Research Article, Int J Cardiovasc Res Vol: 7 Issue: 2
Cytokine Expression and Hypertension Comorbidity in HIV/AIDS Patients at Kenyatta National Hospital HIV Care Centre, Nairobi, Kenya
Angeline Chepchirchir1,2*, Joshua Nyagol3 and Walter Jaoko4
1School of Nursing Sciences, University of Nairobi, Kenya
2Institute of Tropical and Infectious Diseases, University of Nairobi, Kenya
3Department of Human Pathology, Unit of Immunology, School of Medicine, University of Nairobi, Kenya
4Department of Medical Microbiology, School of Medicine, University of Nairobi, Kenya
*Corresponding Author : Ms. Angeline Chepchirchir
School of Nursing Sciences, University of Nairobi P.O Box 2558-00202, KNH Nairobi- Kenya
Tel: +254-720440665
E-mail: chepchirchir@uonbi.ac.ke
Received: November 13, 2017 Accepted: March 12, 2018 Published: March 18, 2018
Citation: Chepchirchir A, Nyagol J, Jaoko W. (2018) Cytokine Expression and Hypertension Comorbidity in HIV/AIDS Patients at Kenyatta National Hospital HIV Care Centre, Nairobi, Kenya. Int J Cardiovasc Res 7:2. doi: 10.4172/2324-8602.1000343
Abstract
Chronic inflammation in HIV disease is sustained by immune reconstitution that results from use of combination therapy by patients and manifested by increased expression of inflammatory cytokines. Chronic inflammation in HIV disease is associated with endothelial dysfunction of the cardio vasculature giving rise to reduced elasticity resulting in high blood pressure. This state is marked by increased IL-6 cytokine expression as manifested in patients with type 2 diabetes and hypertension. This study sought to establish cytokine expression in HIV seropositive patients with hypertension comorbidity and correlate with HIV/AIDS progression. We hypothesized that chronic inflammation and presence of hypertension comorbidity presents higher levels of expression of selected interleukins namely IL-6, IL-8 and IL-17A. This study demonstrated that the IL-6 cytokine expression have a positive correlation with the release of IL-8 cytokine as patients with higher IL-6 cytokine values were more likely to have higher IL-8 cytokine levels. This shows that a synergistic effect from both cytokines may accelerate the development of atherosclerosis on blood vessels. Therefore routine measurement of these cytokines may increase prediction of risk of development of hypertension in patients on HIV care.
Keywords: Cytokine expression; Chronic inflammation; Cardiovascular disease
Background
Chronic inflammation in HIV disease is associated with endothelial dysfunction of the cardio vasculature giving rise to reduced elasticity resulting in high blood pressure [1-8]. This state is marked by increased IL-6 cytokine expression as manifested in patients with type 2 diabetes and hypertension [9,10]. Low dose of omega 3 fish oil used by HIV positive clients have not shown any benefits in reduction of IL-6 expression [11]. Elevated IL-6 expression in chronic conditions is associated with poor progression and clinical outcomes of patients [12,13]. Evaluation of IL-6 gene polymorphism has been established to increase risk of cardiovascular disease [14]. It is a marker of endothelial injury and damage to the vasculature [15]. IL-17A has been implicated in large vessel vasculitis (e.g. aorta) and its high level expression is a risk factor for vascular damage and associated complications [16]. Determination of levels of expression of these cytokines for example, IL-6 is useful as a diagnostic marker for risk of myocardial infarction [17]. Pro-inflammatory cytokines (IL-6 & IL-8) measures may be used as an indicator of severity of cardiovascular disease complications such as chronic heart failure [18,19]. Trigger factors of cardiovascular disease include alcohol abuse which is rampant with HIV seropositive patients [20]. Interventions that target inhibition of IL-6 have shown benefits of reduced cardiovascular events [21]. Individuals with prehypertension have been established to have gradual increase in IL-6 expression over a 3 year period with increasing risk of arterial stiffness and arterial fibrillation [22,23]. On the other hand expression of IL- 17A may offer a compensatory adaptation of dilated cardiomyopathy to improve function in the event of viral myocarditis [24]. This study sought to establish cytokine expression in HIV seropositive patients with hypertension comorbidity and correlate with HIV/AIDS progression. We hypothesized that chronic inflammation and presence of hypertension comorbidity presents higher levels of expression of selected interleukins namely IL-6, IL-8 and IL-17A.
Materials and Methods
A sample of one hundred and twenty six HIV seropositive patients were recruited between January and May 2015 through systematic sampling approach at Kenyatta National Hospital HIV Care Centre, Nairobi which offers exclusive health care services for HIV seropositive patients. The patients are referred to the care center from voluntary counseling clinics and hospitals spread out in different locations of Nairobi County and its environs. The facility attracts patients from all regions of the country thus offering a cosmopolitan mix of study participants. It has quality operating systems with good record keeping and follow-up of patients thus offering a good platform for the recruitment of different clusters of patients. The Centre too has a total enrolment of six thousand active clients out of which 4000 are on anti-retroviral therapy. About six hundreds of these clients have both immunosuppression and cardio metabolic disorders which include diabetes, osteoporosis, renal disease and/or arthritis. The participants were screened for blood pressure and assessed for Body Mass index (BMI). A questionnaire was used to examine participants’ lifestyle characteristics and extraction of clinical parameters from patient records. Blood pressure recorded was an average measure of three (3) systolic and diastolic pressure readings taken in intervals of 15 minutes. A systolic reading above 140 mmHg and diastolic above 90 mmHg was considered to constitute high blood pressure.
Design
This was a cross sectional descriptive study that sought to determine the plasma concentrations of pro-inflammatory cytokines in HIV seropositive patients with hypertension comorbidity. Obtained data on the concentrations of expressed cytokines was correlated with WHO HIV/AIDS stage of patients and use of combination therapy in both hypertensive and non-hypertensive participants.
Population
The study population was HIV positive patients seeking care at the Kenyatta National Hospital’s Comprehensive Care Center. The majority of patients have been on follow-up for at least 5 years. However, some patients have been on care for over 10 years. Eligibility criteria included adult age, HIV positive status, Active on care and informed consent. The eligible participants were categorized into hypertensive and non-hypertensive groups. Ethical approval was obtained from the institution’s Research and Ethics review board. Written informed consent was obtained from each participant prior to enrolment.
Data collection
Demographic and clinical data was collected using structured questionnaires and medical records review. The main variables of study included age, gender, smoking history, alcohol use, antiretroviral therapy, CD4+ count, hematocrit, serum creatinine and body mass index (BMI).
Clinical data
Physical assessments for weight and height measures were taken for computation of individual participants’ body mass index (BMI). Clinical review of patient records was done to obtain the recent laboratory test measures for creatinine, hematocrit, WHO HIV/AIDs stage, CD4+ cell count, cART and antihypertensive drug use. Recent data on laboratory tests like CD4+ cell count, hematocrit and serum creatinine measures that were within 3 months of recording were accepted as valid measures and were used in the study.
Laboratory sample collection and processing
A blood sample of 4 mls was collected from each participant during the routine laboratory sample collection. The blood sample was analyzed for selected cytokines’ levels which include; Interleukin1 (IL-1), Interleukin-2 (IL- 2), Interleukin-4 (IL-4), Interleukin-6 (IL- 6), Interleukin- 10 (IL-10), Interleukin-17A (IL-17A), Tumor Necrosis Factor Alpha (TNF-α) and Interferon-gamma (IFN-γ). The BD™ CBA Human Th1/Th2/Th17 and IL-8 Flex Cytokine Kits were used.
Statistical analysis
Statistical Package for Social Sciences (SPSS) version 22 was used in analysis of data. Both descriptive and inferential statistics were applied. Means and proportions for the key variables were obtained while multivariable analysis was done to establish the modulating effect of IL-6, IL-8 & IL-17A expression on the risk of developing hypertension in HIV disease. Chi square test for independence, Spearman’s rank-order correlation and Pearson product-moment correlation coefficient were used to establish associations between variables.
Results
Cytokine expression and participant demographic characteristics:
A total of 126 participants (46 males and 80 females) were enrolled in the study. Their ages ranged in age from 33 – 56 years (M=44.50, SD=5.64). The average age of males (M=45.26, SD=5.50) was higher than females (M=44.06, SD=5.72), p>.05. The association between gender and IL-17A was moderate, positive and statistically significant (p<0.001), demonstrating that gender influences the release of IL-17A and females are more likely to have higher IL-17A levels than males in HIV positive patients (Table 1).
| Hypertensive | Non-Hypertensive | ||
|---|---|---|---|
| Characteristics | (M ± D) | (M ± SD) | 95% CI |
| IL 17A | 75.51 ± 84.89 | 42.23 ± 64.40 | 6.81 – 59.77 |
| **Levene’s F = 23.354, df = 118.877, p < 0.001 | |||
| *T-Test t = 2.489, df = 118.877, p= 0.014 | |||
| Spearman’s rho rs = -0.173, p = 0.052 | |||
| IL 2 | 0.65 ± 2.36 | 0.12 ± 0.91 | 0.09 – 1.16 |
| **Levene’s F = 11.019, df = 83.534, p= 0.001 | |||
| T-Test t = 1.712, df = 83.534, p= 0.091 | |||
| *Spearman’s rho rs = -0.185, p = 0.038 | |||
| IL 4 | 0.03 ± 0.18 | -0.04 – 0.07 | |
| Levene’s F = 1.576, df = 124, p= 0.212 | |||
| T-Test t = 0.642, df = 124, p= 0.522 | |||
| Spearman’s rho rs = -0.084, p = 0.349 | |||
| IL 6 | 2.59 ± 8.13 | 1.94± 6.38 | -0.94 – 4.23 |
| Levene’s F = 2.059, df = 124, p= 0.154 | |||
| T-Test t = 1.259, df = 124, p= 0.210 | |||
| **Spearman’s rho rs = -0.256, p = 0.004 | |||
| IL 8 | 29.67 ± 70.80 | 16.59 ± 40.46 | -7.14 – 33.30 |
| *Levene’s F = 5.390, df = 102.990, p= 0.022 | |||
| T-Test t = 1.283, df = 102.990, p= 0.203 | |||
| Spearman’s rho rs = -0.026, p = 0.769 | |||
| IL 10 | 0.21 ± 0.77 | 0.72 ± 3.42 | -1.41 – 0.38 |
| *Levene’s F = 5.586, df = 65.705, p= 0.020 | |||
| T-Test t = 1.142, df = 65.705, p= 0.257 | |||
| Spearman’s rho rs = -0.013, p = 0.886 | |||
| TNFa | 0.10 ± 0.39 | 0.08 ± 0.42 | -0.12 – 0.17 |
| Levene’s F = 0.366, df = 124, p= 0.546 | |||
| T-Test t = 0.348, df = 124, p= 0.729 | |||
| Spearman’s rho rs = -0.092, p = 0.306 | |||
| INFy | 6.87 ± 35.66 | 0.00 ± 0.00 | -1.96 – 15.71 |
| **Levene’s F = 1.196, df = 64.000, p= 0.003 | |||
| *T-Test t = 1.553, df = 64.000, p= 0.125 | |||
| *Spearman’s rho rs = -0.217, p = 0.015 | |||
Levene’s test of homogeneity of variances was used.
Independent-samples t-test was used.
Spearman’s Rank-Order Correlation coefficient was used.
**p<0.01
*p<0.05
Table 1: Descriptive characteristics of hypertension status and cytokines (IL 17A, IL 2, IL 4, IL 6, IL 8, IL 10, TNFa, INFy) characteristics of participants.
The association between gender and IL-2 was strong, negative and statistically significant (rs(124)=-0.764, p<0.05), demonstrating too that gender influences the release of IL-2 and males are more likely to have higher IL-2 levels than females in HIV positive patients (Table 1).
The association between hypertension status and IL-17A was mild, negative and statistically significant (rs(124)=-0.248, p<0.05), showing that hypertension status influences the release of IL-17A and hypertensive HIV positive patients are more likely to have higher IL- 17A levels than non-hypertensive patients (Table 2).
| Characteristic | IL 17A | IL 2 | IL 4 | IL 6 |
|---|---|---|---|---|
| Age | r(124) = 0.181 p = 0.142 |
r(124) = -0.055 p = 0.897 |
r(124) = 0.463 p= 0.537 |
r(124) = -0.191 p = 0.127 |
| Gender | rs(124) = 0.417** p < 0.001 |
rs(124) = -0.764* p = 0.027 |
- | rs(124) = -0.011 p = 0.929 |
Spearman’s Rank-Order Correlation coefficient was used.
**p<0.01; *p<0.05
Table 2: Association of Participants’ age & gender and selected cytokines; (IL 17A, IL 2, IL 4, IL 6).
Cytokine expression and participant Social characteristics
The association between social behaviour of the patients and IL- 17A was moderate, negative and statistically significant (rs(124)=- 0.387, p<0.05), demonstrating that the social behaviour of patients influences the release of IL-17A and both smokers and alcoholic patients were more likely to have lower IL-17A levels than nonsmokers and non-alcoholic patients.
However, the association between social behaviour of the patients and IL-6 was moderate, positive and statistically significant (rs(124)=0.435, p<0.05), meaning that the social behaviour of patients too influences the release of IL-6 and both smokers and alcoholic patients were more likely to have higher IL-6 levels than non-smokers and non-alcoholic patients (Tables 3 and 4).
| Characteristic | IL 17A | IL 2 | IL 4 | IL 6 |
|---|---|---|---|---|
| BMI | r(124) = 0.223 p = 0.070 |
r(124) = -0.334 p = 0.418 |
r(124) = -0.301 p = 0.699 |
r(124) = -0.187 p = 0.136 |
| Hypertension status | rs(124) = -0.248* p = 0.043 |
rs(124) = 0.247 p = 0.555 |
rs(124) = 0.258 p = 0.742 |
rs(124) = -0.137 p = 0.277 |
| Social behaviour | rs(124) = -0.387* p = 0.018 |
rs(124) = 0.802 p = 0.055 |
rs(124) = . p = . |
rs(124) = 0.435* p = 0.014 |
| Stress levels | rs(124) = -0.161 p = 0.196 |
rs(124) = -0.436 p = 0.280 |
rs(124) = 0.949 p = 0.051 |
rs(124) = 0.024 p = 0.849 |
Spearman’s Rank-Order Correlation coefficient was used.
**p<0.01; *p<0.05
Table 3: Association of Participants’ BMI, Hypertension status, Social behavior & Stress levels and selected cytokines; IL- 17A, IL- 2, IL- 4 & IL- 6.
| Characteristic | IL 8 | IL 10 | TNFa | IFNy |
|---|---|---|---|---|
| BMI | r(124) = -0.085 p = 0.357 |
r(124) = -0.475 p = 0.140 |
r(124) = -0.190 p = 0.684 |
r(124) = -0.663 p = 0.152 |
| Hypertension status | rs(124) = 0.051 p = 0.581 |
rs(124) = 0.346 p = 0.297 |
rs(124) = 0.632 p = 0.127 |
|
| Social behavior | rs(124) = 0.360** p = 0.003 |
rs(124) = 0.949 p = 0.051 |
||
| Stress levels | rs(124) = -0.082 p = 0.382 |
rs(124) = -0.362 p = 0.274 |
rs(124) = -0.612 p = 0.144 |
rs(124) = 0.239 p = 0.648 |
Spearman’s Rank-Order Correlation coefficient was used
**p<0.01; *p<0.05
Table 4: Association of Participants’ selected characteristics of participants and cytokines (IL 8, IL 10, and TNF α, IFN γ)
The social behaviour of the patients and IL-8 showed a moderate, positive and statistically significant association (rs(124)=0.360, p<0.01). This showed that social behaviour of patients influences the release of IL-8 and both smokers and alcoholic patients were more likely to have higher IL-8 levels than non-smokers and non-alcoholic patients (Table 5).
| Characteristic | IL 17A | IL 2 | IL 4 | IL 6 |
|---|---|---|---|---|
| ART use | rs(124) = 0.176 p = 0.154 |
rs(124) = 0.142 p = 0.261 |
||
| WHO stage | rs(124) = -0.003 p = 0.980 |
rs(124) = 0.802 p = 0.055 |
rs(124) = 0.447 p = 0.553 |
rs(124) = 0.182 p = 0.172 |
| CD4 + cell counts | r(124) = 0.187 p = 0.130 |
r(124) = -0.518 p = 0.189 |
r(124) = -0.151 p = 0.849 |
r(124) = -0.268* p = 0.031 |
| Creatinine | r(124) = 0.049 p = 0.714 |
r(124) = 0.132 p = 0.777 |
r(124) = 0.550 p = 0.450 |
r(124) = 0.285* p = 0.033 |
| HCT | r(124) = 0.175 p = 0.234 |
r(124) = 0.660 p = 0.106 |
r(124) = -0.479 p = 0.682 |
r(124) = 0.031 p = 0.837 |
Spearman’s Rank-Order Correlation coefficient was used
**p<0.01; *p<0.05
Table 5: Association of Participants’ selected characteristics and cytokines (IL 17-A, IL-2, IL-4 & IL- 6) levels.
Cytokine expression and participant clinical parameters
The association between CD4+ cell counts of the patients and IL-6 was mild, negative and statistically significant (rs(124)=-0.268, p<0.05). This observation implies that the CD4+ cell counts of patients influences the release of IL-6 and patients with lower CD4+ cell counts were more likely to have higher IL-6 levels than patients with higher CD4 +cell counts (Table 5). On the other hand a higher level of IL-6 predisposes the patient to development of arteriosclerosis and increases risk of hypertension.
The association between serum creatinine values of the patients and IL- 6 was mild, positive and statistically significant (rs(124)=0.285, p<0.05), demonstrating that the creatinine levels of patients influenced the release of IL-6 and patients with higher creatinine values were more likely to have higher IL-6 levels than patients with lower creatinine values (Table 5).
The association between creatinine values of the patients and IL-8 too was mild, positive and statistically significant a (rs(124)=0.191, p<0.05). This demonstrates that creatinine values of patients’ influences the release of IL-8 and patients with higher creatinine values were more likely to have higher IL-8 levels than patients with lower creatinine values (Table 6). High creatinine levels on the other hand, is an indicator of poor kidney function and a predisposing factor to development of hypertension in HIV positive patients.
| Characteristic | IL 8 | IL10 | TNFa | IFNy |
|---|---|---|---|---|
| ART use | rs(124) = 0.023 p = 0.803 |
rs(124) = 0.400 p = 0.223 |
||
| WHO stage | rs(124) = 0.124 p = 0.203 |
rs(124) = -0.685* p = 0.042 |
rs(124) = -0.711 p = 0.073 |
rs(124) = 0.105 p = 0.895 |
| CD4 counts | r(124) = -0.178 p = 0.053 |
r(124) = -0.247 p = 0.464 |
r(124) = -0.369 p = 0.415 |
r(124) = -0.334 p = 0.517 |
| Creatinine | r(124) = 0.191* p = 0.049 |
r(124) = -0.197 p = 0.611 |
r(124) = 0.022 p = 0.967 |
r(124) = -0.440 p = 0.458 |
| HCT | r(124) = -0.114 p = 0.288 |
r(124) = 0.270 p = 0.518 |
r(124) = 0.218 p = 0.638 |
r(124) = 0.785 p = 0.116 |
Spearman’s Rank-Order Correlation coefficient was used
**p<0.01;*p<0.05
Table 6: Association of Participants’ selected characteristics and cytokines’ levels; IL-8, IL-10, TNF-α and IFN-γ.
The association between WHO HIV/AIDS staging of the patients and IL-10 was strong, negative and statistically significant (rs(124)=- 0.685, p<0.05), so we can infer that the WHO staging of patients influenced the release of IL-10 and patients with early WHO HIV/ AIDS staging were more likely to have increased IL-10 levels than patients with advanced WHO HIV/AIDS staging (Table 6).
Correlation between IL-6 & IL-8 expressions in participants
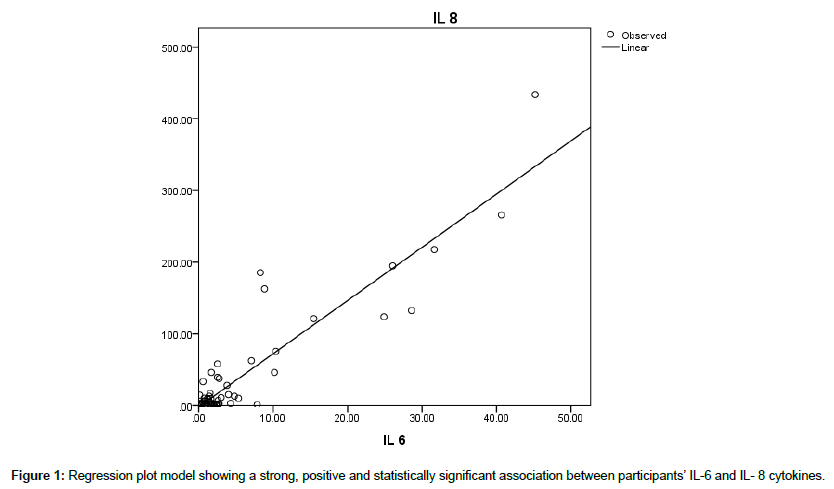
The association between IL-6 and IL-8 cytokines values was strong, positive and statistically significant (rs(124)=0.917, p<0.001). This observation demonstrates that the IL-6 cytokine expression influences the release of IL-8 cytokines; and patients with higher IL-6 cytokines values were more likely to have higher IL-8 cytokines levels than patients with lower IL-6 cytokines values (Table 7). This presents a synergistic effect as both cytokines have an effect on development of atherosclerosis and risk of development of hypertension in HIV positive patients. A Curve Estimation Linear Regression model too elicited a statistically significant association between IL-6 and IL-8 cytokines, p<0.001 (Figure 1).
| Characteristic | Hypertension status | IL 17A | IL 6 |
|---|---|---|---|
| IL 17A | rs(124) = -0.248* p = 0.043 |
- | - |
| IL 6 | rs(124) = -0.137 p = 0.277 |
r(124) = -0.265 p = 0.099 |
- |
| IL 8 | rs(124) = 0.051 p = 0.581 |
r(124) = -0.153 p = 0.234 |
r(124) = 0.917** p < 0.001 |
**p<0.01; *p<0.05
Table 7: Association of Participants’ selected characteristics and cytokines’ levels; (IL-17A, IL-6 and IL-8).
Participant hypertension status and mean distribution of cytokines expression
The association between hypertension status of the patients and IL 2 was mild, negative and statistically significant (rs(124)=-0.185, p<0.05). This observation demonstrates that hypertension status of participants’ influences levels of their IL-2 cytokines and that hypertensive participants are more likely to have higher IL-2 cytokines counts than non-hypertensive counterparts (Table 1). A high level of IL-2 in hypertension means poor sensitization to presence of foreign antigens in the body and thus poor immunity. The association between hypertension status of the patients and IL 6 was mild, negative and statistically significant (rs(124)=-0.256, p<0.01), thus we can infer that the hypertension status of the participants’ influenced levels of their IL-6 cytokines and that hypertensive participants’ are more likely to have higher IL-6 cytokines levels (Table 1).
The association between hypertension status of the patients and INF γ was mild, negative and statistically significant (rs(124)=-0.217, p<0.05), so we can infer that the hypertension status of the participants’ influenced levels of their INF-γ and that hypertensive participants’ are more likely to have higher INF-γ than non-hypertensive participants (Table 1).
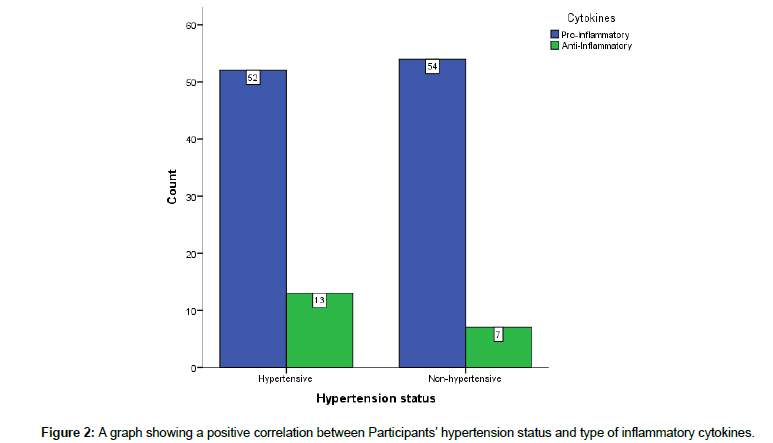
The study did not demonstrate a statistically significant association between hypertension status, pro-inflammatory and anti-inflammatory cytokines of the participants (χ2 test; p=0.228). This observation meant that there is no clear directional influence on a group of cytokines but rather hypertension disorder influences individual cytokines (Figure 2).
Monitoring of IFN-γ as a potential marker of risk of hypertension comorbidity in HIV disease
On determination of the most reliable cytokine to establish risk of hypertension in HIV disease, INF-γ was the only cytokine type that elicited both statistically significant mean differences between hypertensive (M=6.87, SD=35.66) and non-hypertensives participants (M=0.00, SD=0.00), t(124)=1.553, p>.05 and a statistically significant association between the groups (rs(124)=-0.217, p<0.05). This observation demonstrates that INF-γ could be the most reliable cytokine type for determining the participants’ risk of developing hypertension in HIV disease (Table 1).
INF-γ has a statistically significant mean differences between hypertensive and non- hypertensive participants (p<0.003) and a negative correlation with hypertension status (rs=-0.217, p<0.015).The association of IFN-γ and hypertension status is positive and constant thus monitoring INF-γ expression in HIV patients is potentially useful in determining risk of developing hypertension in this population.
The other cytokines with statistically significant mean differences in hypertensive and non- hypertensive groups of participants are IL- 17A and IL-2 (p<0.001) respectively. However, IL-17A has a mild negative non - statistically significant correlation with hypertension status (rs=-0.173, p=0.052). IL2 and IL6 both presented a mild negative statistically significant correlation with hypertension status (rs=-0.185, p=0.038; rs=-0.256, p=0.004) respectively.
There was an increase in BMI levels and CD4+ cell counts for the pro-inflammatory cytokines than for anti-inflammatory cytokines, while an increase in serum creatinine and haematocrit (HCT) measures favoured expression of anti-inflammatory cytokines. The shift in cytokine type expression is dependent on the clinical characteristics of participants (Table 8).
| Characteristic | BMI | CD4 Counts | Creatinine | HCT |
|---|---|---|---|---|
| Cytokines | rs(124) = -0.024 p = 0.791 |
rs(124) = -0.082 p = 0.359 |
rs(124) = 0.019 p = 0.873 |
rs(124) = 0.036 p = 0.728 |
**p<0.01
*p<0.05
Table 8: Association of Participant Clinical characteristics and (Pro-Inflammatory and Anti-Inflammatory) cytokines.
BMI on the other hand had a mild negative non- statistically significant correlation with cytokine release among participants (rs=-0.024, p<0.791). This observation implies that increasing BMI, an indicator of improved and normal immune function is a marker of reduced inflammation among participants. CD4+ cell count on the other hand has similar effect as that of BMI on cytokine release (p<0.359)
Serum creatinine and HCT had a mild positive non-statistically significant correlation(s) with cytokine release among the participants. This suggests that increase in creatinine levels affect partially the release of cytokines because itself is a marker of poor kidney function (p<0.728).
There was no statistically significant difference in the expression of inflammatory and anti- inflammatory cytokines between the hypertensive and non-hypertensive participants (p<0.23). The WHO HIV/AIDS stage of participants too did not present any statistical significance in expression of inflammatory nor non- inflammatory cytokines among the hypertensive and non- hypertensive participants respectively (p<0.49).
Discussion
A hypertension prevalence of 23.2% established in this study is similar to findings from other related studies. This observation increases the patients’ morbidity resulting in compounded inflammation and poly pharmacy [25,26].
The risk of developing hypertension in HIV disease may be predicted by periodic analysis of cytokine profiles of HIV patients. The dominance of either humoral or cellular immune response in respect to the strength of TH1 or TH2 expression, presents an increased risk of metabolic disorders and subsequently complications associated with it which include hypertension.
It was observed in this study that advanced progression of HIV disease as marked by low CD4+ cell count and Th2 cytokine expression is a culmination of dysfunctional immune response and marked endothelial injury that predisposes patients to cardiovascular disease [27]. Dominant cellular immunity on its own does cause much damage as it reacts to existing microbial invasion resulting in net effect of adjacent tissue destruction. The resultant endothelial injury predisposes patients to organ failure due to chronic injury.
The study demonstrated statistically significant increase in the production of IL-6 and IL-17A which have been demonstrated in other studies to cause renal and vascular dysfunction and lead to blood pressure elevation [28]. Hypertension comorbidity may present as a primary complication of inflammatory process on endothelial linings of cardio vasculature or secondary to metabolic syndrome.
Contrary to findings of a comparative study that showed that hypertensive immunosuppressed patients had lower IL-10 compared to non- hypertensive immunosuppressed cases [29,30]. The observation in this study was statistically insignificant, P<0.886. This may be a pointer of altered immune response to HIV virus in participants. This phenomenon allows HIV virus to thrive in its replication processes, transmission and progression.
Higher BMI readings had a positive correlation with expression of IL-6 in this study. This observation is similar to findings in related studies with marked expression of IL-6 being linked to atherosclerosis. Initial increase in BMI in HIV positive patients presents a positive improvement of a patient on care but on the other hand it presents the risk of development of hypertension.
Conclusion
The variation in either TH1 or TH2 cytokine expression affects the pattern of change in clinical parameters of HIV patients on care, response to cART and predisposition to metabolic disorders.
Monitoring of CD4+ Cell count and viral load has been the main pointers of extent of HIV/AIDS progression and health status of a patient. However, the risk of occurrence of metabolic disorders is not predicted by these clinical parameters. Therefore, monitoring and analysis of cytokine expression may help to predict patients’ pathways in their response to cART therapy and risk of metabolic disorders.
This study demonstrated that the IL-6 cytokine expression have a positive correlation with the release of IL-8 cytokine as patients with higher IL-6 cytokine values were more likely to have higher IL-8 cytokine levels. This shows that a synergistic effect from both cytokines may accelerate the development of atherosclerosis on blood vessels. Therefore routine measurement of these cytokines may increase prediction of risk of development of hypertension in patients on HIV care.
IFN-γ levels too was observed to independently depict the risk of hypertension in patients on cART and is too a potential marker to predict patients’ susceptibility.
IFN-γ, IL-6 and IL-8 increase on longitudinal follow up is highly diagnostic of increased risk of developing hypertension. These cytokines requires further scientific establishment of the actual link with hypertensive disease as well as means to prevent and control their pathological effects on cardiovascular structures.
References
- Salter ML, Lau B, Mehta SH, Go VF, Leng S, et al. (2013) Correlates of elevated interleukin-6 and C-reactive protein in persons with or at high-risk for HCV and HIV infections. J Acquir Immune Defic Syndr.
- Armah KA, McGinnis K, Baker J, Gibert C, Butt AA, et al. (2012) HIV status, burden of comorbid disease, and biomarkers of inflammation, altered coagulation, and monocyte activation. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 55: 126-136.
- Armah KA (2013) Prehypertension, Hypertension, and the Risk of Acute Myocardial Infarction in HIV-Infected and -Uninfected Veterans. Clin Infect Dis 58: 121-129.
- Salter ML, Lau B, Go VF, Mehta SH, Kirk GD. (2011) HIV infection, immune suppression, and uncontrolled viremia are associated with increased multimorbidity among aging injection drug users. Clin Infect Dis 53: 1256-1264
- Akbaraly TN, Shipley MJ, Ferrie JE, Virtanen M, Lowe G, et al. (2015) Long-term adherence to healthy dietary guidelines and chronic inflammation in the prospective Whitehall II study. Am J Med 128: 152-160 e4.
- Vaucher J, Marques-Vidal P, Waeber G, Vollenweider P (2014) Cytokines and hs-CRP levels in individuals treated with low-dose aspirin for cardiovascular prevention: a population-based study (CoLaus Study). Cytokine 66: 95-100.
- Peck RN, Shedafa R, Kalluvya S, Downs JA, Todd J, et al., (2014) Hypertension, kidney disease, HIV and antiretroviral therapy among Tanzanian adults: a cross-sectional study. BMC Med 12: 125.
- Aburawi EH, AlKaabi J, Zoubeidi T, Shehab A, Lessan N, et al. (2016) Subclinical Inflammation and Endothelial Dysfunction in Young Patients with Diabetes: A Study from United Arab Emirates. PLoS One 11: e0159808.
- Lukic L, Lalic NM, Rajkovic N, Jotic A, Lalic K, et al. (2014) Hypertension in obese type 2 diabetes patients is associated with increases in insulin resistance and IL-6 cytokine levels: potential targets for an efficient preventive intervention. Int J Environ Res Public Health 11: 3586-3598.
- Oliveira JM, Rondó PH, Lima LR, Fortun ES, Yudkin JS (2015) Effects of a Low Dose of Fish Oil on Inflammatory Markers of Brazilian HIV-Infected Adults on Antiretroviral Therapy: A Randomized, Parallel, Placebo-Controlled Trial. Nutrients 7: 6520-6528.
- Matsuo K, Hasegawa K, Yoshino K, Murakami R, Hisamatsu T, et al. (2015) Venous thromboembolism, interleukin-6 and survival outcomes in patients with advanced ovarian clear cell carcinoma. Eur J Cancer 51: 1978-1988.
- Su D, Li Z, Li X, Chen Y, Zhang Y, et al. (2013) Association between serum interleukin-6 concentration and mortality in patients with coronary artery disease. Mediators Inflamm 2013: 726178.
- Jin SG, Chen GL, Yang, Zhao MY (2015) Gene-gene interactions among CX3CL1, LEPR and IL-6 related to coronary artery disease in Chinese Han population. Int J Clin Exp Pathol 8: 5968-5973.
- Ribeiro V, Bosquetti B, Gonçalves SM, Bucharles SG, Rempel L, et al. (2014) Uremic serum inhibits in vitro expression of chemokine SDF-1: impact of uremic toxicity on endothelial injury. J Bras Nefrol 36: 123-131
- Saadoun D, Garrido M, Comarmond C, Desbois AC, Domont F, et al. (2015) Th1 and Th17 cytokines drive inflammation in Takayasu arteritis. Arthritis Rheumatol 67: 1353-1360.
- Liebetrau C, Hoffmann J, Dörr O, Gaede L, Blumenstein J, et al., (2015) Release kinetics of inflammatory biomarkers in a clinical model of acute myocardial infarction. Circ Res 116: 867-875.
- Fedacko J, Singh RB, Gupta A, Hristova K, Toda E, et al. (2014) Inflammatory mediators in chronic heart failure in North India. Acta Cardiol 69: 391-398.
- Collier P, Watson CJ, Voon V, Phelan D, Jan A, et al. (2011) Can emerging biomarkers of myocardial remodelling identify asymptomatic hypertensive patients at risk for diastolic dysfunction and diastolic heart failure? Eur J Heart Fail 13: 1087-1095.
- Holmes MV, Dale CE, Zuccolo L, Silverwood RJ, Guo Y, et al., (2014) Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. BMJ 349: 4164.
- Makrilakis K, Fragiadaki K, Smith J, Sfikakis PP, Kitas GD (2015) Interrelated reduction of chemerin and plasminogen activator inhibitor-1 serum levels in rheumatoid arthritis after interleukin-6 receptor blockade. Clin Rheumatol 34: 419-427.
- Kim M, Jung S, Kim SY, Lee SH, Lee JH (2014) Prehypertension-associated elevation in circulating lysophosphatidlycholines, Lp-PLA2 activity, and oxidative stress. PLoS One 9: e96735.
- Wu N, Xu B, Xiang Y, Wu L, Zhang Y, et al. (2013) Association of inflammatory factors with occurrence and recurrence of atrial fibrillation: a meta-analysis. Int J Cardiol 169: 62-72.
- Xie Y, Li M, Wang X, Zhang X, Peng T, et al. (2013) In vivo delivery of adenoviral vector containing interleukin-17 receptor a reduces cardiac remodeling and improves myocardial function in viral myocarditis leading to dilated cardiomyopathy. PLoS One 8: e72158
- Chastain DB, King TS, Stover KR (2016) Infectious and Non-infectious Etiologies of Cardiovascular Disease in Human Immunodeficiency Virus Infection. Open AIDS J 10: 113-126.
- Chastain DB, Henderson H, Stover KR (2015) Epidemiology and management of antiretroviral-associated cardiovascular disease. Open AIDS J 9: 23-37.
- de Larrañaga GF, Petroni A, Deluchi G, Alonso BS, Benetucci JA (2003) Viral load and disease progression as responsible for endothelial activation and/or injury in human immunodeficiency virus-1-infected patients. Blood Coagul Fibrinolysis 14: 15-18.
- Harrison DG, Marvar PJ, Titze JM (2012) Vascular inflammatory cells in hypertension. Front Physiol 3: 128.
- Gorenec L, Zidovec Lepej S, Grgic I, Planinic A, Iscic Bes J, et al. (2016) The comparison of Th1, Th2, Th9, Th17 and Th22 cytokine profiles in acute and chronic HIV-1 infection. Microb Pathog 97: 125-130.
- de Medeiros RM., Valverde-Villegas JM, Junqueira DM, Graf T, Lindenau JD, et al. (2016) Rapid and Slow Progressors Show Increased IL-6 and IL-10 Levels in the Pre-AIDS Stage of HIV Infection. PLoS One 11: e0156163.
- McKay HS, Bream JH, Margolick JB, Martínez-Maza O, Phair JP, et al. (2016) Host factors associated with serologic inflammatory markers assessed using multiplex assays. Cytokine 85: 71-79.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi