Review Article, Int J Cardiovasc Res Vol: 9 Issue: 2
Does Oral Anticoagulation Therapy Reduce Thromboembolic Events or Mortality In Patients with Device-detected Subclinical Atrial Fibrillation? A Review
Garly R. Saint Croix1, Lourdes Chacon2, Dhanya Baskaran3 and Hakop Hrachian1
1Division of Cardiology, Columbia University, Mount Sinai Medical Center, United States
2Department of Medicine, Texas Heart Institute
*Corresponding Author: Dr. Garly R. Saint Croix
Division of Cardiology, Columbia University, Mount Sinai Medical Center, 4300 Alton Rd, Miami Beach, United States
Tel: +17868126137
E-mail: saintcroix.garly@gmail.com
Received: February 02, 2020 Accepted: February 24, 2020 Published: March 02, 2020
Citation: Saint Croix GR, Chacon L, Baskaran D, Hrachian H (2020) Does Oral Anticoagulation Therapy Reduce Thromboembolic Events or Mortality In Patients with Device-detected Subclinical Atrial Fibrillation? A Review. Int J Cardiovasc Res 9:2. doi: 10.37532/icrj.2020.9(2).395
Abstract
Atrial fibrillation is the most prevalent arrhythmia occurring in 1.5- 2% of the general population, and accounting for approximately 30% of all strokes. In 20-45% of atrial fibrillation-related strokes, the arrhythmia is not documented and the patient is asymptomatic from a cardiovascular standpoint, prior to the stroke. Subclinical Atrial Fibrillation (SCAF) is defined as asymptomatic or clinically silent atrial fibrillation. Patients with SCAF exhibit no symptoms during the episode of atrial fibrillation. SCAF is frequently detected by continuous electrocardiographic monitoring in patients without history of atrial fibrillation. Several studies have shown that atrial tachyarrhythmias incidentally detected by implantable cardiac devices such as Implantable Cardiac Defibrillator (ICD) and pacemaker, are associated with a two-fold increase in the risk of death or stroke. While there is strong evidence for the benefit of Oral Anticoagulation (OAC) therapy in reducing stroke risk in patients with clinically diagnosed atrial fibrillation, information is lacking regarding benefit in those with subclinical atrial fibrillation. The aim of this current review is to present the prevalence and predictors of SCAF and to assess the impact of anticoagulation on all-cause mortality and thromboembolic events in patients with implantable devices.
Keywords: Anticoagulation; Subclinical atrial fibrillation; Cardiology
Introduction
Atrial fibrillation is the most prevalent arrhythmia occurring in 1.5-2% of the general population, and accounting for approximately 30% of all strokes [1,2]. In 20-45% of atrial fibrillation-related strokes, the arrhythmia is not documented and the patient is asymptomatic from a cardiovascular standpoint, prior to the stroke [3]. SCAF is defined as asymptomatic or clinically silent atrial fibrillation. Patients with SCAF exhibit no symptoms during the episode of atrial fibrillation [4]. SCAF is frequently detected by continuous electrocardiographic monitoring in patients without history of atrial fibrillation [5].
Several studies have shown that atrial tachyarrhythmias incidentally detected by implantable cardiac devices such as Implantable Cardiac Defibrillator and pacemaker, are associated with a two-fold increase in the risk of death or stroke [6]. While there is strong evidence for the benefit of Oral Anticoagulation therapy in reducing stroke risk in patients with clinically diagnosed atrial fibrillation, information is lacking regarding benefit in those with subclinical fibrillation [7]. The aim of this current review is to present the prevalence and predictors of SCAF and to assess the impact of anticoagulation on all-cause mortality and thromboembolic events in patients with implantable devices.
Type of Atrial High Rate Events and Pathophysiology of SCAF
Atrial High Rate Events (AHRE) are common in patients who have Cardiac Implantable Electronic Devices (CIEDs). There is a wide range of these events and to quantify them, the term atrial fibrillation burden has emerged. The degree of burden is proportional to the risk of having clinical atrial fibrillation as well as its complications including thromboembolic events, heart failure and death. ARHE are supraventricular tachyarrhythmias characterized that refers to episodes of greater than 6 minutes on patients who do not have clinically detected atrial fibrillation (Table 1) [8].
| Study (Year) | N | AF Definition | Monitoring Duration | AF Yield |
|---|---|---|---|---|
| Bhatt (2011) | 62 | 30 seconds | MCOT 28 days | 24% AF>5 min 9% |
| Flint (2012) | 236 | 5 seconds | MCOT 30 days | AF<30 sec 4% AF>30 sec 7% |
| Gaillard (2010) | 98 | 32 seconds | TTM 30 days | 9% |
| Gladstone (2014) | 572 | 30 seconds | Event Monitor 30 days vs 24 Holter | 16.1% in event monitor vs 3.2% Holter |
| Kamel (2013) | 20 | 30 seconds | MCOT 21 days | 0% |
| Miller (2013) | 156 | 30 seconds | MCOT 30 days | Overall 17% AF<30 sec 12% AF>30 sec 4% |
| Tayal (2006) | 56 | Any duration | MCOT 21 days | AF<30 sec 18% AF>30 sec 5% |
Table 1: Atrial detection rate using external monitor.
There are several types of AHREs. These include sinus tachycardia, atrial tachycardia, multifocal atrial tachycardia, atrial flutter, sinus node re-entry tachycardia, inappropriate sinus tachycardia and atrial fibrillation. It is not uncommon for devices to also inappropriately label rhythms as AHRE due to premature atrial complexes, or oversensing in the atrial channel, or artifactual, such as those resulting from far-field signals or noise. For example, in the Subclinical Atrial Fibrillation study (ASSERT) 82.7% of AHREs were true AF/ AT and 17.3% were false positives therefore visual confirmation of thisrecording is important.
Patients with atrial fibrillation maybe either symptomatic or asymptomatic. SCAF are atrial heart rhythm episodes, silent, repetitive tachyarrhythmia of short duration (e.g: minutes), especially occurring in patients with electronic cardiac devices with an incidence of 30% with arrhythmia screening methods and cardiovascular condition of the explored patients [9]. SCAF remains a potential deleterious factor for stroke given there is a possible association between AHRE as well clinical AF, and SCAF confers an arrhythmic burden and embolic risk (Table 2) [10]. In the ASSERT trial, SCAF showed an 13% increased risk of ischemic stroke or systemic embolism, an incidence of at least one AHRE in 34.7% of patients without any previous history of AF during 2.5 years of follow-up.
| Study | Study size | Mean Age (years) | Duration of monitoring (months) | Definition of AF | Time to Diagnosis (days) | AF detection rate (%) |
|---|---|---|---|---|---|---|
| Cotter | 51 | 52 | 8 | 2 minutes | 48 | 25 |
| CRYSTAL AF (ICM arm) | 221 | 61.6 | 6 12 36 |
>30 seconds | 41 84 252 |
9 12 30 |
| Etgen | 22 | 65.8 | 12 | >6 minutes | 152 | 27 |
| Jorfida | 54 | 67.8 | 14.5 | >5 minutes | 162 | 46 |
| Poli | 74 | 66.4 | 12 | >2 minutes | 105 | 33 |
| Ritter | 60 | NA | 10 | >30 seconds | 64 | 17 |
| Rojo-Martinez | 111 | 67 | 9 | 2 Minutes | 102 | 33 |
| SURPRISE | 5 | 54 | 19 | >2 minutes | 109 | 16 |
| Ziegler | 1247 | 65.3 | 6 | 2 minutes | 58 | 12 |
Table 2: Atrial detection rate using implantable loop recorder.
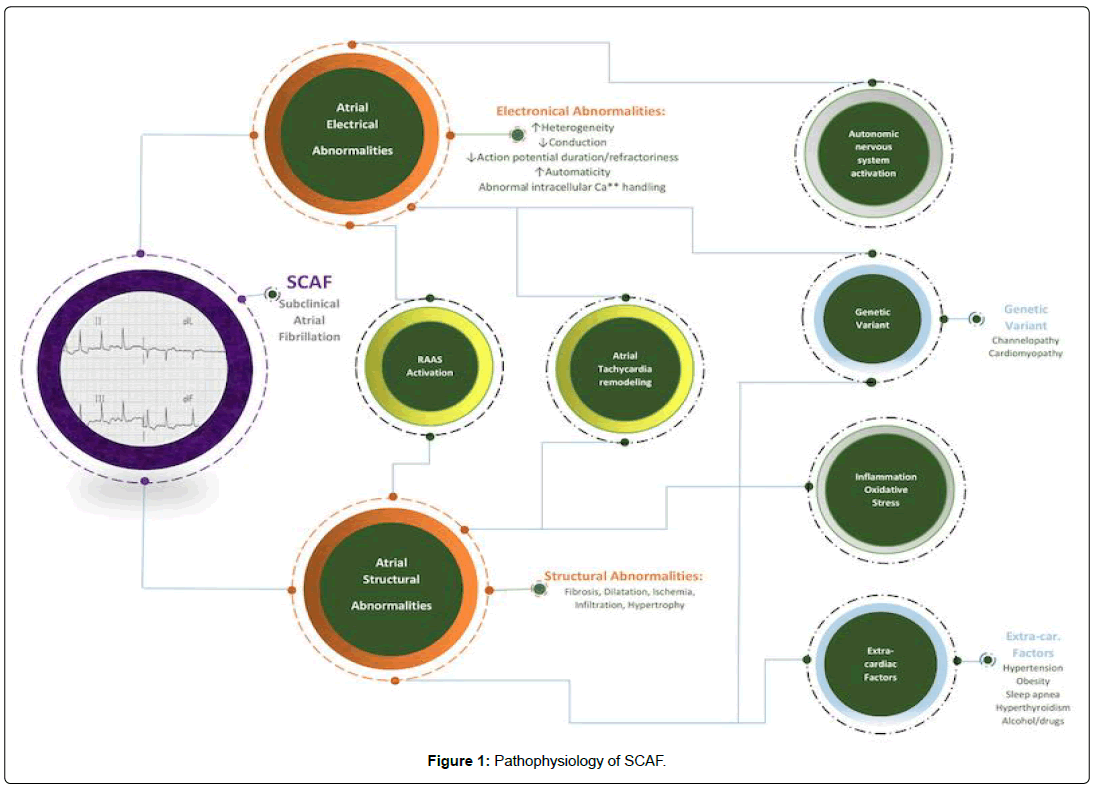
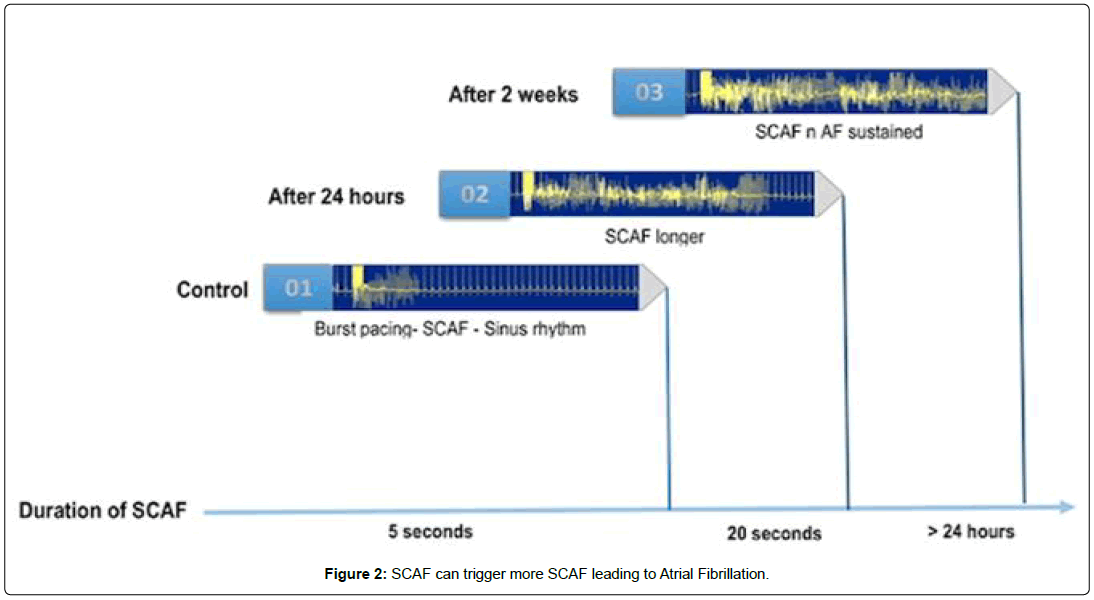
SCAF has similar pathophysiology as symptomatic atrial fibrillation, The cellular and molecular mechanisms contributing to AF has been described as a complex pattern of electrical, structural, and CA2+ handling remodeling, producing a vulnerable substrate for AF. Structural remodeling consisting of left atrial fibrosis hasn been observed in both clinical and experimental paradigms as one of the common pathways to develop AF. Fibrosis likely resulting from several factors working simultaneously becomes a substrate for reentry by increasing the heterogeneity of conduction in the atria. There are extensive interactions between cardiomyocytes and fibroblasts. The cardiomyocytes secreted molecules such as ROS, TGF-B, PGDF, and CTDGF regulates fibroblast properties and ECM. Ausma et al. In a goat model demonstrated that structural remodeling of AF includes changes in atrial histological properties, size, and cellular morphology due to myolysis, glycogen accumulation, disturbance in gap junctions, changes in mitochondrial shape and fragmentation in the sarcoplasmic reticulum. The altered autonomic function also has a role in the genesis and maintenance AF by multiple mechanisms, including vagally mediated action potential duration shortening, adrenergically mediated CA2+ loading delayed after depolarizations promotion, and hyperphosphorylation of the ryanodine receptor. There is subsequently some time before the clinical appearance of AF, which delineates the different phases of the disease pathogenesis and which can be an area for potential diagnostic and therapeutic options for AF. SCAF is associated with structural and electrical remodeling and leads to subsequent atrial fibrillation [11]. A pacing model in goats that causes remodeling concluded that the more there is a burst pacing (a rapid, multiple electrical pulses aiming to pace the heart faster than its intrinsic rate), the longer the duration of SCAF will be [12]. This will lead to further remodeling and incidence of clinical paroxysmal atrial fibrillation. Besides, Goldberger et al. showed several techniques for evaluating atrial myopathy, including MRI, echocardiography ECG waveform analysis, and biomarker analyses that might lead to identifying specific pathways to understand the mechanisms underlying SCAF better[13]. The etiologies of SCAF occurrence. In most patients, SCAF results interaction between multiple factor operating simultaneously (Figure 1) such as extracardiac factors (hypertension, obesity, sleep apnea, hyperthyroidism, alcohol, and drugs), atrial structural abnormalities (fibrosis, dilation, ischemia, inflammation, infiltration, hypertrophy, and atrial tachycardiac remodelling), atrial electrical abnormalities triggered by autonomic nervous system activation (increased heterogeneity, decreased conduction, decreased action potential duration/refractoriness, increased automaticity, and abnormal intracellular calcium handling) and genetic variants (channelopathy, cardiomyopathy) (Figure 2) [14,15].
Prevalence and Detection of SCAF
SCAF is defined as relatively short episodes of fibrillation usually detected with long-term, continuous monitoring [16]. This entity is becoming more common not only in patients with pacemakers, but more broadly in elderly individuals and it is present in 25-30% of all individuals greater than 65 years [17]. This is important since AF is a growing epidemic with predictions of 8 to 12 million Americans affected by this disease by 2050 [18].
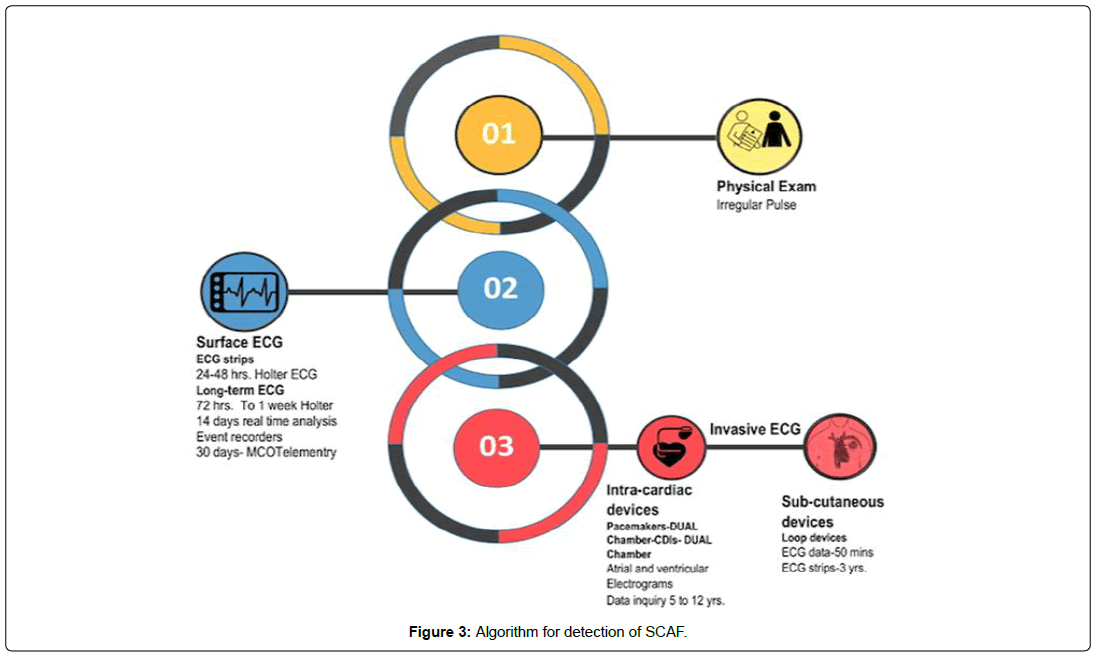
There are several methods to diagnose SCAF starting with finding of an irregularly irregular rhythm during a regular cardiac examination coupled with pulse monitoring. Other methods include, a 12-lead Electrocardiogram (EKG) strip which provides more specific details and a 24-48-hour holter monitoring. Long term and surface EKG include 72 hours to 1-week holter, 12-day real-time analysis event recorders, 30-day mobile cardiac outpatient telemetry (MCOT). Invasive EKG techniques usually refer to the intracardiac device implantation including pacemaker (dual chamber), ICDs (dual chamber) that report atrial electrocardiograms for 5 to 12 years on average. Some subcutaneous implantable loop recorders can also provide EKG data for up to 3 years (Figure 3). Previous trials have shown that atrial detection rate is feasible with either external monitor or with implantable loop recorder (Tables 3 and 4). In a prospective study of 236 patients, Flint et al. defined SCAF as atrial fibrillation for 5 seconds and monitored the patients for a total of 30 days via a MCOT. In this study, an overall incidence of 11% of atrial fibrillation was noted, Atrial fibrillation duration less than 30 seconds was 4% and more than 30 seconds was 7% [19]. Similarly, a cohort study of 1,247 patients with cryptogenic stroke assessed the presence of SCAF by using implantable loop recorder for 6 months and defined atrial fibrillation as episodes that lasted ≥ 2 minutes [20]. In this group, atrial fibrillation detection rate was 12%.
| Trial | AF prior to stroke (at any time) | AF prior to stroke (<30 days) | New AF after stroke |
|---|---|---|---|
| ASSERT (Brambatti M et al Circulation 2014 Mar 14) |
18/51 (35%) | 4/51 (8%) | 8/51 (16%) |
| IMPACT (all) Martin DT, ACC Session, 2014, March 29 |
20/69 (29%) | n.a. | 9/69 (13%) |
| TRENDS (Daoud EG, et al Heart Rhythm 2011;8:1416-23) |
20/40 (50%) | 9/40 (22%) | 6/40 (15%) |
Table 3: Temporal proximity of SCAF and stroke.
| Study | n | Main findings | Conclusion |
|---|---|---|---|
| MOST | 312 patient subgroups with sinus node dysfunction and pacemakers programmed to log AHRE | Median follow-up, 2.3 years | Patients with AHREs exceeding 5 minutes in duration are more than twice as likely to die or have a stroke and 6 times as likely to develop atrial fibrillation |
| The presence of AHRE was an independent predictor of the following: Total mortality (HR, 2.48; 95% CI, I .25-4.91: p=0.0092): death or nonfatal stroke (HR, 2.79; 95% CI, I.51-5.15; p=0.0011); and atrial fibrillation (HR. 5.93; 95% CI. 2.88-2.2; p=0.0001) |
|||
| TRENDS | 2 486 patients with (greater or equal sign) 1 stroke risk factors with pacemakers or defibrillators | Mean follow-up, 1.4 years | Thromboembolism risk is a quantitative function of AHRE burden |
| Annual thromboembolism risk was 1.1% for no-burden, 1.1% for low-burden, and 2.4% for high-burden subsets of 30-d windows | AHRE burden (greater or equal sign) 5.5 h on any of 30 prior days doubled thromboembolism risk | ||
| ASSERT | 2 580 patients (greater or equal sign) 65 years of age with hypertension and no history of AF, with pacemaker or ICD | Mean follow-up, 2.5 years | Subclinical atrial tachyarrhythmias, without clinical AF, occurred frequently in patients with pacemakers and were associated with a significantly increased risk of ischemic stroke or systemic embolism |
| Subclinical atrial tachyarrhythmias were associated with an increased risk of ischemic stroke or systemic thromboembolism (HR, 2.49; 95% CI, I .28-4.85; p=0.007) even after adjustment for predictors of stroke (HR, 2.50; 95% CI, I.28-4.89; p=0.008) | |||
| IMPACT | 2 718 patients with dual-chamber and biventricular defibrillators | Median follow-up, 2 years | Intermittent anticoagulation based on remotely detected AHRE did not prevent thromboembolism |
| 2-Arm RCT: (I) start and stop anticoagulation on the basis of remote rhythm monitoring vs. (2) usual office-based follow-up | Primary events (2.4 vs 2.3 per 100 patient-years) did not differ between trial arms (HR, 1.06; 95% CI, 0.75-1.51; p=0.732); in patients with AHRE, thromboembolism rate was 1.0 vs 1.6 per 100 patient-y (p=0.251); no temporal relationship between AHRE and stroke was seen | ||
| SOS AF | Pooled analysis of data from 5 prospective studies; 10 016 patients with pacemakers and ICDs without permanent AF, with at least 3 months of follow-up | Median follow-up, 2 years | Daily AHRE burden is associated with an increased risk of thromboembolism even after adjustment for anticoagulant use and CHADS score |
| Increased risk of stroke with a maximum daily AHRE threshold of (greater or equal sign) minutes (HR, 1.76; 95% CI, 1.02-3.02; p=0.041) but the highest risk was with (greater or equal sign) 1 hour (HR, 2.11; 95% CI, 1.00-3.64; p=0.008); when controlling for stroke risk factors and oral anticoagulation use at baseline, the risk persisted (HR, 1.90; 95% CI, 1.00-3.61; p=0.047) | |||
| RATE | 5 379 patients with pacemakers or ICDs | Median follow-up, 1.9 years | Adjusted short AHREs (terminating within a single electrogram) were not associated with increased risk of clinical events when compared to no AHRE |
| Patients with only short AHREs were associated with lower adjusted incidence of composite clinical events including stroke (HR, 0.86; 95% CI, 0.77-0.97; p=0.01) when compared to no AHRE; long AHREs were associated with incident clinical events (HR, 1.68: 95% CI, 1.49-1.88; p<0.001) and stroke or TIA (HR, 1.51; 95% CI, 1.03-2.21; p=0.03) | Long AHREs (extending beyond a single electrogram) were associated with an increased incidence of stroke |
Table 4: Previous trials assessing the risk of SCAF and stroke.
In the ASSERT 1 trial (Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing), a prospective cohort design study with a mean follow up period of 2.8 years, at least 1 atrial tachyarrhythmia was detected by an implanted device among 10.1% of patients within 3 months and in an additional 24.5% of patients within approximately 2.5 years [21]. In the TRENDS study, atrial tachyarrhythmias occurred in 7.3% of the patients during more than 10% of the surveillance period and an additional 20% patients had at least one episode of atrial tachyarrhythmias during the 1.1-year of follow up [22].
Whenever there is a diagnosis of atrial fibrillation, starting anticoagulation becomes essential to prevent thromboembolic events depending on CHADS2VASC score (Congestive Heart Failure, Hypertension, Age >75, Diabetes, Stroke or TIA, Vascular Disease, Age>65, Female) score [23]. A caveat is that a small portion of the arrhythmias may not correspond to atrial fibrillation and atrial flutter since the atrial lead of the CIED can over- or under-sense atrial inputs or spurious arrhythmias [24]. ASSERT trial investigators found that in atrial tachyarrhythmias with duration greater than 5 minutes, 17.3% of episodes labeled as atrial fibrillation were false positives, compared to 3.3% of those episodes lasting greater than 6 hours [25]. Pollak et al. found that 11% of episodes greater than 5 minutes were not true atrial tachycardia or atrial fibrillation [26].
Accurately detecting SCAF remains essential for prevention planning of atrial fibrillation complications. Short AHREs are prone to be artifacts. In addition, some atrial high rate episodes can reflect other atrial arrhythmias not necessarily requiring stroke prevention therapy.
Predictors of SCAF
Identifying real predictors of SCAF is challenging given the paucity and heterogeneity of data on this subject. In the ASSERT I trial, subclinical atrial tachyarrhythmias, without clinical atrial fibrillation, occurred frequently in patients with pacemakers and were associated with a significantly increased risk of ischemic stroke or systemic embolism [27]. However, in ASSERT II, it was noted that the incidence of SCAF is similar in both patients with and without prior stroke. This substantially weakens the case that SCAF detection after stroke is linked to causality [28].
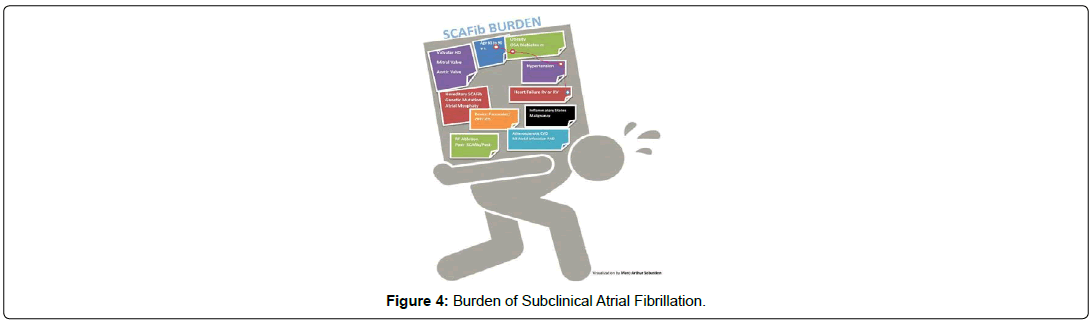
The main predictors of SCAF remain older age, high BMI and metabolic syndrome, and structural heart disease. An episode of SCAF lasting more than 1 hour leads to 13% increased risk of SCAF progression [29]. In addition, there are populations of silent atrial fibrillation that contribute to the SCAF burden including genetic mutations, cardiovascular risk factors (Myocardial infarction, Peripheral artery disease, arrhythmias, valvular disease, inflammatory states, risk for malignancy (Figure 4). It is unclear if left atrial enlargement is a risk factor SCAF as it for atrial fibrillation. It is reasonably to say that this is likely the case given that Left Atrial Appendage is related to electronic and anatomic changes in the setting of remodeling, which are important in progression of AF.
SCAF and Thromboembolic Events
The association between atrial fibrillation and an embolic event is essential to assess because this helps determine if an episode of atrial fibrillation is the exact cause. There are several trials that look at SCAF and incidence of thromboembolic events (Figure 5). The ASSERT trial I included 2,580 patients greater than 65 years old with hypertension and no history of atrial fibrillation, with pacemaker and ICD and had a mean follow up of 2.5 years. This study found that subclinical atrial tachyarrhythmias were associated with an increased risk of ischemic stroke or systemic thromboembolism (HR 2.49; 95% CI: 1,28-4.85; p=0.007) [30]. It concluded that subclinical atrial tachyarrhythmias, without clinical atrial fibrillation, occurred frequently in patients with pacemakers and were associated with a significantly increased risk of ischemic stroke or systemic embolism. The TRENDS study assessed 2,486 patients with 1 or more stroke risk factors with pacemakers or defibrillators and aimed to assess relationship between devicedetected atrial high rate episodes and thromboembolic events. The mean follow-up was 1.4 years and the annual thromboembolism risk was 1.1% for no-burden, 1.1% for low-burden, and 2.4% for high-burden subsets of 30-day windows. This study confirmed that thromboembolism risk is a quantitative function of AHRE burden and AHRE burden greater than 5.5 hours on any of 30 prior days was found to double thromboembolism risk.
In summary, several studies repeatedly demonstrate that SCAF increases the incidence of thromboembolic events. However, the theory that these episodes of SCAF correlate with the clinical events of stroke warrants further investigation. In other words, if stroke occurs immediately after atrial fibrillation (whether symptomatic or not), that will give future directions for monitoring and treating these high atrial rates events instantaneously. Early detection of SCAF could actually give the opportunity to slow down the progression of the disease and prevent high-risk patients from developing longerterm atrial fibrillation and its consequences. More aggressive monitoring to detect SCAF would be warranted, particularly if early therapy is shown to prevent progression and/or complications of thromboembolic events.
Some studies try to assess the temporal proximity of SCAF and stroke. Sparks et.al. initially illustrated that after 20 minutes of atrial tachyarrhythmias, there is prothrombogenic electrical remodeling of the human atria [31]. Nevertheless, several studies have later demonstrated the opposite by showing that there is no temporal relationship between atrial fibrillation and embolic events since the events happened outside of atrial fibrillation episodes [32,33]. In the ASSERT trial, 73% of patients had dissociation with a mean lag period of 46. 7 ± 71.9 days and their thromboembolic events was triggered by other causes or were cryptogenic [34]. This study found that 35% patients had atrial fibrillation to stroke at any time, 8% had AF in less than 30 days prior to stroke and 16% had new AF episode after stroke. Martin DT et.al. showed that there is a lack of temporal relationship between the detection of atrial fibrillation and the stroke event. In other word, the incidence of AF didn’t correlate with the thromboembolic events. Furthermore, in the IMPACT trial, 29% patients had silent atrial fibrillation episodes prior to stroke at any time, 13% had new atrial fibrillation after stroke. Hence, there is no clear temporal proximity of silent atrial fibrillation episodes to thromboembolic events and as a result, intermittent OAC is not a good strategy given this dissociation. A more comprehensive and individualized assessment of risks and benefits should always be used to guide initiation of oral anticoagulant treatment.
Management of Patients with SCAF
Currently, there is no specific algorithm or guideline recommendations addressing whether anticoagulation needs to be started in individuals with SCAF. It is unclear if anticoagulation lowers thromboembolic events given that there are more stroke events during incidental atrial fibrillation, as shown with several trials including the ASSERT trial [35].
Major trials are currently ongoing to investigate differences between SCAF from typical, clinical atrial fibrillation; to evaluate stroke risk factors and markers as well as to define the role of oral anticoagulation (Table 5). The ARTESIA study (Apixaban for the Reduction of Thrombo-Embolism in patients with device-detected sub-clinical Atrial fibrillation- NCT01938248) is a randomized double-blind trial with 4,000 enrolled patients which will assess the benefit of Apixaban compared to aspirin in these participants [36]. The SILENT study (SCAF and Stroke Prevention Trial- NCT02004509) is a randomized clinical trial, single center, parallel trial with 2,054 enrolled participants, which will evaluate OAC compared to conventional management in silent atrial fibrillation. The expectation is that anticoagulation therapy of SCAF directed by CIED intensive monitoring will reduce the incidence of stroke and systemic embolism comparing to patients with non-diagnosed SCAF [37]. The NOAH-AFNET trial 6 (Nonvitamin K anticoagulants in patients with atrial high rate episodes) is a double-blind randomized multi-center clinical trial of 3,400 participants in Europe. It plans to assess the benefit of edoxaban compared to aspirin in reducing thrombo-embolic risk in patient with atrial high rate episodes (NCT02618577). The primary outcome measure will be the time from randomization to the first occurrence of stroke, systemic embolism or cardiovascular death for a period of 28 months [38]. The Danish Loop Study (Atrial Fibrillation detected by continuous EKG monitoring Using Implantable Loop Recorder to Prevent Stroke in High-risk Individuals-NCT02036450) intends to randomize 6,000 patients (with age>70 and at least one of these disease : diabetes, hypertension, heart failure, previous stroke) with a loop recorder implanted in a 1:3 randomization to be treated with standard of care and assessing time to stroke or peripheral embolic episode during 3 years of follow up.
| Study | Inclusion criteria | Randomization/Design | Size (N) | Endpoint | Estimated completion date |
|---|---|---|---|---|---|
| ARTESIA Apixaban for the reduction of thrombo-embolism in patients with device-detected subclinical atrial fibrillation clinicaltrials.gov NCT01938248 |
Permanent PM, ICD or CRT CHA2DS2-VASc score of ≥ 4 Age ≥ 65 At least one-episode symptomatic AF ≥ 6-min (Atrial rate>175/min if an atrial lead is present) but no single episode >24h in duration. NO points with clinical AF |
Apixaban 5 (or 2, 5) mg X 2 vs Aspirin 81 mg X1 daily |
4,000 | 1.Composite of - ischemic stroke - Systemic embolism 2.Major bleeding |
2019 |
| NOAH vitamin AFNET 6 Non- K antagonist oral Anticoagulants in patients with atrial high rate episodes Clinicaltrials.gov NCT0261577 |
Permanent PM or ICD Age ≥ +additional CHA2DS2-VASc score point of ≥ 2, i.e. CHA2DS2-VASc ≥ 3 At least one episode of AHRE ≥ 6 min (Atrial rate>180/min if an atrial lead is present), but no single episode>24 h in duration. NO pts with overt AF |
Edoxaban 60 (30 if renal ins) mg X1 vs Aspirin 100 mg X1 daily. Double-blinded Double dummy |
3,400 | Composite of time to - First stroke - systemic embolism - CV death |
2019 |
| The (Danish) LOOP study Clinicaltrials.gov NCT02036450 |
Age>70 years and at least one of the following diseases: - Diabetes - Hypertension - Heart failure - Previous stroke |
ILR or standard treatment of care (ratio 1:3) |
6,000 | Composite of - ischemic stroke - systemic embolism |
2020 |
| SILENT (Subclinical Atrial Fibrillation and Stroke Prevention Trial – NCT02004509) | Age ≥ 18 years CHADS2 score ≥ 2 Sinus rhythm Cardiac implantable electronic device |
Intensive monitoring arm (Group I) or control group Routine schedule arm (Group II) in a 1:1 ratio. |
2,054 | 1.Composite f - stroke - systemic embolism 2. subclinical AF rate, total mortality, CV mortality, MI, CV hospitalization and bleeding rates. |
2020 |
Table 5: Summary of ongoing trials on SCAF.
It is important to clearly define what duration of SCAF would be impacted through OAC initiation. The MOST trial (Atrial Diagnostics Ancillary Study of the Mode Selection Trial) illustrated an increased risk of stroke and death in patients with pacemaker (n=2,010) when the duration of an atrial fibrillation episode that was greater than 5 minutes [39]. Swiryn et al. in their study consisting of 5,379 patients, have shown that compared with patients without documented atrial fibrillation and atrial flutter, short episodes of atrial tachycardia or atrial flutter (defined as less than 5 minutes) were not associated with increased risk of clinical events [40]. In addition, the 2014 clinical practice guidelines on atrial fibrillation recommend the use of CHADS2VASC score for decision regarding when to start anticoagulation in patients nonvalvular atrial fibrillation [41].
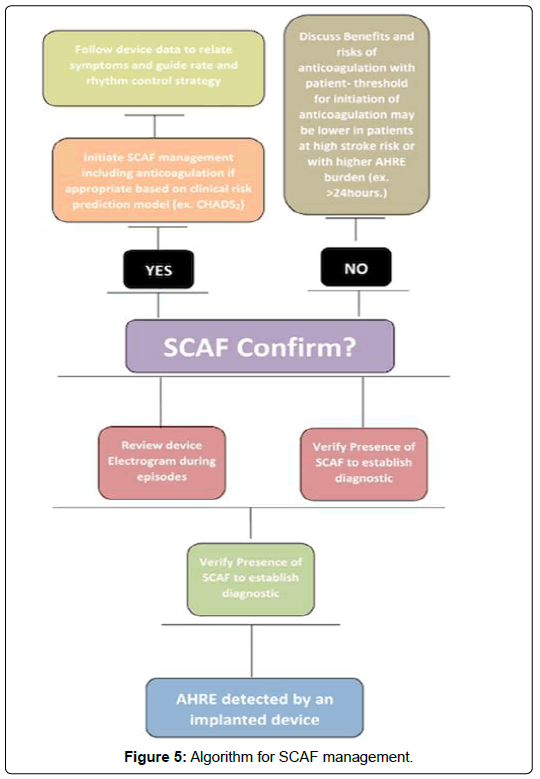
However, there is no agreement about the exact duration and frequency of asymptomatic atrial fibrillation that would be considered critical and necessitating therapy [42]. According to primary literature, the level of atrial fibrillation burden seems to be more symptomatic and with higher burden of thromboembolic events when episodes last more than 5 minutes but less than one hour in one day [43,44]. Overall, there is no evidence to support that OAC use in patients with SCAF will decrease thromboembolic events and there is no current guideline-driven decision algorithm of benefit from anticoagulation in SCAF between 5 minutes and less than one hour. All patients should be considered unique and should be properly risk stratified regarding the benefits of OAC potentially based on a proposed algorithm. Whenever there is ARHEs on CIED, confirm the presence of clinical or subclinical atrial fibrillation. If there is clinical atrial fibrillation, initiate rate and rhythm control strategies based on clinical risk prediction model with CHAD2VASC score. If there is subclinical atrial fibrillation, discuss benefits and risks of anticoagulation with patient. The threshold for initiation of anticoagulation should be lower in patient at high stroke risk or with higher AHRE burden such as episodes greater than 5 hours. For symptomatic episodes greater than 1-hour, paroxysmal atrial fibrillation should be officially classified and medically managed based on the CHADVASC score [45].
Conclusion
SCAF detected on CIEDs and decision to start anticoagulation to prevent thromboembolic events continue to present an important dilemma. There is evidence that SCAF is associated with a higher risk of stroke and systemic thromboembolism. However, anticoagulation initiation based on remotely detected atrial fibrillation should be done with appropriate clinical assessment of cardiovascular risk factors and bleeding risk. Uncertainty still exists about the frequency, duration and overall burden of silent atrial fibrillation, risk of multiple shorter episodes of SCAF compared to less frequent but longer episodes. As a result, despite the proposed algorithm in this review, there is no current data showing that medical management SCAF is of benefit to the patient. Hence, further information is needed to know how much atrial fibrillation burden should prompt treatment, when to initiate anticoagulation and best medical therapies for SCAF. Current ongoing trials including ARTESIA (NCT 01938248), SILENT (NCT02004509), NOAH-AFNET (NCT02618577) will hopefully provide further guidance for standardization of atrial fibrillation detection algorithms and address these concerns and consequences by assessing the clinical efficacy, safety and cost-effectiveness of oral anticoagulation therapy in patients with SCAF detected by implantable devices.
References
- Furberg CD, Psaty BM, Manolio TA, Gardin JM, Smith VE, et al. (1994) Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular health study). Am J Cardiol 74: 236-241.
- Hannon N, Sheehan O, Kelly L, Marnane M, Merwick A, et al. (2010) Stroke associated with atrial fibrillation – incidence and early outcomes in the North Dublin population stroke study Cerebrovasc Dis 29: 43-49.
- Martin DT, Bersohn MM, Waldo AL, Wathen MS, Choucair WK, et al. (2015) Randomized trial of atrial arrhythmia monitoring to guide anticoagulation inpatients with implanted defibrillator and cardiac resynchronization devices. Eur Heart 36: 1660-1668.
- Mahajan R, Perera T, Elliott AD, Twomey DJ, Kumar S, et al. (2018) Subclinical device-detected atrial fibrillation and stroke risk: a systemic review and meta-analysis. Euro Heart J 19: 1407-1415.
- Flaker GC, Belew K, Beckman K, Vidaillet H, Kron J, et al. (2005) Asymptomatic atrial fibrillation: demographic features and prognostic information from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am Heart J 149: 657-663.
- Healey JS, Martin JL, Duncan A, Connolly SJ, Ha AH, et al. (2013) Pacemaker-detected atrial fibrillation in patients with pacemakers: prevalence, predictors and current use of oral anticoagulation. Can J Cardiol 29(2): 224-228.
- Laupacis A, Albers G, Dalen J, Dunn MI, Jacobson AK, et al. (1998) Antithrombotic therapy in atrial fibrillation. Chest 114: 579S-589S.
- Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, et al. (2012) 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation – developed with the special contribution of the European Heart Rhythm Association. Europace 14: 1385-1413.
- Kishore A, Vail A, Magid A, Dawson J, Lees KR, et al. (2014) Detection of atrial fibrillation after ischemic stroke and transient attack: a systemic review and meta-analysis. Stroke 45: 620-626.
- Gorenek B, Bax J, Bariani G, Chen SA, Dagres N, et al. (2017) Device-detected subclinical atrial tahyarrhythmias: definition, implications and management. An European Heart Rhythm Association consensus document. Europace 19: 1556-1578.
- Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, et al. (2009)Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation 119: 1758-1767.
- Shanmugan N, Boerdlein A, Proff J, Ong P, Valencia O, et al. (2012) Detection of atrial high-rate events by continuous Home Monitoring: Clinical significance in the heart failure – cardiac resynchronization therapy population. Europace 14: 230-237.
- Goldberger JJ, Arora R, Green D, Greenland P, Lee DC, et al (2015) Evaluating the atrial myopathy underlying atrial fibrillation: identifying the arrhythmogenic and thrombogenic substrate. Circulation 132: 278-291.
- Boos CJ, Anderson RA, Lip GY (2006) Is atrial fibrillation an inflammatory disorder?. Eur Heart J 27(2): 136-149.
- Kirubakaran S, Chowdhury RA, Hall MCS, Patel PM, Garratt CJ, et al. (2014) Fractionation of electrograms is caused by colocalized conduction block and connexin disorganization in the absence offibrosis as af becomes persistent in the goat model. Heart Rhythm 12: 397- 408.
- Reiffel JA, Verma A, Kowey PR, Halperin JL, Gersh BJ, et al. (2017) Incidence of previously undiagnosed atrial fibrillation using insertable cardiac monitors in a high-risk population: The reveal AF Study. JAMA Cardiol 919: 1120–1127.
- Brambatti M, Connolly SJ, Gold MR, Morillo CA, Capucci A, et al. (2014) On behalf of the ASSERT investigators. Improved relationship between subclinical atrial fibrillation and embolic events. Circulation 129: 2094-2099.
- Dewire J, Calkins H (2013) Update on atrial fibrillation catheter ablation technologies and techniques. Nat Rev Cardiol 10: 599-612.
- Flint AC, Banki NM, Ren X, Rao VA, Go AS, et al. (2012) Detection of paroxysmal atrial fibrillation by 30-day event monitoring in cryptogenic ischemix stroke: the Stroke and Monitoring for PAF in Real Time (SMART) Registry. Stroke 43: 2788-2790.
- Ziegler PD, Rogers JD, Ferreira SW, Nichols AJ, Sarkar S, et al. (2015) Real-word experience with insertable cardiac monitors to find atrial fibrillation in cryptogenic stroke. Cerebrovasc Dis 40: 175-81.
- Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, et al. (2012) Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 366: 120-129.
- Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD, et al. (2009) The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk. The TRENDS Study. Circ Arrhythmia Electrophysiol 2: 474–480.
- Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, et al. (2006) ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation a report of the american college of cardiology/american heart association task force on practice guidelines and the european society of cardiology committee for practice guidelines (writing committee to revise the 2001 guidelines for the management of patients with atrial fibrillation). J Am Coll Cardiol 48: 854-906.
- Zyl MV, McLeod C (2017) Bernard gersh;approach to device-detected subclinical atrial fibrillation. J South Africa Heart Ass 14: 86-95.
- Kaufman ES, Israel CW, Nair GM, Armaganijan L, Divakaramenon S, et al. (2012) Positive predictive value of device-detected atrial high-rate episodes at different rates and du- rations: an analysis from ASSERT. Heart Rhythm 9: 1241– 1246.
- Pollak WM, Simmons JD, Interian A, Atapattu SA, Castellanos A, et al. (2001) Clinical utility of intraatrial pacemaker stored electrograms to diagnose atrial fibrillation and flutter. Pacing and Clinical Electrophysiology 24: 424–429.
- Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, et al. (2012) Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 366: 120-129.
- Ester MM, Healey JS (2016) CSSR.04: Hi Impact EP registries and clinical trials. American heart association scientific sessions 12-16, New Orleans.
- Gorenek B, Bax J, Boriani G, Chen SA, Dagres N, et al. (2017) Device-detected subclinical atrial tachyarrhythmias: definition, implications and management-an european heart rhythm association (EHRA) consensus document, endorsed by heart rhythm society (HRS), asia pacific heart rhythm society (APHRS) and sociedad latinoamericana de estimulacion cardiaca y electrofisiologia (SOLEACE). Europace 19: 1556-1578.
- DeCicco AE, Finkel JB, Greenspon AJ, Frisch DR (2014) Clinical significance of atrial fibrillation detected by cardiac implantable electronic devices. Heart Rhythm 11: 719-724.
- Sparks PB, Jayaprakash S, Mond HG, Vohra JK, Grigg LE, et al. (1999) Left atrial mechanical function after brief duration atrial fibrillation. J Am Coll Cardiol 33: 342-349.
- Boriani G, Glotzer TV, Santini M, West TM, De Melis M, et al. (2014) Device-detected atrial fibrillation and risk for stroke: An analysis of .10000 patients from the SOS AF project (Stroke prevention strategies based on atrial fibrillation information from implanted devices). Eur Heart J 35: 508-516.
- Benezet-Mazuecos J, Iglesias JA, Cortés M, Rubio JM, de la Vieja JJ, et al. (2018) Silent atrial fibrillation in pacemaker early post-implantation period: an unintentionally provoked situation?. EP Europace 20: 758-763.
- DeCicco AE, Finkel JB, Greenspon AJ, Frisch DR (2014) Clinical significance of atrial fibrillation detected by cardiac implantable electronic devices. Heart Rhythm 11: 719-724.
- Kaufman ES, Israel CW, Nair GM, Armaganijan L, Divakaramenon S, et al. (2012) Positive predictive value of device-detected atrial high-rate episodes at different rates and du- rations: an analysis from ASSERT. Heart Rhythm 9: 1241-1246.
- Lopes RD, Alings M, Connolly SJ, Beresh H, Granger CB, et al. (2017) Rationale and design of the Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected Sub-Clinical Atrial Fibrillation (ARTESiA) trial. Am Heart J. 189: 137-145.
- Filho MM, et al. SILENT - Subclinical AtrIal FibrilLation and StrokE PreveNtion Trial (SILENT). ClinicalTrials. Gov Identifier: NCT02004509.
- Kirchhof P, Blank B, Calvert M, Camm J, Chlouverakis G, et al. (2017) Probing oral anticoagulation in patients with atrial high rate episodes: rationale and design of the non–vitamin K antagonist oral anticoagulants in patients with Atrial High rate episodes (NOAH–AFNET 6) trial. Am Heart J 190: 12-18.
- Glotzer TV, Hellkamp AS, Zimmerman J, Sweeney MO, Yee R, et al. (2003) Atrial high rate episodes detect- ed by pacemaker diagnostics predict death and stroke: report of the Atrial diagnostics ancillary study of the Mode Selection Trial (MOST). Circulation 107: 1614-1619.
- Swiryn S, Orlov MV, Benditt DG, DiMarco JP, Lloyd-Jones DM, et al. (2016) Clinical implications of brief device-detected atrial tachyarrhythmias in a cardiac rhythm management device population: results from the registry of atrial tachycardia and atrial fibrillation episodes. Circulation 134: 1130-1140.
- January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, et al. (2014) American college of cardiology/american heart association task force on practice G. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the american college of Cardiology/American heart association task force on practice guidelines and the heart rhythm society. J Am Coll Cardiol 64: e1-e76.
- Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, et al. (2014) Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Eur Heart J 31: 2369-2429.
- Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD, et al. (2009) The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk. The TRENDS Study. Circ Arrhythmia Electrophysiol 2: 474-480.
- Boriani G, Glotzer TV, Santini M, West TM, De Melis M, et al. (2014) Device-detected atrial fibrillation and risk for stroke: An analysis of .10000 patients from the SOS AF project (Stroke prevention strategies based on atrial fibrillation information from implanted devices). Eur Heart J 35: 508-516.
- Coppens M, Eikelboom JW, Hart RG, Yusuf S, Lip GYH, et al. (2012) The CHA2DS2-VASc score identifies those patients with atrial fibrillation and a CHADS2 score of 1 who are unlikely to benefit from oral anticoagulant therapy. Eur Heart J 34:170-176.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi