Commentary, J Infect Dis Immune Ther Vol: 2 Issue: 1
Do Microbes Care About Human Heart?
Pushpanathan Muthuirulan*
Laboratory of Gene Regulation and Development, Program in Cellular Regulation and Development, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland,United States
*Corresponding Author : Pushpanathan Muthuirulan
Laboratory of Gene Regulation and Development, National Institutes of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland 20892, United States
Tel: +1301-674-3108
E-mail: pushpanathan31@gmail.com
Received: December 14, 2017 Accepted: January 15, 2018 Published: January 22, 2018
Citation: Muthuirulan P (2018) Do Microbes Care About Human Heart? J Infect Dis Immune Ther 2:1.
A Commentary on
Understanding the functional interactions between microbes and human metabolism could potentially reduce the risk of Heart Disease.
Abstract
Heart disease, or cardiovascular disease (CVD) is widely recognized as an inflammatory disease. CVD encompasses a wide range of conditions in humans, including diseases in heart blood vessel such as coronary artery disease (CAD), abnormal heart rhythm (arrhythmias) and congenital heart defects (CHD) [1]. According to the Center for Disease Control and Prevention (CDC), CAD is among the most common type of heart disease to humans, which is highly prevalent in the United States (http://www.cdc.gov/heartdisease/).
Keywords: Microbes; cardiovascular disease
Commentary Report
Heart disease, or cardiovascular disease (CVD) is widely recognized as an inflammatory disease. CVD encompasses a wide range of conditions in humans, including diseases in heart blood vessel such as coronary artery disease (CAD), abnormal heart rhythm (arrhythmias) and congenital heart defects (CHD) [1]. According to the Center for Disease Control and Prevention (CDC), CAD is among the most common type of heart disease to humans, which is highly prevalent in the United States (http://www.cdc.gov/heartdisease/). CAD occurs mainly due to accumulation of low–density lipoproteins and cholesterol in arteries resulting in ‘atheromatous plaque’ –a disease condition called Arteriosclerosis. Plaque buildup in arterial walls can cause ischemia (restriction of blood), leading to obstruction of peripheral arteries, congestive heart failure, heart attack and stroke in humans. Another cause of heart disease is an arrhythmia, a condition where the heart beats too fast (tachycardia) or too slow (bradycardia) or too early (premature contraction) or too irregularly (fibrillation). Heart disease may also be caused by problems in the structure of heart that is present at birth, known as CHD. CHD changes the normal flow of blood through the heart. Recent statistics suggest that 8 out of every 1,000 newborns are affected with CHD. According to the National Heart, Lung and Blood Institutes (NHLBI) at National Institutes of Health, every year more than 35,000 babies in the United States are born with CHD and more than 1 million adults are living with CHD (https://www.nhlbi.nih.gov/healthtopics/ congenital-heart-defects). CVD are the leading cause of death worldwide. What causes CVD? How CVD can be inherited from parents to their newborns? Are microbes can be the cause CVD? Just like genes, whether microbes can be inherited from parents to new born? How to treat and manage CVD? –The answer to these questions still remains an enigma. However, recent research has provided strong evidence that microbes can contribute to non–infectious chronic diseases such as cancer and CVD [2]. Microbes live all around and inside of us. More than a hundred trillion microbial cells live in just a human gut, outnumbering the total human cells by 10-fold suggesting that gut is the major reservoir of microbes than any other organs in human body. Most microbes residing within human are commensals or often beneficial (as symbionts), some are opportunistic pathogens that may cause life threatening diseases to patients, especially in case of immunocompromised conditions. With the advent of high throughput Next-Generation sequencing technologies, researchers had substantiated the role of microbes in CVD and have identified that gut microbiota of patient with symptomatic atherosclerosis was enriched in bacterial genera, Ruminococcus and Collinsella. Metabolic profiling studies have also uncovered the enrichment of genes associated with peptidoglycan biosynthesis in CVD patients than the healthy peers–suggesting that increased peptidoglycan production by resident pathogens an in infected host may contribute to symptomatic atherosclerosis through regulation of host inflammatory pathways. The people with lower risk of CVD harbored specialized group of bacteria such as Eubacterium, Roseburia and Bacteroidetes, which all possessed an anti− inflammatory properties−suggesting that these beneficial group of bacteria helps the human body to fight against the inflammatory response such as free radical damage to cells and thereby believe to reduce the risk of CVD [3].
Gut microbes utilize the dietary components that are not readily absorbed by the small intestine as growth substrates that influences human physiology, metabolism, nutrition and immune function. Most gut microbes have beneficial effects to humans, however under certain circumstances these microbes convert beneficiary dietary compounds, such as choline, into metabolites that are highly detrimental to human health. Researchers have demonstrated that gut microbial metabolism of choline results in the production of trimethylamine (TMA), which is absorbed and further metabolized by flavin monooxygenases 1 and 3 (FMO 1 and FMO3) in the host liver to generate Trimethylamine-N-oxide (TMAO). Increased TMAO levels in serum has positive correlation with impaired renal function, colorectal cancer and CVD. TMAO has also implicated its role in artherosclerosis by promoting forward cholesterol transport and by inhibiting reverse cholesterol transport. Researchers have also unmasked the detrimental effects of human gut bacteria, which converts the common nutrient, L-carnitine found in red meat into TMAO that has been found to increase the risk of death from heart disease [4]. In recent years, there has been growing interest among researchers in identifying potential inhibitors that block production of TMAO to reduce the risk of CVD.
The primary complications associated with atherosclerosis is the sudden rupture of stable atheroma or atheromatous plaque, leading to a life–threatening atherothrombotic lesion. The risk of plaque disruption is highly related to intrinsic properties of individual plaques (their vulnerability) and extrinsic forces acting on plaques (rupture triggers). The intrinsic properties mainly predispose plaques to rupture, whereas the extrinsic forces may precipitate disruption if vulnerable plaques are present. Recent studies have unmasked the presence of bacterial species, Pseudomonas aeruginosa in carotid arterial plague as biofilm deposit that contribute to enhanced risk of plague rupture [5]. The biofilms present within atherosclerotic lesions are susceptible to induction of dispersion response, characterized by the coordinated release of degradative enzyme by bacteria that destabilizes the plague leading to enhanced risk of plague rupture and thrombogenesis. It has been recognized earlier that stress can also trigger plague rupture. Onset of stress increases the plasma concentration of hormone, norepinephrine, which interacts with iron transport protein transferrin in serum leading to the release of free iron. This sudden increase in free iron has potential impact and induces biofilm dispersion of resident pathogens in an infected host leading to plague rupture and thrombogenesis.
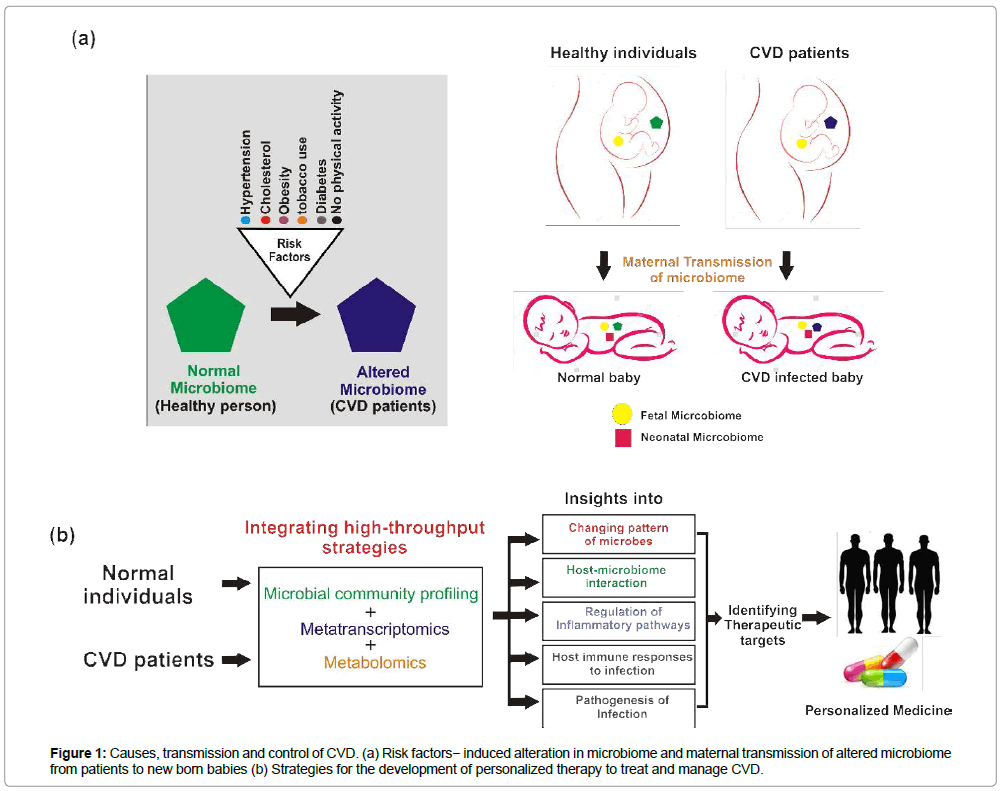
Besides the implications of gut microbes in CVD, the release of bacterial and viral DNA elements into the circulation could also be either the cause or consequence of CVD incidence. Unlike other organs, human blood was presumed to be sterile, and microbes were thought to occur in circulation only during sepsis. Recent study on circulating human microbiome by deep shotgun metagenome sequencing revealed greater microbial diversity in CVD patients than the healthy individuals. The genus Propionibacterium was predominant among CVD patient’s whereas, Pseudomonas was the dominant genus among healthy individuals. Furthermore, phages for Propionibacterium, Pseudomonas and Rhizobium also constituted major fraction of bacteriophages in the circulation of CVD patients [6]. In human, there have been changing patterns in the type of microorganisms (altered microbiome) based on the underlying risk factors such as hypertension, cholesterol, overweight/obesity, tobacco use, lack of physical activity and diabetes that extensively promotes the risk of CVD. Maternal transmission of microbes in humans has attracted considerable attention in the recent years that helps to understand the vertical transmission of microbiome from mother to infant. Recent studies have suggested that infants incorporate an initial microbiome from birth and receives copious supplementation of maternal microbes through birth and breast feeding [7]. Like genetic inheritance of genes, neonatal complications of CVD might also be due to vertical transmission of altered microbiome from infected mothers to off springs (Figure 1).
The knowledge about the functional interactions between human microbiome and immune system is still at the stage of infancy. However, understanding the microbial community structure and their functional interactions with immune systems of both CVD patients and healthy individual will provide deeper insights into the mechanistic relationship between host-microbiome interactions and unravel the potential risks of human developing CVD. The way of integrating strategies such as microbial community profiling, transcriptomics and metabolomics can be efficient, which will provide global understanding of changing pattern of microorganisms, hostmicrobiome interactions, regulation of inflammatory/infection pathways, and the interactions between microbiome and immune systems . These approaches will help us to understand the major underlying cause for CVD and also identify potential therapeutic targets for the development of personalized therapy to manage and treat CVD.
References
- Nabel EG (2003) Cardiovascular disease. N Engl J Med 349: 60-72.
- HA Halpin, Varela MM, Moreno JMM (2010) Chronic disease prevention and the New Public Health. Public Health Reviews32: 120.
- Karlsson FH, Fak F, Nookaew I, Tremaroli V, Fagerberg B, et al. (2012) Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat commun 3: 1245.
- Koeth RA, Wang Z, Levison BS, Buffa JA, Sheehy BT, et al. (2013) Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat med 1: 576-585.
- Lanter BB, Sauer K, Davies DG (2014) Bacteria present in carotid arterial plaques are found as biofilm deposits which may contribute to enhanced risk of plaque rupture. M Bio 5: e01206-14.
- Dinakaran V, Rathinavel A, Pushpanathan M, Sivakumar R, Gunasekaran P, et al. (2014) Elevated levels of circulating DNA in cardiovascular disease patients: metagenomic profiling of microbiome in the circulation. Plos one 105221.
- Funkhouser LJ, Bordenstein SR (2013) Mom knows best: the universality of maternal microbial transmission. PLoS Biol 11: e1001631.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi