Research Article, J Plant Physiol Pathol Vol: 9 Issue: 2
Early Detection of Fusarium Oxysporum Infection Using Binomial Logistic Regression Models from Visible-Near Infrared Reflectance Spectroscopy
Juan Carlos Marin-Ortiz1*, Lilliana Maria Hoyos-Carvajal1, Verónica Botero-Fernandez2 and Lucio Flavio de AlencarFigueiredo3
1National University of Colombia, Department of Agricultural Sciences, Medellín050034, Colombia
2National University of Colombia, Department of Geosciences and Environment, Medellín050034, Colombia
3University of Brasilia, Department of Botany, Brasília 70910-900, Brazil
- *Corresponding Author:
- Juan Carlos Marín-Ortiz
National University of Colombia, Department of Agricultural Sciences, Medellín 050034, Colombia
E-mail: juancarlosmo@gmail.com
Received Date: January 19, 2020; Accepted Date: February 18, 2021; Published Date: February 26, 2021
Citation: Marin-Ortiz JC, Hoyos-Carvajal LM, Botero-Fernandez V, de Alencar Figueiredo LF (2021) Early Detection of Fusarium Oxysporum Infection Using Binomial Logistic Regression Models from Visible-Near Infrared Reflectance Spectroscopy. J Plant Physiol Pathol 9:2.
Copyright: © All articles published in Journal of Plant Physiology & Pathology are the property of SciTechnol, and is protected by copyright laws. Copyright © 2020, SciTechnol, All Rights Reserved.
Abstract
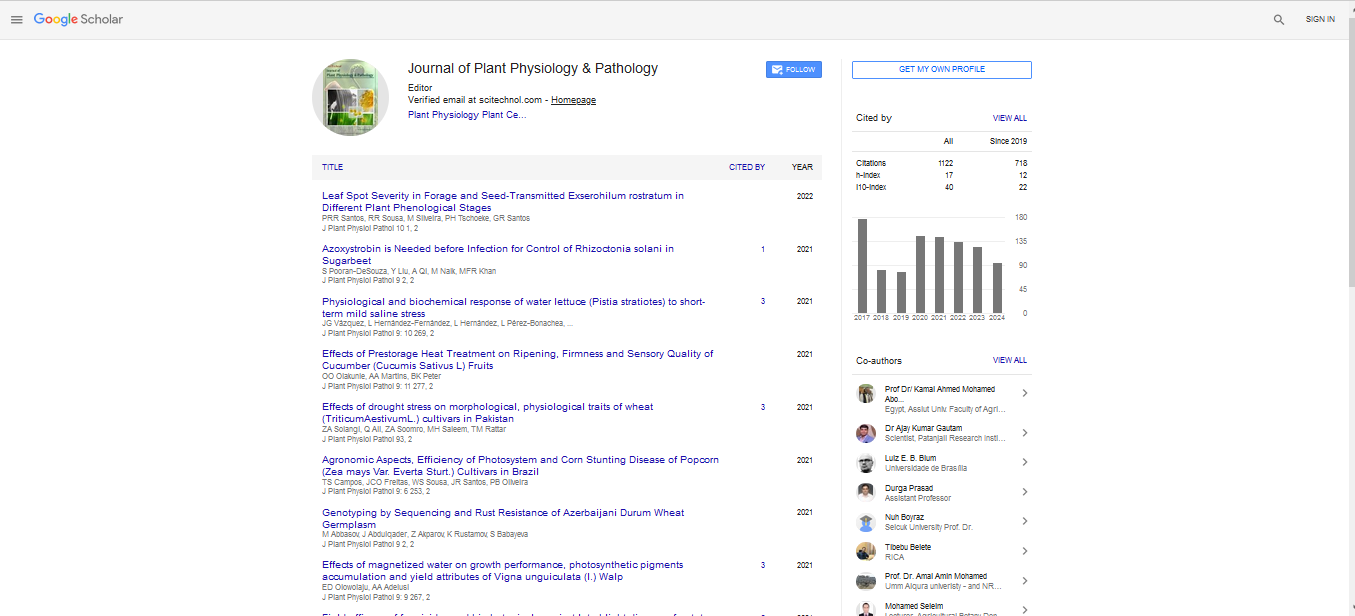
Vascular wilt is a serious threat to a large number of economicallyimportant crops. The evaluation of the disease incidence is done visually, which makes it subjective and delayed, besides, it requires the destructive sampling. The application of the binomial logistic regression models (BLRM) to predict the Fusarium infection using spectra reflectance data in the visible and near-infrared (VIS/NIR) spectral range has not been attempted so far in any of its hosts. The aim of this research was to develop a methodology based on BLRM that allow the incidence detection of Fusarium infection in tomato plants using reflectance spectra. The study was carried out during the asymptomatic period of the disease with two tomato varieties, one tolerant and one susceptible to all races of Fusariumoxysporum. They were developed 16 BLRM, one model per sampling (every three days), which were highly significant (p <0.001) and showed high goodness of fit after 6 days postinfection (DPI). Three key wavelengths that are reliable for fusarium wilt detection wasidentified in a greenhouse setting: reflectances at 430 nm, 550 nm and 750 nm (R430, R550 and R750). In the models developed in tolerant plants only the variables R970 in incidence at 3 dpi (I3dpi) and R704 in incidence at 9 DPI (I9dpi)were not significant.According to the results obtained, the BLRMs generated from the reflectance data in tomato plants have a higher prediction yield.The BLRMs models developed in this research have potential use for rapid detection and non-destructive estimation of vascular wilt incidence in plants during the disease incubation period.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi