Research Article, Int J Cardiovasc Res Vol: 6 Issue: 5
Could the Size of Ductus Arteriosus be an Independent Predictor for the changes in Left Ventricular Function Post Percutaneous closure! Evaluation by Tissue Doppler and Speckle-derived Strain Rate Echocardiography
El-shedody SA and El-Sherbeny WS*
Cardiovascular Medicine Department, Faculty of Medicine, Tanta University, Egypt
*Corresponding Author : Wafaa S. El-Sherbeny
Cardiovascular Medicine Department, Faculty of Medicine, Tanta University, Egypt
Tel: 3337544/040
E-mail: wfelsherbeny@gmail.com
Received: July 24, 2017 Accepted: September 09, 2017 Published: September 13, 2017
Citation: El-shedody SA, El-Sherbeny WS (2017) Could the Size of Ductus Arteriosus be an Independent Predictor for the changes in Left Ventricular Function Post Percutaneous closure! Evaluation by Tissue Doppler and Speckle-derived Strain Rate Echocardiography. Int J Cardiovasc Res 6:5. doi: 10.4172/2324-8602.1000325
Abstract
Aim: Evaluate the changes of left ventricular (LV) systolic and diastolic function in children after percutaneous closure of patent ductus arteriosus (PDA) and to correlate these changes in relation to the size of the ductus. Methods: 30 children provisional diagnosed with PDA and planned for percutaneous trans-catheter PDA closure. Conventional 2D, Doppler and tissue Doppler imaging (TDI) and speckled-derived strain rate echocardiography were obtained pre-closure, early (I day), and late (1 month) post-PDA closure.
Results: Mean age was 26.0 ± 32.17 months (9 male and 21female). The mean PDA diameter was 3.11 ± 1.09 mm. Ejection fraction (EF) and fractional shortening (FS)significant reduced in the day after PDA closure compared with pre closure status (P<0.05), then improved significantly after one month compared with early post- closure measures(p<0.05),but no significant change when compared with pre-closure measure, (P>0.05). There is a negative correlation between the PDA size and changes in left ventricular diastolic dimensions, EF and FS early after PDA closure. Significant reduction in both early and late mitral diastolic flow velocities (EM and AM) after PDA closure (both one day and one month) ,early tissue Doppler velocity of lateral mitral annulus (E`lateral) significant decrease early after PDA closure(P<0.05), but one month after closure E` lateral significantly increased.(P=0.03), EM/E` lateral had a significant reduction in one month after closure compared with pre-closure and early post–closure (P=0.001). There was a significant decrease in global and regional longitudinal strain at early after PDA closure (P=0.001), although there was a significant improvement in all measures in the subsequent one month after closure.
Conclusion: Size of PDA considered as an independent predictor for the acute decrease in left ventricular function early after PDA closure which recovers completely within one month.
Keywords: Echocardiography; Percutaneous closure; Tissue doppler; Myocardial deformation
Introduction
Patent ductus arteriosus (PDA) is a part of the normal fetal life that should be closed postnatal. Its patency in the postnatal life considered as a pathology which should be managed, either trans catheter or surgically [1].
Percutaneous closure of PDA is a well-established, safe, and effective procedure of choice for treatment of PDA whenever feasible [2]. Trans catheter PDA closure is superior to surgical repair regarding a shorter hospital stay, less morbidity and mortality [3]. The clinical consequences of the ductal patency depend on its size. The size of PDA determines the magnitude of left to right shunting and as a consequence the degree of left ventricular volume overload and remodeling [4]. That in turn induces changes in left ventricular function both systolic and diastolic. PDA closure could be defined as an acute reduction in the preload that is why the changes of left ventricular function both systolic and diastolic are issue of interest for the past decade [4].
TDI and myocardial deformation imaging using speckle tracking echocardiography are new modalities, that provides direct quantitative information about myocardial motion and deformation, which is in comparison to the ejection fraction (EF) is more geometry in dependent [5]. These techniques have advantage of directly viewing the myocardium and measuring regional and global myocardial function, and the ability to detect early myocardial dysfunction induced by different diseases [6].
This study was conducted to evaluate the changes of left ventricular systolic and diastolic function in children with PDA immediately after percutaneous closure of PDA and one month later using two-dimensional (2D) echocardiography, TDI and speckledderived strain rate (SDSR) echocardiography and to correlate these changes in relation to the size of the ductus.
Patients and Methods
Patient’s characteristic
This prospective study include 30 children with PDA (mean age was 26.0 ± 32.17 months), those were planned for trans catheter closure of PDA at the Cardiology department, Tanta university hospital, Egypt. All patients had clinical and or echocardiographic evidence of hemodynamically significant PDA. All underwent trans catheter closure in the duration between January 2015 to January 2016. Exclusion criteria were irreversible pulmonary hypertension, coexistence of other congenital heart malformation either affect the hemodynamic judge of the study or require surgical intervention, vascular disease, and bleeding disorder. After approval from institutional ethical committee, an informed consent was obtained from all participants parents in this research.
Methods
All patients who were scheduled for trans catheter PDA closure underwent 2D echocardiography, Doppler and TDI and SDSR and measurements obtained 24 h prior to PDA closure (pre-closure), and one day after(early), and one month later (late) post-PDA closure.
Echocardiography
Standard 2D echocardiogram following the segmental analysis from all echocardiographic windows (subcostal, apical, parasternal and suprasternal) was performed using vivid 9. General electronics (GE) with 3.5 to 5 (MHz) passed array transducers. In agitated infants and children, sedation with chloral hydrate aqueous solution (50-100 mg/Kg) 15 minutes prior to the study was done.
The M-Mode measurements included left ventricular enddiastolic dimension (LVEDD), and left ventricular end-systolic dimension (LVESD). Ejection fraction (EF) and fraction of shortening (FS) were all measured in the parasternal long-axis view. Also the left atrial diameter is measured at end systole (maximal size) while aortic diameter is measured at diastole in parasternal long-axis view, left atrial/aortic ratio was measured.
Color Doppler is a very sensitive modality in detecting the presence of a PDA and used to estimate the degree of ductal shunting. Even an extremely tiny patent ductus can be detected by a color flow signal entering the pulmonary artery near the origin of the left pulmonary artery. It is used to estimate the geometry of the ductus, its left pulmonary end and aortic ampulla and direction of the shunt.
Continuous wave Doppler echocardiography used to record PDA gradient which reflect the estimated pulmonary pressure and the size of the ductus.
Diastolic function analysis
The mitral inflow signal was acquired in the apical four-chamber view. The pulsed Doppler sample volume was 2 mm, placed at the mitral valve tip, we measured: the early peak diastolic flow velocity (E) and atrial filling velocity (A) and E/A ratio was calculated.
Tissue doppler echocardiography
The tissue Doppler velocities were obtained from the apical four chamber view. A pulsed wave Doppler sample gate of 1-2 mm was used and was positioned at the lateral and septal mitral annuls. In general, the higher the myocardial velocities the better the myocardial function. Off-line analysis used the average readings in 2-3 cardiac cycles of: peak systolic (S`) myocardial velocity, the peak early diastolic (E`) myocardial velocity, the peak late diastolic (A`) myocardial velocity and the ratio of mitral inflow E wave to the tissue Doppler E` wave (E/E`) was measured which correlates with LV end diastolic pressure.
Strain rate and speckled echocardiography
The two-dimensional (2D)gray scale imaging were recorded from the apical four – two and three chamber views, using frame rate of 80-100 frame/second the narrowest sector including the LV apex and myocardial walls in different views with vivid 9 GE. Three consecutive cardiac cycles were obtained and digitally stored for offline analysis.
Strain analysis
Off line measurement of the 2D strain were obtained, speckle tracking was performed using the semi-automatic AFI algorithm (Automated Function Imaging, Version 112, GE Healthcare, Horten, Norway) that analyses myocardial motion by tracking frame-toframe speckle changes in 2D images. The region of interest (ROI) was eventually adjusted to ensure tracking of the whole myocardial thickness if the tracking suboptimal the endocardial border will be retracted, the end –systolic strain values were measured at aortic valve closure. The algorithm calculated average longitudinal peak systolic strain (LPSS) for each of the LV 18 segments, displaying segmental strain plots in each apical view, computed average peaks for each view, and global LPSS (GLPSS), displayed in bull’s eye format [7].
The global LV longitudinal strain was calculated by averaging all the measured segmental values. The average longitudinal strain of the different left ventricular walls (lateral, septal, inferior, posterior, anterior, and anteroseptal) was calculated by averaging the apical, mid and basal end systolic strain segmental values in each wall.
Cardiac catheterization
After the consent of the parents, the procedure were performed under general endotracheal anesthesia, antibiotic one hour before the procedure, intravenous heparin just after the vascular accesses complete hemodynamic study had been done for assessment of pulmonary vascular resistance and shunt quantification. Aortography was done in lateral and right anterior oblique (RAO) projection to analyze the length of the ductus, its diameter at its narrowest end. After sizing the PDA, the moderate to large ductus >2 mm at its pulmonary end had been closed by amplatzer duct occlude, the restrictive ones less than in 2 mm at its narrowest point had been closed by PFM coil, however the devices were deployed by the standard technique. After device release, control aortography was done to assess the degree of residual shunt if any, and to assess the position of the device.
Statistical analysis
The full detailed form is: SPSS 20, IBM, Armonk, NY, United States of America.
Quantitative data were expressed as mean ± standard deviation (SD). A one-way analysis of variance (ANOVA) when comparing between more than two means. Post Hoc test was used for multiple comparisons between different variables. Pearson’s correlation coefficient (r) test was used for correlating data.
Results
Thirty patients (9 male and 21 female) had successful Trans catheter closure with no major complications, no significant residual shunt no obstruction to the aorta or to any pulmonary branches. All had been discharged home one day after the procedure. The patients mean age was 26.03 ± 2.17 months (range 7-120 months). The mean PDA diameter was 3.11 ± 1.09 mm, (range: 1.2 -5.2 mm).
Echocardiography
M-mode and 2D echocardiography was done before, one day and one month after PDA closure. Table 1 shows the significant reduction in LVEDD, EF and FS one day after closure compared with preclosure status, (P<0.05), LVEDS show no significant reduction, there was a significant decrease in LVEDD and LVEDS one month after closure compared with pre-closure measures. EF and FS improved significantly after one month compared with early post-closure measures (p<0.05), but no significant change in systolic function when compared with pre-closure measure (p>0.05). LA/AO ratio significantly reduced one day after PDA closure compared with preclosure status (p<0.05), and remarkable reduction was observed one month after closure compared with the pre-closure status.
| Before Mean ± S. D | 24 hours post | 1 month post | P1 | P2 | P3 | |
|---|---|---|---|---|---|---|
| LVEDD (mm) | 31.24 ± 5.82 | 28.40 ± 5.39 | 26.53 ± 4.98 | 0.045* | 0.001* | 0.185 |
| LVEDS (mm) | 18.09 ± 3.37 | 17.67 ± 3.19 | 15.87 ± 2.87 | 0.607 | 0.008* | 0.030* |
| FS (%) | 41.89 ± 5.78 | 37.14 ± 5.30 | 40.13 ± 6.15 | 0.002* | 0.238 | 0.047* |
| E F(%) | 74.73 ± 4.99 | 68.80 ± 6.72 | 73.40 ± 6.51 | 0.001* | 0.401 | 0.005* |
| LA / AO Ratio | 2.09 ± 0.57 | 1.75 ± 0.38 | 1.59 ± 0.33 | 0.004* | 0.001* | 0.163 |
FS fractional shortening, EF ejection fraction, LA/AO: left atrial diameter/aortic annulus
*Significant P-value
P1 Pre-closure versus 24 h post-closure
P2 Pre-closure versus 1 month post-closure
P3Value 24 h post-closure versus 1 month post-closure
Table 1: Echocardiographic parameters before and after PDA closure.
Tissue doppler echocardiographic results
Table 2 shows a significant reduction in both mitral E and A values after PDA closure (both one day and one month) (p<0.05) compared with pre closure status. But the reduction of E/A ratio not reach statistically significant. E` lateral significant decrease early after PDA closure as compared to before closure (p<0.05), but one month after closure there was a significant increase in E` lateral compared with one day after closure of PDA (Figure 1A,B), E`/A` lateral ratio decrease significant in early post closure compared to pre closure (p= 0.012) but one month after closure there was insignificant increase in E`/A` ratio (p=0.11). EM/E` lateral ratio did not change in early post closure, but it had a significant reduction in one month after closure compared with pre-closure and early post–closure (p=0.001).
| Before Mean ± S. D | 24 hours post | 1 month post | P1 | P2 | P3 | |
|---|---|---|---|---|---|---|
| EM (cm/s) | 108.48 ± 16.34 | 78.20 ± 19.70 | 71.32 ± 20.75 | 0.001* | 0.001* | 0.128 |
| AM (cm/s) | 75.72 ± 11.46 | 59.24 ± 14.84 | 53.52 ± 13.84 | 0.001* | 0.001* | 0.187 |
| EM / AM ratio | 1.46 ± 0.27 | 1.44 ± 0.35 | 1.41 ± 0.5 | 0.358 | 0.124 | 0.268 |
| E' Lateral (cm/s) | 11.52 ± 1.11 | 10.82 ± 1.01 | 11.25 ± 1.15 | 0.027* | 0.324 | 0.034* |
| E' Septal (cm/s) | 9.37 ± 0.87 | 8.98 ± 0.89 | 13.59 ± 0.94 | 0.128 | 0.001* | 0.001* |
| S' Lateral (cm/s) | 7.39 ± 1.18 | 5.29 ± 1.74 | 6.41 ± 1.17 | 0.001* | 0.015* | 0.006* |
| S' Septal (cm/s) | 6.29 ± 1.01 | 4.03 ± 1.14 | 5.38 ± 1.10 | 0.001* | 0.004* | 0.001* |
| A' Lateral (cm/s) | 10.63 ± 2.15 | 10.59 ± 1.97 | 10.51 ± 2.32 | 0.362 | 0.189 | 0.305 |
| A' Septal (cm/s) | 10.47 ± 2.25 | 10.41 ± 1.85 | 10.38 ± 2.16 | 0.328 | 0.174 | 0.521 |
| E' / A' Lateral | 1.62 ± 0.62 | 1.35 ± 0.65 | 1.48 ± 0.59 | 0.012* | 0.094 | 0.112 |
| EM / E' Lateral | 9.16 ± 1.45 | 8.78 ± 1.62 | 5.10 ± 1.43 | 0.197 | 0.001* | 0.001* |
| EM / E' Septal | 11.72 ± 2.38 | 8.84 ± 2.59 | 4.88 ± 1.69 | 0.001* | 0.001* | 0.001* |
*Significant P.value
P1 Pre-closure versus 24 h post-closure
P2 Pre-closure versus 1 month post-closure
P3 Value 24 h post-closure versus 1 month post-closure
Table 2: The comparison between the Doppler and tissue Doppler measurements before and after PDA closure.
Effect of PDA size on LV function and dimensions
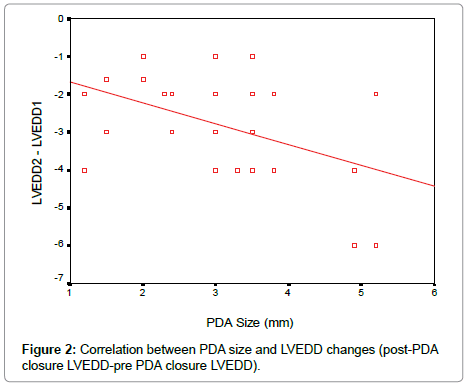
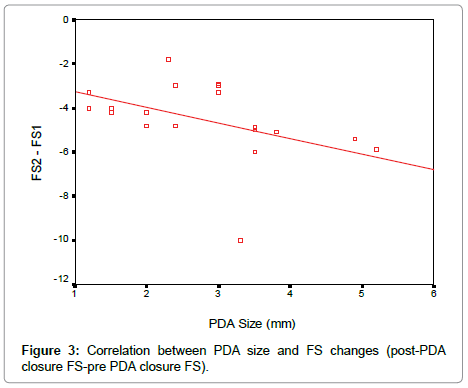
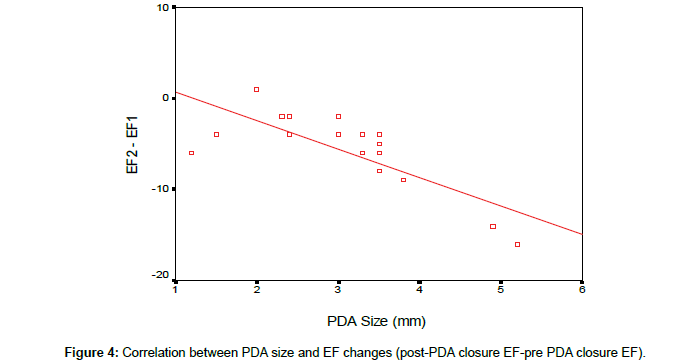
According to our results, there was a significant reduction in LVEDD and LV systolic function early after PDA closure. To determine the effect of PDA size on the changes in LV function and dimensions, linear regression analysis was done as shown in the Table 3. There is a negative correlation between the PDA size and changes in EF, FS and left ventricular diastolic dimensions early after PDA closure (Figures 2-4).
| LVEDD difference | FS difference | EF difference | ||||
|---|---|---|---|---|---|---|
| R | P | R | P | R | P | |
| Size | - 0.445 | 0.014* | - 0.422 | 0.020* | - 0.654 | 0.004* |
Table 3: Analyze the effect of PDA size on left ventricular function and dimension.
Longitudinal strain results
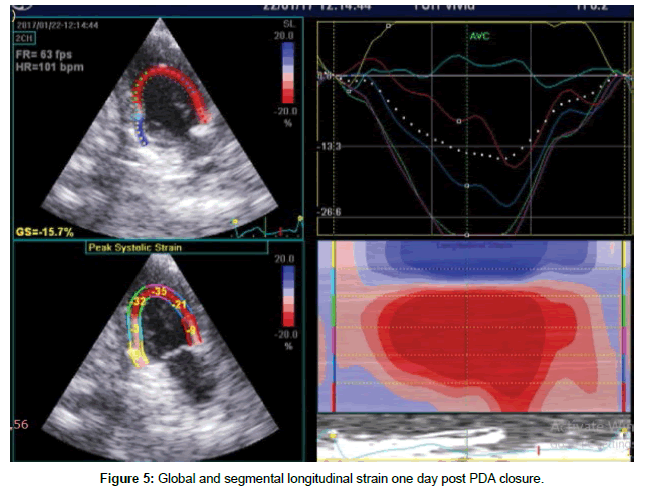
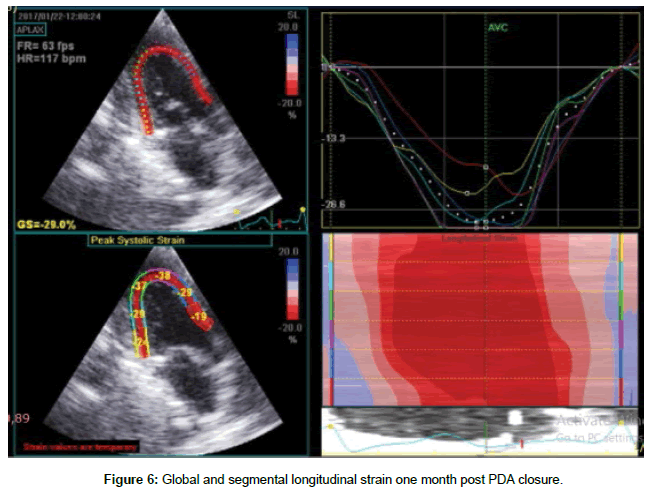
Table 4 shows global and segmental longitudinal strain results in pre-closure, early and late post- closure status. There was a significant decrease in global and udinal strain at early post –closure compared with pre-closure measures (p=0.001), although there was a significant improvement in all measures in the subsequent one month after closure except for inferior wall, the values of global and segmental longitudinal strain at one month post-closure were significantly lower than pre-closure ones, except for lateral and anteroseptal walls. The difference was statistically insignificant (Figures 5 and 6).
| Before Mean ± S. D | 24 hours post | 1 month post | P1 | P2 | P3 | |
|---|---|---|---|---|---|---|
| Septal | 20.95 ± 1.98 | 13.42 ± 2.83 | 18.71 ± 2.38 | 0.001* | 0.001* | 0.001* |
| Inferior | 22.68 ± 2.13 | 18.79 ± 1.79 | 19.03 ± 1.71 | 0.001* | 0.001* | 0.628 |
| Lateral | 18.93 ± 1.87 | 15.69 ± 1.94 | 18.02 ± 2.90 | 0.001* | 0.125 | 0.001* |
| Anterior | 18.63 ± 1.68 | 15.49 ± 1.96 | 17.05 ± 1.89 | 0.001* | 0.001* | 0.002* |
| Posterior | 22.99 ± 2.04 | 17.89 ± 1.67 | 19.34 ± 2.72 | 0.001* | 0.001* | 0.012* |
| Anteroseptal | 21.25 ± 2.77 | 18.85 ± 2.19 | 20.84 ± 2.85 | 0.001* | 0.543 | 0.004* |
| Global strain | 21.12 ± 2.06 | 12.15 ± 1.85 | 17.71 ± 1.77 | 0.001* | 0.001* | 0.001* |
Table 4: The comparison between the results of global and segmental longitudinal strain before and after PDA closure.
Cardiac catheterization results
All the enrolled patients had successful trans catheter closure with no major complications, no significant residual shunt, no obstruction to the flow in the aorta nor to any of the pulmonary branches, 18 patients had been closed by the standard technique using Amplatzer duct occlude I (ADO I) (AGA Medical Corp., Golden Valley, Minnesota)the Device size is chosen so that the smaller end of the device is at least 2 mm larger than the narrowest portion of the PDA four patients had been closed by using PDAR (PFM, Cologne, Germany) and eight patients had been closed by Nit- Occlud coil (PFM, Cologne, Germany) coil is selected with distal coil diameter no more than 2 mm larger than aortic ampulla, distal coil diameter at least 3 to 4 mm larger than narrowest diameter and length of the configured coil is no longer than PDA length. All the patients show marked relieve of their symptoms relieve of tachypnea and sweating and the effort on lactations, except two patients had huge shunt because of large non restrictive ductus, after closure develop tachypnea which needs medications: Lasix, capoten and sildenafile, for two weeks then the patient improved and the medications discontinued.
Discussion
A hemodynamically significant PDA has to be closed either surgically or by trans catheter intervention at any age. Trans catheter closure of PDA carries a lots of advantages when compared to surgical ligation [3,8] being less invasive with no surgical scar, short hospital stay and low morbidity with comparable success rate [3]. The trans catheter closure of PDA has advanced rapidly and many devices and methods have been developed to allow trans catheter closure of virtually all PDAs, regardless of size or configuration. It is necessary to consider both the morphology as well as size of the ductus in order to select the proper occluded device to use for PDA occlusion. All patients included in this study had been closed successfully with no residual shunt, no obstruction to any of the pulmonary branches with free flow into the descending aorta.
Many authors were interested to evaluate the changes in left ventricular function which are expected to improve after PDA closure, but some patients may show evidence of drop in the left ventricular systolic function. (4) Even so the improvement in the diastolic function??
This study reported that early deterioration of left ventricular systolic function after successful PDA closure. At early post- PDA closure, there was a significant reduction in LVEDD and left ventricular systolic function (EF and FS) which improved after one month. Our explanation for this observation is that hemodynamically significant PDA associated with left to right shunt, which increases the left ventricular preload. based on the frank-starling`s law, LV volume overload due to PDA is required to increase LV volume and (EF) stretching the length of LV muscle fiber. PDA closure results in sudden changes in the loading condition, acute reduction in the preload, reduction of LV volume overload, there by reducing the LV fiber length and EF, another explanation for this observation is sudden increase in after load due to termination of blood flow through low resistance pulmonary circulation after PDA closure leads to systolic dysfunction.
These results was in agreement with the study of Tilahun et al. [9] who study transient LV systolic dysfunction following surgical closure of large PDA among children and adolescents and reported that patients with large PDA underwent the procedure at older age had a significant reduction in LVEF and FS early post- operative (5 days) compared to pre –operative status, then normalization of LV systolic function after 5 weeks and recommend possible initiation of ACEI for those with significant systolic dysfunction. Other studies on children underwent PDA closure was in agreement with our study [4,8]. Our study showed complete spontaneous resolution of LV systolic dysfunction within one month which is consistent with study made by Galal et al. [8] and Eerola et al. [10]. In contrast to our results, Jeong et al. [11] reported persistent LV systolic dysfunction in 11% of adult patients who underwent PDA closure; this discrepancy might be related to the difference in patient’s age group. A longer duration of left ventricular volume overload may cause irreversible structural changes in the left ventricular myocardium, thus leading to imperfect recovery after reduction in loading condition.
In our study, there was a negative correlation between the PDA size and changes in EF, FS and left ventricular dimensions the larger the PDA size the more reduction in LVEDD, FS and EF early post PDA closure which is consistent with observations made by Amoogzar et al. [12].
Kim et al. [13] in their retrospective study found that QP/Qs >1.6 and pulmonary artery pressure /systemic artery pressure >0.32 predicted the reduced post-closure left ventricular systolic function.
Assessment of left ventricular diastolic function in patients with PDA is misjudged due to the increase in preload and mitral inflow, Choony et al. [14] in their study reported that the reduction in the filling pressure changes the flow velocity profile in a manner that mimics the abnormalities with impairment of LV diastolic function, therefore when interpreting Doppler derived indexes of LV diastolic function, the influence of preload must taken into account.
Also in absence of definite criteria for diastolic dysfunction in children with PDA, descriptive analysis was used in this study. In current study, there was a significant reduction in mitral E and A after PDA closure both in early and late post-closure status but E/A ratio not significantly decreased, this due to the reduction in left ventricular preload after PDA closure and because of the Doppler velocities are load dependent results in decrease in EM and AM in both early and late post closure state, this was in the agreement with the studies of Noori et al. [15] and Gupta et al. [4]. TDI is relatively independent of loading condition; we used this modality for assessment of diastolic function.
Our TDI results revealed that E` lateral was significantly decreased early after PDA closure (p<0.05), but after one month E` lateral increased significantly as compared to 24 hrs (p<0.05), E`/ A`lateral ratio diminished early after PDA closure (p=0.01) but after one month non significantly increased, this due to E` velocities have influenced by both left ventricular relaxation and restoring forces. The decrease in systolic function and restoring force in early post closure time lead to decrease E`lateral. As the left ventricular systolic function recovered, diastolic function of the left ventricle (E`, E`/A`ratio ) also improved in late post –closure status.
The decrease in EM and E` lateral in early post closure time, lead to EM/ E` lateral ratio did not change, however, after one month EM still diminished because of the diminished in the preload but E` lateral increased due to improved systolic function, therefore EM/E` lateral significantly reduced (p=0.001), this results in concordant with the study of Amoogzar et al. [12].
Changes in longitudinal systolic strain
Theoretically, a larger left ventricle should deform less with lower strain values to generate the same LV output but due to increased stroke volume and left ventricular output in pre- closure status, strain values were the highest in pre-closure time resulting from the increased pre-load, El-khuffash et al. [16] found that there is a strong relationship between LV systolic strain and left ventricular output confirming that the increased global longitudinal strain values largely resulted from the increased preload. The decrease in systolic strain values at early post-closure time parallel to deterioration in left ventricular systolic function. All LV segmental and global strain values (except for inferior wall) increased one month after PDA closure as the left ventricular systolic function recovered. These results were in accordance with the study by EL-Khuffash et al. [16]. They study the acute changes in myocardial systolic function in preterm infants after PDA ligation, found that the global longitudinal strain values significantly decreased one hour after the procedure then increased 18 hour postoperative but still significantly lower than preoperative values.
Our result was in the same line with the results of Amoogzar et al. [12] in their study on 21 children with PDA evaluating left ventricular function using speckle- derived strain rate echocardiography at pre-closure, 1 day and 1 month post –closure, found that global and segmental longitudinal strain measures reduced significantly early after PDA closure but it improved remarkably after one month.
Conclusion
Size of PDA considered as an independent predictor for the acute decrease in left ventricular function early after PDA closure which recovers completely within one month.
Study limitations
Small sample size limited its power in eliciting differences in the variables affecting the left ventricular dysfunction. The parameters used for the evaluation of left ventricular systolic function, though in concordance with previous studies, were preload dependent which could influence the interpretations in the post –closure status.
References
- Schneider DJ (2012) The patent ductus arteriosus in term infants, children, and adults. Semin Perinatol 36: 146-153.
- Zhang CJ, Huang YG, Huang XS, Huang T, Huang WH, et al. (2012) Transcatheter closure of large patent ductus arteriosus with severe pulmonary arterial hypertension in adults: immediate and two-year follow-up results. Chin Med J125: 3844-3850
- Masura J, Tittel P, Gavora P, Podnar T (2006) Long term outcome of transcatheter patent ductus arteriosus closure using Amplatzer duct occluders. Am Heart J 151: 755.e7-10.
- Gupta SK, Krishnamoorthy K, Tharakan JA, Sivasankaran S,Sanjay G, et al. (2011) Percutaneous closure of patent ductus arteriosus in children: immediate and short-term changes in left ventricular systolic and diastolic function. AnnPediatr Cardiol 4:139-144.
- Dragulescu A, Mertens LL (2010) Developments in echocardiographic techniquesfor the evaluation of ventricular function in children. Arch CardiovascDis 103: 603-614.
- Mohammed A, Mertens L, Friedberg MK (2009) Relations between systolic and diastolic function in children with dilated and hypertrophic cardiomyopathy as assessed by tissue Doppler imaging. J Am Soc Echocardiogr 22: 145-51.
- Teske AJ, De Boeck BW, Melman PG, Sieswerda GT, Doevendans PA, et al. (2007) Echo cardiographic quantification of myocardial function using tissue deformation imaging, a guide to image acquisition and analysisusing tissue Doppler and speckle tracking. Cardiovasc Ultrasound 5: 27.
- Galal MO, Amin M, Hussein A, Kouatli A, Al-Ata J, et al. (2005) Left ventricular dysfunction after closure of large patentductus arteriosus. Asian Cardiovasc Thorac Ann 13: 24-29.
- Tilahun B, Tefera E (2013) Transient left ventricular systolic dysfunction following surgical closure of large patent ductus arteriosus among children and adolescents operated at the cardiaccentre. Ethiop J Cardiothorac Surg 8: 139.
- Eerola A, Jokinen E, Boldt T, Pihkala J (2006) The Influence of Percutaneous Closure of Patent Ductus Arteriosus on Left Ventricular Size and FunctionA Prospective Study Using Two and Three-Dimensional Echocardiography and Measurements of Serum Natriuretic Peptides. J Am Coll Cardiol 47: 1060–1066.
- Jeong YH, Yun TJ, Song JM, Park JJ, Seo DM, et al. (2007) Left ventricular remodeling and change of systolic function after closure of patent ductus arteriosus in adults: device and surgical closure. Am HeartJ 154: 436-440.
- Amoogzar H, Shakiba AM, Derakhshan D, Ajami G, Cheriki S, et al. (2015) Evaluation of left ventricular function by tissue Doppler and speckle-derived strain rate echocardiography after percutaneous ductus closure. Pediatr Cardiol 36: 219–225.
- Kim YH, Choi HJ, Cho Y, Lee SB, Hyun MC (2008) Transient left ventricular dysfunction after percutaneous patent ductus arteriosus closure in children. Korean Circ J 38: 596-600
- Choong CY, Herrmann HC, Weyman AE, Fifer MA (1987) Preload dependence of Doppler-derived indexes of left ventricular diastolic function in humans. J Am CollCardiol 10: 800-808.
- Noori S, Friedlich P, Seri I, Wong P (2007) Changes in myocardial function and hemodynamics after ligation of the ductus arteriosus in preterm infants. J pediatr 150: 597-602.
- El-Khuffash AF, Jain A, Dragulescu A, McNamara PJ, Mertens L (2012) Acute changes in myocardial systolic function in preterm infants undergoing patent ductus arteriosus ligation: a tissue Doppler and myocardial deformation study. J Am Soc Echocardiogr 25: 1058-1067.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi