Research Article, J Plant Physiol Pathol Vol: 6 Issue: 5
Disease Progression and Productivity of Melon Plants with Different Cultural Management Techniques
Mateus Sunti Dalcin*, Paulo Henrique Tschoeke, Dalmarcia de Souza Carlos Mourão, Pedro Raymundo Argüelles Osorio, Raimundo Wagner de Sousa Aguiar, Alex Sander Rodrigues Cangussu and Gil Rodrigues dos Santos
Federal University of Tocantins, Gurupi Campus, 77402-970 Gurupi, TO, Brazil
*Corresponding Author : Mateus Sunti Dalcin
Federal University of Tocantins, Gurupi Campus, 77402-970 Gurupi, TO, Brazil
Tel: +55 63 999664960
E-mail: m2d@uft.edu.br
Received: September 07, 2018 Accepted: September 25, 2018 Published: October 02, 2018
Citation: Dalcin MS, Tschoeke PH, Mourão DSC, Osorio PRA, Aguiar RWS, et al. (2018) Disease Progression and Productivity of Melon Plants with Different Cultural Management Techniques. J Plant Physiol Pathol 6:5. doi: 10.4172/2329-955X.1000188
Abstract
Studies of several agricultural crops have been concluded with the aim of achieving greater environmental balance by reducing the use of pesticides, as well as promoting the viability of cultural management techniques that diminish the proliferation of phytopathogens. In the melon growing industry, studies of this sort are scarce. Therefore, the objective of this work was to evaluate the influence on disease progression and melon crop productivity of various production systems, including fertilization, spacing, pesticide use, and pruning of branches and culling of fruits. Organic fertilization was efficient for the management of phytopathogens due to the presence of antagonistic microorganisms. Minimizing pesticide use did not affect crop productivity.
Keywords: Cucumis melo L.; Production systems; Cultural control; Stagonosporopsis cucurbitacearum; Fusarium oxysporum f. sp. melonis
Introduction
Currently, varieties of melon adapt to various regions such that they maintain their productive characteristics, providing stability in terms of fruit quality as well as providing greater profit to the farmers.
Despite the economic importance of the melon crop to Brazil, melon productivity can be considered low compared to its productive potential, and the various producing regions are affected by several factors [1-3] Climatic factors such as temperature, relative humidity, precipitation and solar radiation exert influence on growth, development, and fruit quality and melon productivity. The melon is a typical plant of regions of warm climate, requiring, for its development and production, a temperature above 20°C [4]. Therefore, research is required to define the optimal management technologies capable of increasing productivity and quality, allowing Brazilian melon production to compete on an equal footing in the national and international markets.
Among the biotic factors affecting melon growth is gummy stem blight, or canker of the stem, caused by the fungus Stagonosporopsis cucurbitacearum (Fr.) Aveskamp, Gruyter and Verkley, also known as Didymella bryoniae (Auersw.) Rehm [5]. The disease causes seedling tipping, leaf lesions and cankers of stems and stalks, compromising the development of plants and reducing yield and fruit quality [6]. Another disease, known as Fusarium wilt, caused by the fungus Fusarium oxysporum f. sp. melonis (Leach and Currence) Snyd and Hans is also responsible for serious economic damage [7]. The pathogen is difficult to control because it survives in soil in resistant structures, as well as in crop residues and in infected seeds [8].
Due to the seriousness of the damage caused by these diseases, a proper management is necessary for the development of plants and improvement of crop production. Although chemicals are the most widely used form of treatment. Continuous use of fungicides is not advisable as a long-term solution because of the negative effects of pesticides on the environment [9] and increasing pathogen resistance to chemical treatments [10,11].
To reduce the use of fungicides, it is necessary to use more efficient applications and cultural techniques that minimize the environment conducive to the development of pathogens. According to the literature, there are few studies evaluating the effects of various management techniques and the progression of melon diseases.
The objective of this work was to develop control methods that minimize the use of fungicides, creating a rational management of the crop that hinders the proliferation of diseases, maintaining an acceptable level of productivity and fruit quality.
Materials and Methods
Local and climatic conditions
Were conducted two trials in an area located in the phytosanitary sector of the Federal University of Tocantins-UFT, municipality of Gurupi (11°44’44.866”S; 49°3’8.968”W 278 m altitude), State of Tocantins, from July to September 2014 and June to September 2015. The area is found in the Cerrado biome and the soil is classified as dystrophic red-yellow latosol [12] with medium texture.
According to the Köppen classification, the region’s climate is Aw, defined as tropically hot and humid with rainy seasons in summer and dry seasons in winter. The average annual temperature is around 26°C. Average temperature range is very small, with a monthly average temperature of at least 20°C and a maximum monthly average of 33°C. The average annual precipitation is 1,632 mm, with the highest rainfall from October to March and lowest from April to September [13]. Were conducted the initial soil preparation with two heavy harrows and a leveling harrow, and we used a rotary hoe for the survey of the beds (0.70 m width at the upper base, 0.90 m width at the lower base and height 0.30 m). The beds were protected with double-sided plastic canvas (black/white) with a thickness of 150 μm, leaving the white face exposed were employed drip irrigation (3.6 L hour-1) aiming to achieve field capacity and fixed watering. Were sowed five seeds per pit at 2 cm depth, and performed plant thinning at fifteen days, leaving one plant per pit. The cultivar used was the Hibrix® hybrid.
Characterization of the treatments
The experimental design was a randomized complete block design with four replicates and five treatments (production systems):
T1 – Conventional System I (CS I): Planting spacing 2 × 0.5 m. Fertilization at 1000 kg ha-1 with formulation 5-25-15 (N-P-K), 262 kg ha-1 potassium chloride (60% K) and 454 kg ha-1 urea (44% N) total coverage, divided in four parts. We applied the recommended commercial doses of fungicides Cerconil® (methyl thiophanate, 0.7 g L-1+chlorothalonil, 1.7 g L-1) and Score® (difenoconazole, 0.3 g L-1) and the insecticides Decis® (deltamethrin, 1.0 ml L-1) and Evidence® (imidacloprid, 0.3 g L-1) weekly. There was no thinning.
T2 – Conventional System II (CS II): similar to T1, differing only in that were maintained two branches and two fruits per plant.
T3 – Integrated Production System I (IPS I): Planting spacing 2 × 1 m, fertilization according to the soil analysis, where 640 kg ha-1 of 5-25-15 (N-P-K) was applied to the plantings in both years, 114.3 kg potassium chloride (60% K) and 318 kg urea (44% N), divided in four parts. Two branches were maintained per plant and all fruits were retained. Cerconil® (methyl thiophanate, 0.7 g L-1+chlorothalonil, 1.7 g L-1) and Score® (difenoconazole, 0.3 g L-1) and insecticides Decis® (deltamethrin, 1.0 ml L-1) and Evidence® (imidacloprid, 0.3 g L-1) was applied. Were monitored every three days, recording the incidence of diseases and pests according to the norms established for collection and respecting the level of damage defined for each pest according to the relevant literature.
T4 – Integrated Production System II (IPS II): similar to T3, differing in that were maintained only two fruits per plant.
T5 – Organic Production System (OPS): Plant spacing 2 × 0.5 m, fertilization with 20 t ha-1 cattle manure, without application of pesticides, leaving two branches and two fruits per plant. Characterization of manure: pH: 7.8; (K: 1200; P: 357.2) mg dm-3; (Ca: 4.7; Mg: 7.6) cmolc dm-3; M.O.: 11 dag kg-1. Manure was collected to analyze the presence of microorganisms. Were added 99.0 ml of sterilized distilled water to the organic sample, obtaining a stock solution of 10 g L-1. Each sample was homogenized for 5 minutes with a magnetic stirrer at 150 rpm. Subsequently, we added 1.0 mL of the stock solution to 99.0 mL of sterilized distilled water to give a final dilution of 10-3, i.e., one gram of soil per 99 ml sterilized distilled water. From this final concentration 1.0 mL was pipetted and deposited in the center of Petri dishes containing potato-dextrose-agar (PDA) culture medium. The solution was spread on the surface of the culture medium and the plates were sealed, identified and incubated at 25°C ± 2°C with photoperiod of 12 hours for 7 days. After this time, we identified the fungi by magnifying glass and common optical microscope. Bacteria grown in PDA medium were spiked into Luria Bertani medium (LB) in test tubes and placed in a shaker at 200 rpm for 72 hours. Then Gram-stained slides were prepared to identify the bacteria.
Disease evalution
To evaluate the progression of diseases in melon plants, we recorded the severity in relation to the time, and the plants were evaluated every seven days using a scoring scale described by Santos et al. [14] as follows: 0 – healthy plants ; 1 – plants with less than 1% of the leaf area affected; 3 – plants with 1- 5% of the leaf area affected; 5-plants with 6-25% of the leaf area affected ; 7 – plants with 26-50% of the leaf area affected; 9 – plants with more than 50% of the leaf area affected. Subsequently, the scores attributed to the disease in the leaves were converted to percentages of diseased leaf area by the midpoint of each score. The number of evaluations of each trial was set according to the onset of disease. At the end of the evaluations, the severity data were converted into the Area under Disease Progression Curve (AUDPC), according to the method described by Shaner and Finney [15].
Data analysis
The fruits were harvested and weighed, and we calculated the total soluble solids (TSS) content. Productivity was expressed as t ha-1 and TSS in °Brix. The data were subjected to analysis of variance (ANOVA) and Tukey’s test was applied at 5% probability for comparison of means. T test was used to comparing estimates for contrasts of interest.
Results and Discussion
Disease incidence
In 2014, in July and September the temperature ranged between 15.2°C and 37.7°C, and the relative humidity of the air ranged from 48.7% to 66.8%. During the course of the test, cumulative precipitation of 65 mm was found distributed in several phases of the culture cycle.
During the 2015 growing season, the temperature ranged from 14.6°C to 38.9°C and relative humidity ranged from 44.9% to 71.7%. During this period, there was no rain during the test period.
The climatic variables in 2014 prevalent at 65 days after planting (DAP), favored the appearance of gummy stem blight caused by the fungus Stagonosporopsis cucurbitacearum. These climatic conditions favored the infection of the pathogen and allowed the progression of the disease during most of the treatments until the harvest period.
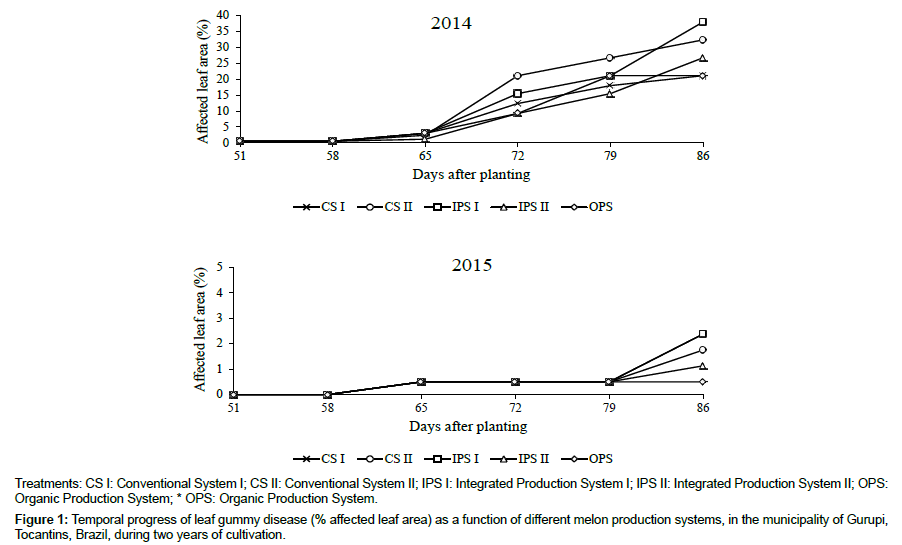
The severity of gummy stem blight on the leaves (Figure 1) increased in all treatments at 65 DAP, when the first rains occurred, causing initial infections in the plant leaves. The maximum final values of affected leaf area (at 86 DAP) ranged from 21.1% for Conventional System I and Organic Production System to 38% for Integrated Production System I. Keinath [16], observed that there was infection despite irregular leaf wetting, and the pathogen was capable of causing an increase in the severity of the disease in the plant. This phenomenon was also described by Santos et al. [14]. They reported that, during periods unfavorable to the disease when water was present only for a short period of time, there was sufficient water for the fungus to penetrate through natural openings of leaves and branches, or through caused different injuries. These authors commented that under these conditions, the values of affected leaf area did not exceed 40% in the treatments most affected. Dalcin et al. [17] found that the severity of this disease increased even at the end of the crop cycle, when the melon fruits had virtually completely formed. Rennenberger and Keinath [18] also demonstrated the high capacity of Stagonosporopsis to infect several genera of cucurbitaceae.
Figure 1: Temporal progress of leaf gummy disease (% affected leaf area) as a function of different melon production systems, in the municipality of Gurupi, Tocantins, Brazil, during two years of cultivation.
In the experiment conducted in 2015, we also detected gummy stem blight on the leaves, however, it was of low severity in the plant leaves (Figure 1). The area under the disease progression curve did not differ significantly (P>0.05) between treatments compared within each year (Table 1). However, different values are observed between harvests, with the year 2015 having lower values of the AULBPC.
| Production System | 2014 | 2015 | ||
|---|---|---|---|---|
| AULBPC Leaf Blight | DPSB (%) Stem Blight | AULBPC Leaf Blight | DPF (%) Fusariosis | |
| CS I | 117.3 a | 20 | 28.8 a | 18 |
| CS II | 131.3 a | 15 | 28.0 a | 35 |
| IPS I | 129.5 a | 28 | 29.8 a | 30 |
| IPS II | 105.0 a | 50 | 26.3 a | 28 |
| OPS | 117.3 a | 0 | 24.5 a | 0 |
| CV% | 10.47 | 12.01 | ||
Means followed by the same lowercase letter in the column do not differ statistically from each other by the Tukey test at the 5% probability level.
Table 1: Area Under Leaf Blight Progress Curve (AULBPC), percentage of dead plants by stem blight (DPSB), and dead plants by fusariosis (DPF) in different melon production systems, in Gurupi, Tocantins, Brazil.
Santos et al. [10] reported that the pathogen Didymella bryoniae (S. cucurbitacearum) infected and damaged not only to stem but also the leaves of the plants. This was influenced by leaf wetness, an essential condition for the development of the disease. The value of AULBPC was measured by leaf severity assessment showed higher levels of the disease in 2014, when the disease occurred more severely in the leaves. In the same year, the disease also occurred in the stems, causing death in all treatments, except in the treatment with organic fertilization where there was high infestation of Bacillus and Trichoderma. Lecomte et al. [19] and Cao et al. [20] assert that the biological control of Fusarium oxysporum through these microorganisms is efficient.
The lack of difference in control of the disease in leaves due to the application of fungicides among the treatments in 2014 can be explained by the considerable resistance of S. cucurbitacearum to fungicides reported by several authors [10,11].
In 2015, there was a low incidence of gummy stem blight on the leaves, however, there was a high incidence of Fusarium wilt, caused by the fungus Fusarium oxysporum f. sp. melonis (Leach and Currence) Snyd and Hans. With regard to spatial distribution, the disease was distributed in sectors within the experimental plots. There were wilted and dead plants already on 30 DAP, extending from the period of flowering until 50 DAP, with fruits in development. Yang et al. [21] observed that melon root exudates significantly promoted spore germination and mycelial growth of F. oxysporum f. sp. melonis resulting in a high incidence of fusariosis, thus confirming the influence of the roots of the plants on the development of the pathogen. This fact may lead us to infer that successive melon cultivation in the same area may have stimulated and/or favored the high incidence of this pathogen in plants.
On the other hand, observing the results obtained in relation to the dead plants by Fusarium and/or Stagonosporopsis, we believe that fertilization with manure probably contributed to the suppression of these pathogens in root and stem tissues. Organic fertilization made the environment favorable to the development of fungal and antagonistic bacteria, inhibiting the development of soil-dwelling pathogens such as those of the genus Fusarium spp. [22-24] and D. bryoniae [25]. In the present study, was evaluated microbiota in manure and found microorganisms considered antagonists such as fungi of the genus Trichoderma sp. and bacteria of the genus Bacillus sp. This agrees with results described in the literature [26,27]. Thus, believe that organic fertilization with bovine manure was effective in controlling plant mortality, both by S. cucurbitacearum and by F. oxysporum.
Agronomic evaluation
The results obtained in the two trials in different years suggested that the different levels of disease incidence in the plants may have influenced agronomic characteristics and crop yields (Table 2).
| Production System | 2014 | 2015 | ||
|---|---|---|---|---|
| PROD (t ha-1) |
AFW (kg) | PROD (t ha-1) | AFW (kg) | |
| CS I | 15.6 a | 0.622 a | 17.8 ab | 0.85 abc |
| CS II | 14.4 ab | 0.899 a | 12.9 ab | 1.02 ab |
| IPS I | 10.3 ab | 0.763 a | 16.2 ab | 0.80 bc |
| IPS II | 8.2 b | 0.975 a | 18.7 a | 1.09 a |
| OPS | 15.2 a | 0.859 a | 11.2 b | 0.71 c |
| CV% | 22.13 | 19.13 | 20.91 | 12.13 |
| Estimates for contrasts of interest | ||||
| CS I vs CS II | 1.2ns | -0.277* | 5.0ns | -0.1634ns |
| IPS I vs IPS II | 2.1ns | -2.213ns | -2.5ns | -0.2882* |
Means followed by the same lowercase letter in the column do not differ statistically from each other by the Tukey test at the 5% probability level. *Significant at 5% probability in the T test. ns: not significant.
Table 2: Average productivity (PROD) and average fruits weight (AFW) of melon as a function of different production systems in two years of cultivation, in Gurupi, Tocantins, Brazil.
In the 2014 harvest, lower productivity among treatments was obtained by IPS II, producing only 8.2 t ha-1, significantly different (P<0.05) from those of CS I and OPS with 15.6 t ha-1 and 15.2 t ha-1, respectively. The mortality of plants caused by the fungus Stagonosporopsis severely affected the productivity of this treatment. Santos et al. [28] stated that gummy stem blight caused large reductions in yield and fruit quality. In the 2015 crop, the conventional systems maintained productivity stability. In IPS II treatment, there was an increase in productivity due to a decrease in plant mortality. Greater number of plants meant higher production. Were observed that a greater application of pesticides in the conventional treatments (CS I, CS II), did not provide higher productivity than did the integrated systems (IPS I, IPS II), nor was there a significant difference (P>0.05) in AFW.
However, the OPS had a reduction in productivity even though it was not affected by plant mortality. It should be borne in mind that the manure used in the 2015 harvest was the same manure used in the 2014 planting season. Thus, this fertilizer had been exposed for a long time to the climatic elements (rain and sun) that cause degradation and loss of nutrients, primarily nitrogen. Azeez and Averbeke [29], reported that bovine manure released large amounts of nitrogen after 120 days, after contact with the soil and when exposed to rain, leaving the material nitrogen-deficient. The same authors suggested supplementing manure fertilization with inorganic nitrogen sources to meet the needs of the plants. In the present study, the nutritional deficiency of nitrogen was quite evident in the plants, demonstrated by chlorosis in the leaves.
Table 2 also shows the estimates for contrast of interest among similar treatments, differing only in terms of thinning of branches and fruits. Comparing the productivity between the treatments we found no significant difference (P>0.05) between them (CS I vs. CS II; IPS I vs. IPS II). Regarding weight (AFW), there was a significant difference by the t test (P<0.05). In the 2014 harvest, CS I and CS II were distinct, as were IPS I and IPS II in 2015. These results suggest that the thinning of branches and fruits can be efficient for the producer aiming to obtain larger fruits for marketing. Salehia et al. [30] showed similar results, where they demonstrated that the pruning and maintenance of two branches in the plant stimulated the production of larger fruits.
The practice of branching influenced the crop cycle, altering the total soluble solids content and delaying the harvest.
Table 3 shows that the harvest was staggered due to the uneven maturity of the fruits between the treatments. The CS I differed statistically (P<0.05) from the others at 65 DAP in both years, presenting physiologically mature fruits at the acceptable value of °Brix for commercialization [31]. Were observed that in the treatments subjected to the thinning of branch thinning (CS II, IPS I, IPS II, OPS) there was a prolongation of the cycle in 15 days. Usually, in tropical regions, the cycle of the cultivar ends at 65 DAP. Miao et al. [32] Performed pruning of branches in melon plants and observed that the practice extended the life of functional leaves, better balancing the growth of the aerial part and the roots and delayed the senescence of the plant.
| Production System | 2014 | 2015 | ||
|---|---|---|---|---|
| 65 DAP | 80 DAP | 65 DAP | 80 DAP | |
| CS I | 11.63 aA | 11.75 aA | 12.5 aA | 12.4 aA |
| CS II | 9.25 bB | 11.88 aA | 9.0 bB | 12.3 aA |
| IPS I | 8.63 bB | 11.25 aA | 8.9 bB | 11.8 aA |
| IPS II | 9.63 bB | 11.75 aA | 9.9 bB | 12.1 aA |
| OPS | 9.88 bB | 11.75 aA | 10.0 bB | 11.8 aA |
| CV% | Production System: 10.0 Harvest season: 6.2 |
Production System: 7.54 Harvest season: 7.50 | ||
DAP: Days after Planting
Table 3: Total soluble solids content (°Brix) of melon in different production systems and fruit development season in Gurupi, Tocantins, Brazil.
Conclusion
The incidence and severity of diseases in the melon crop was a determining factor in crop productivity. Fertilization with bovine manure reduced plant mortality with the presence of antagonistic organisms in the microbiota. The thinning of fruits increased the size and mass of the product, however the thinning of branches increased the crop cycle by approximately 15 days.
References
- Santos GR, Ferreira MSV, Pessoa-Filho MACP, Ferreira ME, Café-Filho AC (2009) Host specificity and genetic diversity of Didymella bryoniae from Cucurbitaceae in Brazil. J Phytopathol 157: 265-273.
- Silva MC, Silva TJA, Silva EMB, Farias LN (2014) Productive and qualitative characteristics of traced melon fertilized with nitrogen and potassium. Rev Bras Eng Agríc Ambient 18: 581-587.
- Tschoeke PH, Oliveira EE, Dalcin MS, Silveira-Tschoeke MCAC, Santos GR (2015) Diversity and flower-visiting rates of bee species as potential pollinators of melon (Cucumis melo L.) in the Brazilian Cerrado. Sci Hort 186: 207-216.
- Caldas RMS, Machado J, Andrade JSCO, Wanderley RA (2015) Processing of Melon crops for beekeeping in the backwoods of Moxotó represented by Digital Terrain Model. Geama 1: 1-15.
- Steward JE, Turner AN, Brewer MT (2015) Evolutionary history and variation in host range of three Stagonosporopsis species causing gummy stem blight of cucurbits. Fungal Biol 119: 370-82.
- Santos GR, Café-Filho AC (2005) Reaction of watermelon genotypes to gummy stem blight. Horticul Brasil 23: 945-950.
- Zhao Q, Dong C, Yang X, Mei X, Ran W, et al. (2011) Biocontrol of Fusarium wilt disease for Cucumis melo using bio-organic fertilizer. App Soil Ecol 47: 67-75.
- Medeiros EV, Viana MG, Albuquerque CC, Viana FA, Silva KMB (2012) Ethanolic extract of Senna alata on the control of Fusarium oxysporum, which causes fusarium wilt on melon plants. Rev Bras Eng Agríc Ambient 16: 1166-1170.
- Wolukau JN, Zhou XH, Li Y, Zhang YB, Chen JF (2007) Resistance to Gummy Stem Blight in Melon (Cucumis melo L.) Germplasm and Inheritance of Resistance from Plant Introductions 157076, 420145, and 323498. Hortsci 42: 215-221.
- Santos GR, Café-Filho AC, Reis A (2006) Resistance of Didymella bryoniae to fungicides in Brazil. Fitopatol Bras 31: 476-482.
- Keinath AP (2013) Susceptibility of cucurbit rootstocks to Didymella bryoniae and control of gummy stem blight on grafted watermelon seedlings with fungicides. Plant Dis 97: 1018-1024.
- Santos HG, Almeida J, Oliveira J, Lumbreras J, Anjos L, et al. (2013) Brazilian system of soil classification. Brasília, Embrapa, Brazil.
- Hargreaves GH, Samani ZA (1985) Reference crop evapotranspiration from temperature. Applied Engineering in Agriculture 01: 96-99.
- Santos GR, Zambolim L, Resende JAM, Costa H (2005) Integrated management of watermelon diseases. Viçosa, University in Viçosa, Brazil.
- Shaner G, Finney RE (1997) The effect of nitrogen fertilization on the expression of slow-mildewing resistance in Knox Wheat. Phytopathol 67: 1051-1056.
- Keinath AP (2014) Differential susceptibility of nine cucurbit species to the foliar blight and crown canker phases of gummy stem blight. Plant Dis 98: 247-254.
- Dalcin MS, Tschoeke PH, Aguiar RWS, Fidelis RR, Didonet J, et al. (2017) Severity of gummy stem blight on melon in relation to cultivars, use of fungicides and growing season. Hortic Bras 35:483-489.
- Rennberger G, Keinath AP (2018) Susceptibility of fourteen new cucurbit species to gummy stem blight caused by Stagonosporopsis citrulli under field conditions. Plant Dis 102: 1365-1375
- Lecomte C, Alabouvette C, Edel-Hermann V, Robert F, Steinberg C (2016) Biological control of ornamental plant diseases caused by Fusarium oxysporum: a review. Biolo Cont 101: 17-30.
- Cao Y, Pi H, Chandrangsu P, Li Y, Wang Y, et al. (2018) Antagonism of two plant-growth promoting Bacillus velezensis isolates against Ralstonia solanacearum and Fusarium oxysporum. Scient Rep 8: 1-8.
- Yang RX, Gao ZG, Liu X, Yao Y, Cheng Y (2014) Root exudates from muskmelon (Cucumis melo L.) Induce autotoxicity and promote growth of Fusarium oxysporum f. sp. melonis. Allelopathy J 33: 175-188.
- Zhang Y, Liao X, Zeng X, Huang H, Gao W, et al. (2010) Identification of an antagonistic bacterial strain from duck manure against Rhizoctonia solani. Acta Phytopathol Sin 40: 517-521.
- Pathak AK, Godika S (2010) Effect of organic fertilizers, biofertilizers, antagonists and nutritional supplements on yield and disease incidence in Indian mustard in arid soil. Indian J Agri Sci 80: 652-654.
- Nongkhlaw FMW, Joshi SR (2016) Micrographical Assessment of Antifungal Effect of Endophytic Bacteria. Biolo Sci 86: 9-14.
- Nga NTT, Giau NT, Long NT, Lübeck M, Shetty NP, et al. (2010) Rhizobacterially induced protection of watermelon against Didymella bryoniae. J App Microbiol 109: 567-582.
- Srivastava R, Khalid BA, Singh US, Sharma AK (2010) Evaluation of arbuscular mycorrhizal fungus, fluorescent Pseudomonas and Trichoderma harzianum formulation against Fusarium oxysporum f. sp. lycopersici for the management of tomato wilt. Biolo Cont 53: 24-31
- Calvo P, Orrillo EO, Romero EM, Zúñiga D (2010) Characterization of Bacillus isolates of potato rhizosphere from andean soils of Peru and their potential PGPR characteristics. Braz J Microbiol 41: 899-906.
- Santos GR, Castro Neto MD, Ramos LN, Café-Filho AC, Reis A, et al. (2009) Reaction of melo genotypes to the gummy stem blight and the downy mildew. Hortic Bras 27: 160-165.
- Azeez JO, Averbeke WV (2010) Nitrogen mineralization potential of three animal manures applied on a sandy clay loam soil. Bioresour Technol 101: 5645-5651.
- Salehia R, Kashia A, Leeb JM, Javanpourc R (2014) Mineral concentration, sugar content and yield of iranian ‘Khatooni’ melon affected by grafting, pruning and thinning. J Plant Nutr 37: 1255-1268.
- Costa ND (2008) The melon crop. Brasília, Embrapa, Brazilian Agricultural Research Corporation, Brazil.
- Miao L, Lu H, Zhang Y, Jiang G, Yang X, et al. (2011) Effects of different pruning type on muskmelon fruit quality yield and retardation of early-senescence cultivated in autumn. Nort Horticul 5: 548-556.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi