalase (CAT) activity: Plants of both cultivars showed no significant difference between treatments at the inoculation site (Figures 2A and 2B). However, at the systemic level, Adele plants infected with the PRSV-W showed higher CAT activity from 20 DAI compared to the control (Figure 2C). The Caserta plants responded to the viral infection sooner than those of Adele, i.e. at 16 DAI, showing high activity compared to control until 20 DAI (Figure 2D). Caserta plants infected showed higher CAT compared to Adele from 12DAI.
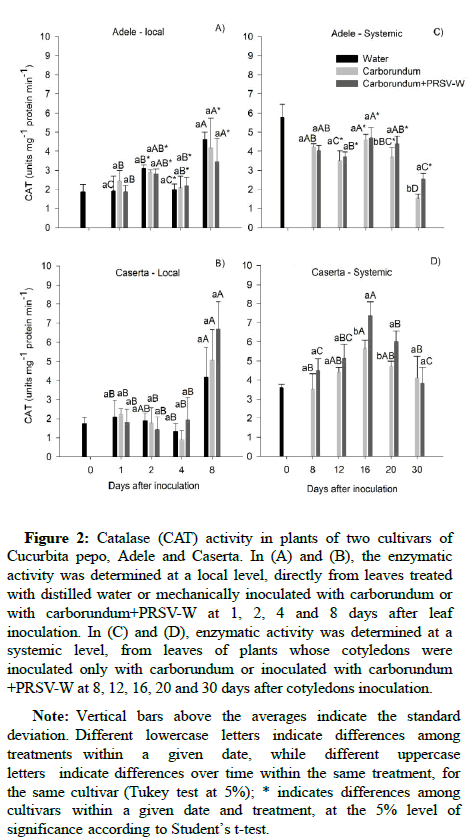
Figure 2: Catalase (CAT) activity in plants of two cultivars of Cucurbita pepo, Adele and Caserta. In (A) and (B), the enzymatic activity was determined at a local level, directly from leaves treated with distilled water or mechanically inoculated with carborundum or with carborundum+PRSV-W at 1, 2, 4 and 8 days after leaf inoculation. In (C) and (D), enzymatic activity was determined at a systemic level, from leaves of plants whose cotyledons were inoculated only with carborundum or inoculated with carborundum +PRSV-W at 8, 12, 16, 20 and 30 days after cotyledons inoculation.
Note: Vertical bars above the averages indicate the standard deviation. Different lowercase letters indicate differences among treatments within a given date, while different uppercase letters indicate differences over time within the same treatment, for the same cultivar (Tukey test at 5%); * indicates differences among cultivars within a given date and treatment, at the 5% level of significance according to Student’s t-test.
Guaiacol Peroxidase (GPX) activity: At the local level, Adele plants infected with the PRSV-W showed higher GPX activity to 1 DAI compared to the controls (Figure 3A). Caserta plants infected with the PRSV-W showed higher GPX activity in all times compared to the plants sprayed with distilled water. GPX activity has progressively increased over time and the Caserta cultivar presented higher activity than Adele at 1 and 2 DAI in plants treated with carborundum and at 8 DAI in those inoculated with the virus (Figures 3A and 3B). At the systemic level, Adele plants inoculated with the virus had higher GPX activity from 16 DAI when compared to the control (Figure 3C). Caserta plants inoculated with the PRSV-W presented higher activity than those not inoculated from 12 DAI onwards (Figure 3D). Over time, enzyme activity increased, regardless of treatment, for both Adele and Caserta. GPX activity was higher in Caserta than in Adele (Figures 3C and 3D).
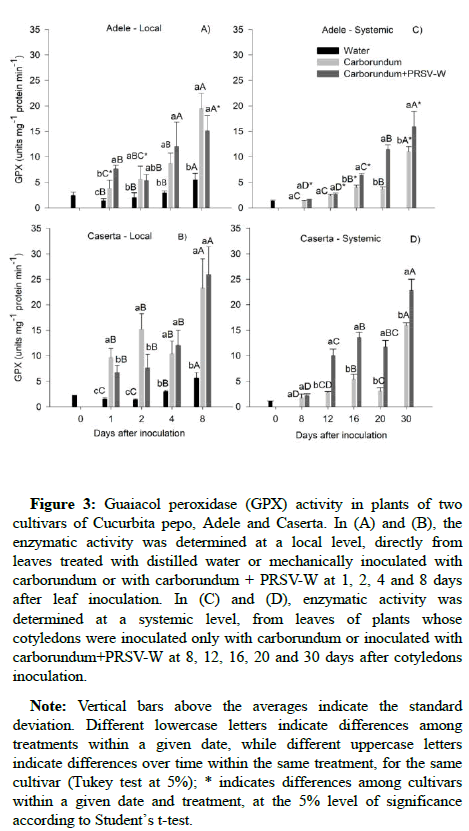
Figure 3: Guaiacol peroxidase (GPX) activity in plants of two cultivars of Cucurbita pepo, Adele and Caserta. In (A) and (B), the enzymatic activity was determined at a local level, directly from leaves treated with distilled water or mechanically inoculated with carborundum or with carborundum + PRSV-W at 1, 2, 4 and 8 days after leaf inoculation. In (C) and (D), enzymatic activity was determined at a systemic level, from leaves of plants whose cotyledons were inoculated only with carborundum or inoculated with carborundum+PRSV-W at 8, 12, 16, 20 and 30 days after cotyledons inoculation.
Note: Vertical bars above the averages indicate the standard deviation. Different lowercase letters indicate differences among treatments within a given date, while different uppercase letters indicate differences over time within the same treatment, for the same cultivar (Tukey test at 5%); * indicates differences among cultivars within a given date and treatment, at the 5% level of significance according to Student’s t-test.
Glutathione Reductase (GR) activity: At the local level, glutathione reductase showed higher activity from the fourth day in inoculated Adele plants when compared to treatments without the virus (Figure 4A). In Caserta activity remained relatively stable up to 4 DAI and showed a great increase at 8 DAI, regardless of treatment. The cultivars differed mainly at 8 DAI, when Caserta plants presented higher enzymatic activity in comparison to Adele (Figure 4A and 4B). Systemically, Adele plants inoculated with the virus had higher enzymatic GR activity at 12, 16 and 30 after inoculation compared to the control (Figure 4C). Inoculated Caserta plants always had higher enzymatic activity in comparison to those treated with carborundum (Figure 4D) or in comparison to the inoculated Adele plants, except at 12 DAI (Figures 4C and 4D). In general, for both inoculated cultivars, there were no much changes in GR activity over time (Figures 4C and 4D).
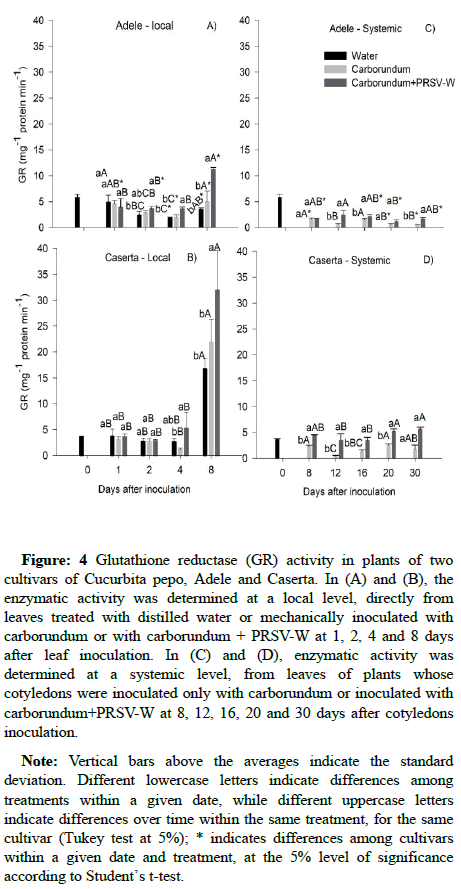
Figure 4: Glutathione reductase (GR) activity in plants of two cultivars of Cucurbita pepo, Adele and Caserta. In (A) and (B), the enzymatic activity was determined at a local level, directly from leaves treated with distilled water or mechanically inoculated with carborundum or with carborundum + PRSV-W at 1, 2, 4 and 8 days after leaf inoculation. In (C) and (D), enzymatic activity was determined at a systemic level, from leaves of plants whose cotyledons were inoculated only with carborundum or inoculated with carborundum+PRSV-W at 8, 12, 16, 20 and 30 days after cotyledons inoculation.
Note: Vertical bars above the averages indicate the standard deviation. Different lowercase letters indicate differences among treatments within a given date, while different uppercase letters indicate differences over time within the same treatment, for the same cultivar (Tukey test at 5%); * indicates differences among cultivars within a given date and treatment, at the 5% level of significance according to Student’s t-test.
Quantification of PRSV-W by RT-qPCR: The RT-qPCR amplification curves were generated by using 10-fold dilutions. Thus, a slope of -3.256 and an efficiency of 102,84% were obtained in this study.
The viral loads in artificially inoculated Adele and Caserta leaves (Local effect) are shown in Figure 5A. There is no statistically significant difference between cultivars for all evaluated times.
The cv. Caserta showed higher viral load than Adele from the first time analyzed (8 days after inoculation) in zucchini leaves systemically infected by PRSV-W. The viral load stabilized in Caserta from 12 days after inoculation, while for Adele, this stabilization occurred later, after 20 days of inoculation. In the last analyzed time, the viral load in the new leaves of the Caserta cultivar was at least 3 times higher than in the Adele ones (Figure 5B).
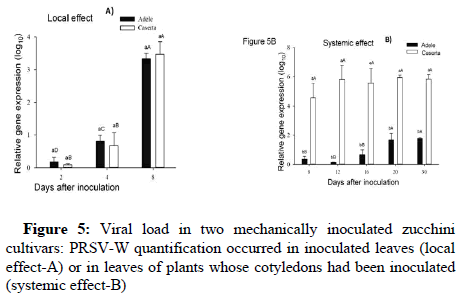
Figure 5: Viral load in two mechanically inoculated zucchini cultivars: PRSV-W quantification occurred in inoculated leaves (local effect-A) or in leaves of plants whose cotyledons had been inoculated (systemic effect-B)
Disease incidence and severity: In the evaluation of the local response all plants used in the first experiment were infected with PRSV-W, regardless of cultivar (Incidence-Table 1). The Caserta plants exhibited characteristic severe symptoms, unlike the Adele plants, which showed mild virus symptoms until the end of evaluations, 30 days after inoculation–DAI (Figure 6). All Caserta plants presented severe symptoms in the first evaluation, 14 DAI. Plants of the cv. Caserta showed higher scores than Adele, about 8 times higher (Figure 6).
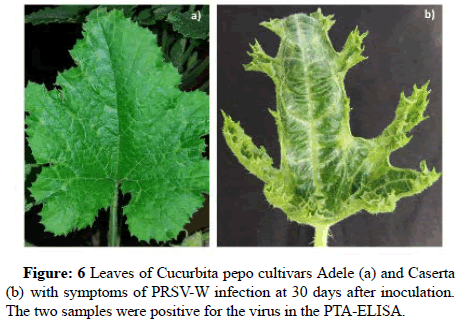
Figure 6: Leaves of Cucurbita pepo cultivars Adele (a) and Caserta (b) with symptoms of PRSV-W infection at 30 days after inoculation. The two samples were positive for the virus in the PTA-ELISA.
Therefore, in the systematic response experiment, symptoms appeared earlier and assessments began at 5 DAI. Even so, the Caserta plants exhibited more severe symptoms than the Adele plants and, after 12 DAI, 100% of the Caserta plants were infected with a maximum severity score and with scores 5 times higher than Adele (Table 1).
Table 1: Result of the evaluation of symptoms (severity) and the incidence of PRSV-W in two mechanically inoculated zucchini cultivars.
Discussion
Cucurbit plants are affected by pathogens that limit their development. To defend themselves, they can modulate their defense mechanisms and are able to fight the pathogens [25]. The C. pepo cv., Adele and Caserta, exhibited different response against PRSV-W infection resulting in different symptomatology, viral load and enzymatic content.
In response to infection, the plants produce Reactive Oxygen Species (ROS) that can cause serious damage to cell structure, thus possibly leading to death. To avoid damage caused by ROS, plants developed an efficient antioxidant defense system with enzymatic and non-enzymatic components that prevent the onset of free radical chain reactions by eliminating and reducing O2 and H2O2 [26].
According to Demidchik, enzymes are present at different cell sites, virtually participate in all biochemical reactions and help to control ROS in plants. Examples of enzymes that have antioxidant effect are SOD, APX, GR and CAT, and the balance between activities them in cells is crucial for determining the level of superoxides and H2O2 [27].
At adequate concentrations, H2O2 has the ability to rapidly diffuse through the plant membrane, aiding in plant defense responses, apparently having a dual effect, causing localized death of host plant cells to restrict pathogens and acting as a diffusible signal for the induction of genes involved in pathogenesis. However, its accumulation at a toxic level inside the cell is harmful [28].
APX is an enzyme that plays an important role in preventing the deleterious effects of H2O2 [29]. In the present study, it was possible to observe that in the absence of the virus, Adele plants showed higher APX activity than Caserta, but when challenged with the virus there was a faster local response in Caserta plants (1 DAI) than in plants to Adele (2 DAI), and this response differs from the control up to 8 DAI, with the exception of 4 DAI. This increase in APX activity at the site of infection in Caserta plants was apparently not related to the reduction in viral load, on the contrary, since increases in virus multiplication at the site of infection were observed throughout the evaluations. One explanation for this result is that the induction of APX can cause a reduction in the level of H2O2 (APX converts H2O2 into water) limiting the signal transduction by this compound in the plant and facilitating the proliferation and dissemination of the virus, as seen at the site of infection.
Systemically, APX activity was high at the beginning of evaluations in inoculated plants of both cultivars, compared to their controls. However, it decreases abruptly in Adele (approximately 50% lower at 30 DAI compared to 8 DAI), which does not occur with such intensity in Caserta. This reduction in enzymatic activity over time may result in a lesser effect of the enzyme on H2O2 and consequently explain the lower load viral observed in Adele plants, since H2O2 may act in signaling the plant's defense responses against the virus. The low symptomology in Adele plants reinforces this systemic effect of APX that may have contributed to the low severity of viral symptoms in these plants, compared to Caserta. These results are in agreement with Fodor, et al. who found that the onset of TMV symptoms in tobacco plants was preceded by a slight decline in APX and other enzymes, and that this reduction in antioxidant capacity and the appropriate accumulation of some ROS contribute to the plant's defense mechanisms [30].
Despite the possible increase in H2O2, due to the decline in APX activity, it is possible that it also influenced CAT activity at the site of infection. Adele and Caserta infected with the virus did not show significant differences in CAT activity in relation to the respective controls, but at the end of the local evaluation, Caserta plants showed higher enzymatic content than Adele, which converges with the results obtained with APX, in which increases of enzymatic activity reduces H2O2 which may contribute to increased viral content. At the systemic level, the effects were similar, inoculated Caserta plants showed greater CAT activity compared to Adele, contributing to the reduction of H2O2 and its effects on an acquired systemic response. Similar results were obtained by Riedle-Bauer when studying antioxidant enzymes in the interaction of cucumber (Cucumis sativus) and zucchini (C. pepo) infected with CMV and ZYMV, respectively [31]. For peroxidases, all isoforms were detected both in cucumber and zucchini, not only functioning as free radical scavengers, but also catalysts for the formation of H2O2. The author points out that the formation of ROS in plants was not sufficient for tissue collapse and restriction of the pathogen, as the increase in antioxidant enzymes prevented cell death.
It is notable that both cultivars infected with the virus had significant increases in GPX activity over time compared to controls. In Adele, GPX and GR activities were started locally earlier than in Caserta. At the systemic level, the activity of GPX and GR is activated earlier in Caserta than in Adele, probably in an attempt to get rid of the virus at the site of infection. Adele had a quick response to the viral infection at the beginning, and this may have contributed to reducing the viral load in this cultivar and making it difficult for the virus to translocate systemically. Possibly, GPX plays an important role in Adele, hindering virus replication within the plant, which does not occur in Caserta, which increases GPX activity only after the virus has established itself within the plant.
In young leaves of both cultivars there was greater GPX activity than in non-infected ones, proving the action of the enzyme not only on inoculated leaves, but also on leaves where the virus was systemically established. However, the systemic increase of virus in Caserta plants triggered earlier and greater responses in GPX and also GR activities (12 and 8 DAI, respectively) when compared to Adele (16 and 12 DAI, respectively). Plants that have suffered stress have greater GPX activity, because the enzyme, to maintain the integrity of plant cells, needs greater activity to reduce the peroxide generated due to the action of the virus within the plant. Similar to the work by Missiura, where GPX had greater activity in four watermelon cultivars (Citullus lanatus Schrad) infected with PRSV-W than in healthy plants [32]. The presence of the virus can induce a stressful condition in the plant that culminates in the formation of ROS and, consequently, lead to greater activity of antioxidant enzymes to combat possible toxic effects of these compounds.
GPX and GR are also involved in H2O2 detoxification. According to Das and Roychoudhury, GPX eliminates H2O2 present in cells not only when plants are under stress conditions, but even under normal conditions [13]. In the present study, a gradual and systemic increase of GPX was observed in both cultivars, which is in agreement with the results obtained by Diaz-Vivancos, et al. in a study with different peach cultivars infected with Plum Pox Virus (PPV), also found an increase in the activity of some enzymes such as APX and GPX in the susceptible and resistant cultivars, however, the increase in activity was greater in the cultivar susceptible [33]. However, it is clear that the increase in enzymes was not enough to prevent the spread of the virus in Caserta plants.
Overall, some enzymes were activated rapidly in Adele in response to the virus in the inoculated leaf and could help in restricting viral replication and movement. On the other hand, in situations where the virus established a high concentration (Caserta, systemically), the increase in the activity of some antioxidant enzymes in younger leaves may be an attempt by the plant to reduce the damage generated by ROS, contributing little to contain the evolution of the virus. Through the results, it can be seen that there is a relationship between the stress generated (load viral) and activities of antioxidant enzymes (Figure 7).
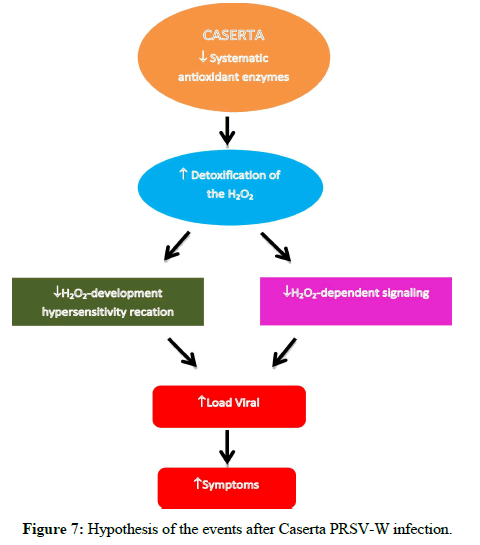
Figure 7: Hypothesis of the events after Caserta PRSV-W infection.
Conclusion
Therefore, this study hypothesizes that viral infection led to the production of ROS systemically in zucchini cultivars, which culminated in an increase in the activity of some antioxidant enzymes and different responses regarding viral load and severity. It is possible that for Caserta the action of these enzymes in the detoxification of H2O2 reduced its effects on cell signaling and, consequently, the reduction of SAR. Linked to this, the elimination of H2O2 by enzymes prevents oxidative explosion and direct toxicity to the virus, leading to viral systemic proliferation and increased severity (Figure 7). On the other hand, based on symptoms, viral load and enzymatic activities, it is clear that Adele plants have greater tolerance to virus replication, especially in younger leaves, since both cultivars did not differ in load viral in inoculated leaves, but, systemically, this cultivar had lower load viral than Caserta.
One of the limiting factors of the present study is that it was not possible to measure the H2O2 levels that could better confirm the hypothesis.
Author Contributions
• Ludiana Canton–Investigation; Methodology; Data curation; Writingoriginal draft; Formal analysis;
• Eduardo Gainete Ramos – Support in conducting experiments and enzyme analyzes;
• David Marques de Almeida Spadotti – Responsible for ELISA tests;
• Jorge Alberto Marques Rezende – Funding acquisition; Methodology; Writing-Review and editing.
• Robson Marcelo di Piero–Conceptualization; Formal analysis; Funding acquisition; Methodology; Project administration; Resources; Supervision; Writing-review and editing.
• Danila Souza Oliveira Coqueiro: Writing-review and editing.
Acknowledgements
Ludiana Canton thanks the National Council for Scientific and Technological Development (CNPq) for the scholarship, while Jorge Alberto Marques Rezende and Robson Marcelo di Piero thank this institution for the researcher grants.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Shuang D, Liu N, Ling F, Chen W, Fang Q Shen (2014) Bio-organic fertilizer application significantly reduces the Fusarium oxysporum population and alters the composition of fungi communities of watermelon Fusarium wilt rhizosphere soil. Biol Fertil Soils 50: 765-774.
- Paris HS, Yonash N, Portnoy V, Mozes-Daube N, Tzuri G. et al (2003) Assessment of genetic relationships in Cucurbita pepo (Cucurbitaceae) using DNA markers. Theor Appl Genet 106:971–978.
[Crossref ][Google Scholar][Pubmed]
- Lecoq H, Wisler G, Pitrat M (1998) Cucurbit viruses: the classics and the emerging. Eval. Enhanc. Cucurbit Germplasm 98: 126–142.
- Whitaker TW, Robinson RW (1986) Squash breeding, in: Basset MJ (Ed.). Breed Veg Crop Westport, AVI, pp. 209–242. [Crossref ][Google Scholar]
- Kurosawa C, Pavan MA, Rezende JAM (2005) Doenças das cucurbitaceas, in: Kimati H, Amorim L, Camargo LEA, Rezende JAM (Eds.). Man. Fitopatol Doenças Das Plantas Cultiv, 4a, pp. 293–294.
- Gonsalves DS, Tripathi JB, Suzuki JY (2010) Papaya Ringspot Virus. The Plant Health Instructor DOI: 10.1094/PHI-I-2010-1004-01.[Google Scholar]
- Walkey DGA (1991) Production of virus-free plants. Appl Plant Virol 270–292.
- Cooper JI, Jones AT (1983) Responses of plants to viruses: proposals for the use of terms. Phytopathol 73: 127-128.
- Doke N (1983) Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiol Plant Pathol 23: 345–357.
- Doke N, Ohashi Y (1988) Involvement of an O2−generating system in the induction of necrotic lesions on tobacco leaves infected with tobacco mosaic virus. Physiol Mol Plant Pathol 32:163-175.
- Resende MLV, Salgado SML, Chaves ZM (2003) Espécies ativas de oxigênio na resposta de defesa de plantas a patógenos. Fitopatol Bras 28: 123–130.
- Sharma P, Dubey RS (2004) Ascorbate peroxidase from rice seedlings: Properties of enzyme isoforms, effects of stresses and protective roles of osmolytes. Plant Sci 167:541–550.
- Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci.
- Mhamdi A, Queval G, Chaouch S, Vanderauwera S, Van Breusegem F. et al (2010)Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models. J Exp Bot 61: 4197-220.
[Crossref ][Google Scholar][Pubmed]
- Mittler R (2002) Oxidative stress. antioxidants and stress tolerance. Trends Plant 7: 405-410.
[Crossref ][Google Scholar][Pubmed]
- Parida A, Das AB, Mohanty PS (2004) Defense potentials to NaCl in a mangrove, Bruguiera parviflora: Differential changes of isoforms of some antioxidative enzymes. J Plant Physiol 161: 531-542.
[Crossref ][Google Scholar] [Pubmed]
- Freitas M, Stadnik M (2015) Ulvan-induced resistance in Arabidopsis thaliana against Alternaria brassicicola requires reactive oxygen species derived from NADPH oxidase. Physiol Mol Plant Pathol 90: 49-56.
- Luiz C, Rocha Neto AC, Franco PO, Di Piero RM (2017) Emulsions of essential oils and aloe polysaccharides: Antimicrobial activity and resistance inducer potential against Xanthomonas fragariae. Trop Plant Pathol 42: 370-381.
- Negrão D, Viana S, Broetto F (2014) Preparo de Tampões e Métodos para Coleta, Procedimentos e Extração de Enzimas em Tecidos Vegetais, in: Broetto F. Métodos de trabalho em bioquímica vegetal e tecnologia de enzimas. Botucatu: IBB, Cultura Acadêmica, pp. 07-11. [Crossref ][Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254.
[Crossref ][Google Scholar][Pubmed]
- Mowat WP, Dawson S (1987) Detection of plant viruses by ELISA using crude sap extracts and unfractionated antisera. J Virol Methods 15: 233-247.
[Crossref ][Google Scholar][Pubmed]
- Bonilha E, Gioria R, Della Kobori PT, Vecchia RF, Piedade SMS et al (2009) Yield of varieties of Cucurbita pepo preimmunized with mild strains of Papaya Ringspot Virus-type W and Zucchini yellow mosaic virus. Sci Agric 66: 419-424.
- Freitas DMS (2007) Novas observações sobre a proteção com estirpes fracas do Papaya Ringspot Virus-type We do Zucchini yellow mosaic virus em plantas de abobrinha-de-moita. Dissertação de Mestrado, Escola Superior de Agricultura Luiz de Queiroz, Universidade de São Paulo, Piracicaba.
- Ferreira DF (2011) Sisvar: a computer statistical analysis system. Ciência e Agrotecnologia (UFLA) 35: 1039-1042.
- Nishad R, Ahmed T, Rahman VJ, Kareem A (2020) Modulation of plant defense system in response to microbial interactions. Frontiers in Microbiology 11: 1298.
[Crossref ][Google Scholar][Pubmed]
- Gullner G, Fodor J, Király L (1995) Induction of glutathione Sâ?ÂÂÂtransferase activity in tobacco by tobacco necrosis virus infection and by salicylic acid. Pestic Sci 45: 290-291.
- Demidchik V (2015) Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ Exp Bot 109: 212-228.
- Apostol I, Heinstein PF (1989) Rapid stimulation of an oxidative burst during elicitation of cultured plant cells. Role in defense and signal transduction. Plant Physiol 90:109-116.
[Crossref ][Google Scholar][Pubmed]
- DÄ?browska GB, Kata A, Goc A, Szechynska-Hebda M, Skrzypek E (2007) Characteristics of the plant ascorbate peroxidase family. Acta Biol Cracoviensia Ser Bot 49: 7-17.
- Fodor J, Gullner G, Adam AL, Barna B, Komives T.et al (1997) Local and systemic responses of antioxidants to tobacco mosaic virus infection and to salicylic acid in tobacco (role in systemic acquired resistance). Plant Physiol 114:1443-1451.
- Riedle-Bauer M (2000) Role of reactive oxygen species and antioxidant enzymes in systemic virus infections of plants. J Phytopathology 148:297-302.
- Missiura FB (2005) Alterações metabólicas promovidas pelo Papaya Ringspot Virus – type W em plantas de melancia. Dissertação (Mestrado) – Universidade de Passo Fundo, Passo Fundo, Universidade de Passo Fundo. [Crossref ][Google Scholar]
- Diaz-Vivancos P, Rubio M, Mesonero V, Periago P, Ros Barcelo S et al (2006) The apoplastic antioxidant system in Prunus: Response to long-term plum pox vírus infection. J Exp Bot 57: 3813-3824.
[Crossref ][Google Scholar][Pubmed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 
