Research Article, Int J Cardiovasc Res Vol: 11 Issue: 5
Effectiveness and Safety of Intravenous Alteplase in Patients with Acute Ischemic Stroke: Results of a Single Centre Study
Kumardeep Paul1*, Nadeem Motlekar2, Mrinalini Singh3 and Jerestyn Khapoliwala4
1Department of oncology , Holy Spirit hospital, Mahakali Caves Rd, Sher E Punjab Colony, Andheri East, Mumbai, Maharashtra, India
2Department of Medical Sciences, Holy Spirit hospital, Mahakali Caves Rd, Sher E Punjab Colony, Andheri East, Mumbai, Maharashtra, India
3Department of Clinical Sciences, Holy Spirit hospital, Mahakali Caves Rd, Sher E Punjab Colony, Andheri East, Mumbai, Maharashtra, India
4Department of Cardiology, Boehringer-Ingelheim Mumbai, Maharashtra, India
*Corresponding Author: Kumardeep Paul, Department of oncology, Holy Spirit hospital, Mahakali Caves Rd, Sher E Punjab Colony, Andheri East, Mumbai, Maharashtra, India, Tel: 918451000000; E-mail: kumardeep.paul01@yahoo.in
Received date: 25 Feb, 2022, Manuscript No. ICRJ-22-55524;Editor assigned date: 28 February, 2022, PreQC No. ICRJ-22-55524 (PQ);Reviewed date: 14 March, 2022, QC No. ICRJ-22-55524; Revised date: 26 April, 2022, Manuscript No. ICRJ-22-55524 (R);Published date: 03 May, 2022, DOI: 10.4172/2324-8602.1000470
Citation: Paul K, Motlekar N, Singh M, Khapoliwala J (2022) Effectiveness and Safety of Intravenous Alteplase in Patients with Acute Ischemic Stroke: Results of a Single Centre Study. Int J Cardiovasc Res 11:5.
Abstract
Objective: To evaluate effectiveness and safety of intravenous alteplase (tPA) for the treatment of acute ischemic stroke.
Materials and methods: In this prospective observational study, adult patients with ischemic stroke were treated with intravenous alteplase. We recorded baseline demographics and NIHS score was calculated at baseline, 2 hours, 24 hours and 7 days. Improvement was assessed by evaluating total NIH stroke score at different time points. Based on the neurological assessment, patients were categorised into three types; Unchanged (U), Improving (I) and Deteriorating (D). Blood pressure was closely monitored until 24 hours after infusion of alteplase. Both neurological assessment and blood pressure was monitored every 15 minutes for the first 2 hours after start of infusion then every 30 minutes for next 6 hours and hourly from the post infusion hour until 24 hours after infusion.
Results: Twenty six patients [male 16 (61.50%); female 10 (38.50)] between 34 to 86 years of age were enrolled in this study. Total NIHS score reduced from 10.77 (± 5.01) at pre-treatment to 4.04 (± 4.00) at 7 days. The improvement in NIHS score at two hours versus pre-treatment (p<0.001), at 24 hours versus 2 hours (p=0.002) and 7 days versus 24 hours (p<0.001) was statistically significant. Clinically no significant change was observed in the blood pressure of the patients till 24 hours after thrombolysis. At the end of 24 hours, 40% patients showed improved status and in 60% patients, status was unchanged.
Conclusion: Intravenous alteplase is effective and safe treatment approach for treatment of acute ischemic stroke. No major complications were observed in this study.
Keywords: Acute ischemic stroke; Alteplase; Effectiveness
Introduction
Stroke is an important health problem worldwide with significant contribution of cases from developing countries [1]. In India, it is one of the leading causes of morbidity and mortality [2]. Uncontrolled hypertension, diabetes, smoking and dyslipidemia are the common risk factors for stroke.
Thrombolytic therapy plays a key role in the treatment of acute ischemic stroke for reducing disability [3]. The objective of using thrombolytic therapy in acute ischemic stroke is recanalization of the blocked blood vessel [4]. Intravenous thrombolysis is approved [5] and practiced worldwide. Intravenous and intra-arterial thrombolysis both are commonly practiced in India. However, thrombolytic therapy is not possible in all patients because of late reporting of the patients for treatment after symptom onset. Moreover, many centers are quipped to administer thrombolytic agents for patients with stroke.
Those who receive timely treatment show favorable results. A study in Indian patients showed favorable outcomes at three months in 67.1% thrombolysis patients. Intravenous thrombolysis has become a standard treatment in eligible patients presenting within this time window [6].
Intravenous alteplase i.e. recombinant tissue plasminogen activator is commonly used thrombolytic treatment for acute ischemic stroke. Use of alteplase up to 4.5 hours from the onset of clinical symptoms of acute ischemic stroke provides beneficial effects. A study from India (n=97) showed mean onset to needle time of 177.2 min. The data on effectiveness of intravenous alteplase in the treatment of ischemic stroke in Indian patients are limited.
Objective:The objective of this study was to evaluate effectiveness and safety of intravenous alteplase (tPA) for the treatment of acute ischemic stroke.
Materials and Methods
In this prospective observational study, adult patients (>18 years of age) with ischemic stroke causing measurable deficit and time of symptom onset to potential treatment of less than three hours and admitted in the hospital from 2015 to 2017 were included. Patients with significant head injury or prior stroke in previous 3 months, symptoms suggestive of subarachnoid haemorrhage, history of arterial puncture at a non-compressible site within previous 7 days, history of previous intracranial haemorrhage, history of intracranial neoplasm, arteriovenous malformation or aneurysm, recent intracranial or intraspinal surgery, elevated blood pressure (systolic>185 mm Hg or diastolic>110 mm Hg), active internal bleeding, acute bleeding diathesis including but not limited to platelet count less than 100,000/mm3, use of heparin in the previous 48 hours, resulting in abnormally elevated aPTT greater than the upper limit of normal, current use of anticoagulant with INR>1.7 or PT>15, current use of direct thrombin inhibitors (e.g. Dabigatran) or factor Xa inhibitors (e.g. Rivaroxaban, Apixaban) with elevated sensitive laboratory tests (such as aPTT, INR, platelet count, and ECT; TT; dabigatran level; or appropriate factor Xa activity assays), blood glucose less than 50 mg/dl, CT demonstrating multipolar infarction (hypodensity>1/3 cerebral hemisphere) were excluded from the current study. The relative contraindications for use of IV tPA were only minor or rapidly improving stroke symptoms (clearing spontaneously), pregnancy, seizure at onset with postictal neurological impairments, history of major surgery or serious trauma within the preceding 14 days, recent gastrointestinal or urinary tract haemorrhage (within 21 days) or history of acute myocardial infarction in previous 3 months. If the time of symptom onset to potential treatment was 3 to 4.5 hours, additional exclusion criteria included patients with age more than 80 years of age, severe stroke (NIHS score more than 25), taking an oral anticoagulant regardless of INR or history of diabetes and prior ischemic stroke.
Alteplase was administered as per the approved dosage [7], i.e. 0.9 mg/kg (not more than 90 mg total dose) infused intravenously over 60 minutes with 10% of the total dose administered as an initial bolus.
Patients were evaluated for severity of condition with NIH stroke scale at baseline, 2 hours, 24 hours and 7 days. The scores for all 11 sub-points were recorded at all these time points. Improvement was assessed by evaluating total NIH stroke score8 at different time points.
Based on the neurological assessment, patients were categorised into three types; Unchanged (U), Improving (I) and Deteriorating (D). Blood pressure was closely monitored until 24 hours after infusion of alteplase. Both neurological assessment and blood pressure was monitored every 15 minutes for the first 2 hours after start of infusion then every 30 minutes for next 6 hours and hourly from the post infusion hour until 24 hours after infusion.
Statistical analysisThe collected data from the case report forms was entered in the Microsoft Excel sheet for analysis. Continuous variables are presented as mean and standard deviation whereas categorical variables are presented as number and percentages. ANOVA test was used to examine difference in the NIH stroke score at different time points. Paired t test was used to test significance in the NIH stroke score and blood pressure at different time points. P value less than 0.05 was considered as statistically significant.
Results
A total of 26 patients were enrolled in this study. The study population consisted of 10 (38.50%) female and 16 (61. 50%) male patients (Table 1).
| Parameter | Result |
|---|---|
| Male n (%) | 16 (61.50%) |
| Female n (%) | 10 (38.5%) |
| Mean (± SD) age in years (overall) (n=26) | 58.59 (± 13.77) |
| Mean (± SD) age of males in years (n=16) | 61.50 (15.23) |
| Mean (± SD) age of females in years (n=10) | 56.56 (13.12) |
| Mean blood sugar level (n=20) | 142.05 (± 46.71) |
Table 1: Baseline characteristics.
Minimum and maximum age of patient in the study cohort was 34 years and 86 years respectively. Fifty percentage patients were less than 60 years of age and above 60 years each. Readings of blood glucose level were available in 20 patients with mean level 142.05 (± 46.71) mg/dl (range 63-240 mg/dl).
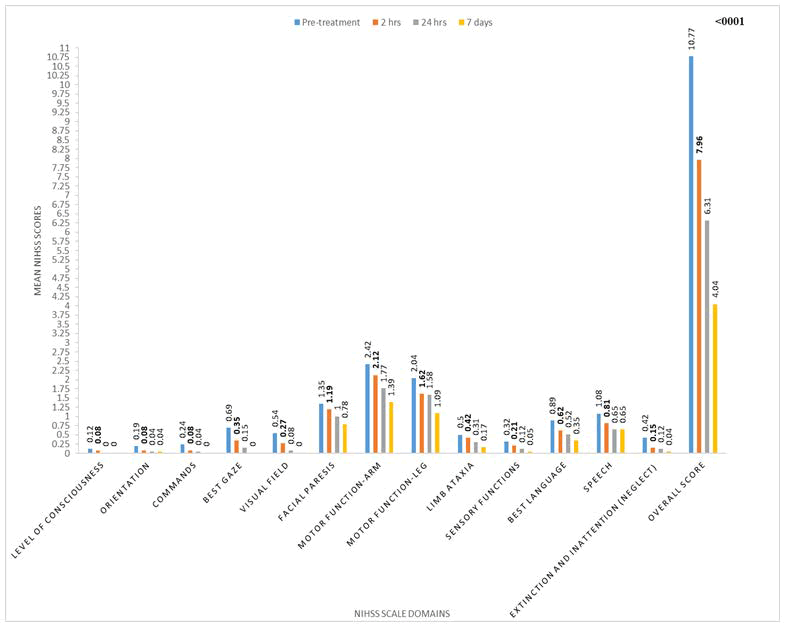
The NIHS score for individual parameters and total score before treatment, at 2 hours, 24 hours and 7 days is given in Graph 1. Total NIHS score reduced from 10.77 (± 5.01) at pre-treatment to 4.04 (± 4.00) at 7 days. The improvement in NIHS score at two hours versus pre-treatment (p<0.001), at 24 hours versus 2 hours (p=0.002) and 7 days versus 24 hours (p<0.001) was statistically significant.
For level of consciousness, orientation, limb ataxia and sensory function there was no significant difference in the NIHS score of pre-treatment versus 2 hours, 2 hours versus 24 hours and 24 hours versus 7 days (Figure 1, Table 2). For commands, the NIHS score significantly improved at 2 hours as compared to pre-treatment score (p=0.043), but the difference between 2 hours versus 24 hours (0.0327) and 24 hour versus 7 days was not significant (p=1.000). Best gaze and visual field score significantly improved at 2 hours compared to pre-treatment. Difference between 24 hours versus 2 hours was also significant for both parameters. For facial paresis, the difference was significant at 24 hours versus 2 hours (p=0.011) and 7 days versus 24 hours (p=0.042). Motor function of arm and leg significantly improved at 7 days versus 24 hours (p=0.008 and p=0.001 respectively). Best language and speech score significantly improved at 2 hours compared to pre-treatment (p=0.016 and p=0.006 respectively).
Systolic blood pressure did not show significant change at most time points till 24 hours except at 30 minutes compared to 15 minutes (p=0.024). Similarly, diastolic blood pressure also was stable at most time points till 24 hours except at 11 to 13 hours (Table 3).
| Time points | Mean (+SD) systolic blood pressure (mm Hg) | Mean (+SD) diastolic blood pressure (mm Hg) | Comparison | P-value | P-value |
|---|---|---|---|---|---|
| (Systolic blood pressure) | (Diastolic blood pressure) | ||||
| 15 min (n=23) | 155.65 (± 28.26) | 92.17 (± 11.26) | - | - | - |
| 30 min (n=23) | 150.87 (± 26.95) | 90.00 (± 12.06) | 15 min vs. 30 min | 0.024 | 0.057 |
| 45 min (n=23) | 152.17 (± 22.15) | 89.35 (± 13.68) | 30 min vs. 45 min | 0.665 | 0.741 |
| 60 min (n=23) | 151.00 (± 21.24) | 88.09 (± 12.16) | 45 min vs. 60 min | 0.581 | 0.497 |
| 1 hr 15 min (n=23) | 150.00 (± 20.89) | 89.57 (± 13.31) | 60 min Vs. 1 hr 15 min | 0.635 | 0.413 |
| 1 hr 30 min (n=23) | 148.26 (± 19.92) | 90.00 (± 12.06) | 1 hr 15 vs. 1 hr 30 min | 0.357 | 0.77 |
| 1 hr 45 min (n=23) | 146.52 (± 20.14) | 89.13 (± 14.11) | 1 hr 30 min vs. 1 hr 45 min | 0.528 | 0.492 |
| 2 hr (n=23) | 146.52 (± 19.21) | 86.96 (± 12.59) | 1 hr 45 min vs. 2 hr | 1 | 0.135 |
| 2.5 hr (n=23) | 146.96 (+ 14.90) | 88.70 (+ 11.00) | 2 hr vs. 2.5 hr | 0.883 | 0.383 |
| 3 hr (n=23) | 147.39 (± 13.56) | 86.09 (± 11.18) | 2.5 hr vs. 3 hr | 0.77 | 0.056 |
| 3.5 hr (n=23) | 145.65 (± 15.02) | 89.13 (± 11.64) | 3 hr vs. 3.5 hr | 0.445 | 0.184 |
| 4 hr (n=23) | 143.91 (+ 18.03) | 86.52 (+ 10.27) | 3.5 hr vs. 4 hr | 0.328 | 0.162 |
| 4.5 hr (n=22) | 143.18 (± 17.29) | 85.91 (± 7.96) | 4 hr vs. 4.5 hr | 0.296 | 0.504 |
| 5 hr (n=22) | 142.27 (± 13.07) | 85.45 (± 8.00) | 4.5 hr vs. 5 hr | 0.628 | 0.747 |
| 5.5 hr (n=22) | 143.91 (± 15.02) | 85.91 (± 7.96) | 5 hr vs. 5.5 hr | 0.406 | 0.771 |
| 6 hrs (n=22) | 144.09 (± 12.97) | 85.00 (± 7.40) | 5.5 hr vs. 6 hr | 0.408 | 0.492 |
| 6.5 hr (n=22) | 143.18 (± 15.24) | 83.18 (± 8.94) | 6 hr vs. 6.5 hr | 0.576 | 0.329 |
| 7 hr (n=22) | 144.09 (+ 14.36) | 84.55 (+ 7.39) | 6.5 hr vs. 7 hr | 0.492 | 0.451 |
| 7.5 hr (n=22) | 144.09 (± 14.03) | 82.27 (± 6.85) | 7 hr vs. 7.5 hr | 1 | 0.135 |
| 8 hr (n=22) | 144.55 (± 14.71) | 84.09 (± 7.96) | 7.5 hr vs. 8 hr | 0.771 | 0.257 |
| 9 hr (n=22) | 141.82 (± 15.63) | 84.09 (± 9.59) | 8 hr vs. 9 hr | 0.186 | 1 |
| 10 hr (n=21) | 141.90 (± 14.70) | 82.38 (± 8.89) | 9 hr vs. 10 hr | 1 | 0.379 |
| 11 hr (n=22) | 140.45 (± 16.47) | 85.45 (± 8.00) | 10 hr vs. 11 hr | 0.452 | 0.11 |
| 12 hr (n=21) | 139.52 (± 18.84) | 81.43 (± 7.93) | 11 hr vs. 12 hr | 0.705 | 0.009 |
| 13 hr (n=22) | 139.09 (± 18.49) | 85.00 (± 9.13) | 12 hr vs. 13 hr | 0.789 | 0.042 |
| 14 hr (n=20) | 140.50 (± 16.69) | 83.50 (± 9.88) | 13 hr vs. 14 hr | 0.234 | 0.591 |
| 15 hr (n=22) | 140.91 (± 15.71) | 83.64 (± 7.90) | 14 hr vs. 15 hr | 0.494 | 1 |
| 16 hr (n=20) | 140.00 (± 17.77) | 83.50 (± 9.33) | 15 hr vs. 16 hr | 0.825 | 1 |
| 17 hr (n=22) | 138.64 (± 18.33) | 82.27 (± 7.52) | 16 hr vs. 17 hr | 0.481 | 0.453 |
| 18 hr (n=20) | 138.50 (± 15.31) | 82.00 (± 9.51) | 17 hr vs. 18 hr | 1 | 1 |
| 19 hr (n=22) | 138.64 (± 13.90) | 81.82 (± 7.33) | 18 hr vs. 19 hr | 0.789 | 0.748 |
| 20 hr (n=20) | 138.50 (± 15.99) | 83.00 (± 9.79) | 19 hr vs. 20 hr | 0.748 | 0.419 |
| 21 hr (n=22) | 138.64 (± 17.54) | 80.91 (± 11.09) | 20 hr vs. 21 hr | 0.815 | 0.09 |
| 22 hr (n=20) | 135.50 (± 17.61) | 79.00 (± 9.12) | 21 hr vs. 22 hr | 0.33 | 0.716 |
| 23 hr (n=22) | 137.73 (± 17.71) | 80.91 (± 11.09) | 22 hr vs. 23 hr | 0.297 | 0.494 |
| 24 hr (n=20) | 136.00 (± 19.84) | 81.50 (± 10.40) | 23 hr vs. 24 hr | 0.186 | 0.33 |
Table 2: Time point-wise analysis of blood pressure.
Overall status of patient at different time points is shown in table 4. At 1 hours, 52% patients showed improved status whereas at the end of 6 hours, 37.5% patients had improved status. After 24 hours, 40% patients showed improved status whereas in remaining 60% patients, status was unchanged(Table 3).
| Time | Improved n (%) | Unchanged n (%) | Deteriorated n (%) |
|---|---|---|---|
| 15 min (n=25) | 3 (12%) | 22 (88%) | 0 |
| 30 min (n=25) | 5 (20%) | 20 (80%) | 0 |
| 45 min (n=25) | 9 (36%) | 14 (56%) | 2 (8%) |
| 1 hr (n=25) | 13 (52%) | 11 (44%) | 1 (4%) |
| 1.15 hr (n=25) | 11 (44%) | 12 (48%) | 2 (8%) |
| 1.30 hr (n=25) | 8 (32%) | 14 (56%) | 3 (12%) |
| 1.45 hr (n=25) | 4 (12%) | 18 (54%) | 3 (12%) |
| 2 hr (n=25) | 5 (20%) | 17 (68%) | 3 (12%) |
| 2.5 hr (n=25) | 10 (40%) | 13 (52%) | 2 (8%) |
| 3 hr (n=25) | 9 (36%) | 16 (64%) | 0 |
| 3.5 hr (n=25) | 7 (28%) | 18 (72%) | 0 |
| 4 hr (n=25) | 6 (24%) | 19 (76%) | 0 |
| 4.5 hr (n=25) | 6 (24%) | 19 (76%) | 0 |
| 5 hr (n=25) | 5 (20%) | 18 (72%) | 2 (8%) |
| 5.5 hr (n=24) | 6 (25%) | 15 (62.5%) | 3 (12.5%) |
| 6 hr (n=24) | 9 (37.5%) | 13 (54.17%) | 2 (8.33%) |
| 6.5 hr (n=24) | 8 (33.33%) | 16 (66.67%) | 0 |
| 7 hr (n=24) | 4 (16.67%) | 20 (83.33%) | 0 |
| 7.5 hr (n=24) | 9 (37.5%) | 15 (62.5%) | 0 |
| 8 hr (n=24) | 7 (29.17%) | 17 (70.83%) | 0 |
| 9 hr (n=25) | 10 (40%) | 14 (56%) | 1 (4%) |
| 10 hr (n=25) | 7 (28%) | 17 (68%) | 1 (4%) |
| 11 hr (n=24) | 10 (41.67%) | 13 (54.17%) | 1 (4.17%) |
| 12 hr (n=24) | 11 (45.83%) | 13 (54.17%) | 0 |
| 13 hr (n=24) | 8 (33.33%) | 16 (66.67%) | 0 |
| 14 hr (n=23) | 7 (30.43%) | 16 (69.56%) | 0 |
| 15 hr (n=23) | 6 (26.09%) | 16 (69.57%) | 1 (4.35%) |
| 16 hr (n=23) | 8 (34.78%) | 15 (65.22%) | 0 |
| 17 hr (n=23) | 8 (34.78%) | 15 (65.22%) | 0 |
| 18 hr (n=23) | 8 (34.78%) | 15 (65.22%) | 0 |
| 19 hr (n=23) | 3 (13.04%) | 20 (86.96%) | 0 |
| 20 hr (n=24) | 4 (16.7%) | 20 (83.3%) | 0 |
| 21 hr (n=24) | 6 (25%) | 18 (75%) | 0 |
| 22 hr (n=25) | 9 (36%) | 16 (64%) | 0 |
| 23 hr (n=25) | 10 (40%) | 15 (60%) | 0 |
| 24 hr (n=25) | 10 (40%) | 15 (60%) | 0 |
Table 3: Overall status of patient.
Discussion
Thrombolytic therapy represents an important intervention in patients with acute ischemic stroke because of its efficacy [8-11]. A Cochrane review has shown that thrombolytic therapy given up to six hours after stroke shows beneficial effects. Treatment within first three hours provides more benefits than treatment given later [12].
In this study we evaluated efficacy and safety of tissue plasminogen activator in patients with acute ischemic stroke. Overall, intravenous rtPA (alteplase) was found to be effective in reducing NIHSS score from baseline to 7 days. The difference in the score was statistically significant at all evaluated time-points. The results suggest confirm the beneficial effects of early thrombolysis in patients with acute ischemic stroke.
Studies showing impact of intravenous thrombolysis on individual NIHS scale items are limited. In this regards, our study provides significant insights. For commands, best gaze, visual field, best language, speech and extinction and inattention (neglect) simultaneous stimulation with visual and tactile stimulus scores significantly improved at 2 hours compared to pre-treatment. Timely thrombolysis seems to have early effect on these functions. Motor function of arm and leg significantly improved at 7 days versus 24 hours. As reported in the literature [13], intravenous tissue-plasminogen activator in our study was found to be effective and useful in most patients with acute ischemic stroke presenting within the suitable time window.
Significant increase in blood pressure is common in patients receiving intravenous thrombolysis [14]. In the SAMURAI rt-PA registry, early variations in systolic blood pressure were shown to be positively associated with symptomatic intracerebral hemorrhage and death in patients receiving intravenous thrombolysis [15]. Similarly, a retrospective analysis suggested that in patients receiving tissue plasminogen activator for stroke, absence of hypertension at the time presentation does not preclude the risk of rise in blood pressure. Therefore, control of blood pressure control is very important in patients treated with tissue plasminogen activator for ischemic stroke. It is recommended be monitored every 15 minutes to 1 hour for 24 hours in these patients [16]. In this study, we evaluated efficacy of intravenous alteplase with close observation of blood pressure. We also evaluated the status of patients at different time points till 24 hours after administration of alteplase. In our study, there were no clinically significant fluctuations in the blood pressure till 24 hours. It is reported that if there is no increase in blood pressure during the first 6 hours, subsequent hypertension over the next 18 hours is unlikely.
Thrombolytic therapy in acute ischemic stroke is associated with risk of brain haemorrhage in about five to ten percentage of patients and it can be fatal. Considering this risk, tissue plasminogen activator should be administered carefully after analysing its risk benefit ratio [17]. In our study, intravenous alteplase was well tolerated without intracranial bleeding or other significant complications.
Hyperglycemia and intracranial haemorrhage are shown to be independent predictors of hyper acute worsening in patients with acute ischemic stroke receiving thrombolysis [18]. Hence, reducing risk of bleeding in hyperglycemic setting is important [19]. In our study, maximum blood glucose in one patient was 240 mg/dl. Hyperglycemia was not of a concern in our study patients.
A study (n=201) patients with acute ischemic stroke receiving thrombolysis within 6 hours of symptom onset showed worsening in 13% patients after 24 hours. Improvement and unchanged status was observed in 39% and 48% patients after 24 hours of thrombolysis. The percentage of patients showing improvement was similar in our study. Another report also suggested improvement (i.e. NIHS score <4) in 41% patients at day 7 [20].
As reported in other studies [21], our results suggest that alteplase is effective and safe for treatment of acute ischemic stroke.
In our study, thrombolysis was well tolerated. No life-threatening or major bleeding complications were observed in any patients.
Small sample size and single centre data are the limitations of our study. Larger studies are required to confirm our observations.
Conclusion
Intravenous alteplase is effective and safe treatment approach for treatment of acute ischemic stroke. No life-threatening bleeding complications were observed in any patient. There were no clinically significant changes in blood pressure till 24 hours.
References
- Banerjee TK, Das SK (2016) Fifty years of stroke researches in India. Ann Indian Acad Neurol 19:1-8.
- Pandian JD, Sudhan P (2013) Stroke epidemiology and stroke care services in India. J Stroke 15:128-34.
- Robinson T, Zaheer Z, Mistri AK (2011) Thrombolysis in acute ischaemic stroke: An update. Ther Adv Chronic Dis 2:119-131.
- Kirmani JF, Alkawi A, Panezai S, Gizzi M (2012) Advances in thrombolytics for treatment of acute ischemic stroke. Neurology 79:119-125.
- Furlan AJ, Katzan IL, Caplan LR (2003) Thrombolytic therapy in acute ischemic stroke. Curr Treat Options Cardiovasc Med 5:171-180.
- Mehta A, Mahale R, Buddaraju K, Majeed A, Sharma S, et al. (2017) Intravenous Thrombolysis for Acute Ischemic Stroke: Review of 97 Patients. J Neurosci Rural Pract 8:38-43.
- Alteplase for Acute Ischemic Stroke in Patients Aged >80 Years (2011) Actilyse Prescribing information. Boehringer Ingelheim Limited. 2020. 2322–2331.
- National Institute of Neurological Disorders and Stroke (2022)Know stroke know the signs. Act in time. NIH Stroke Scale.
- Suwanwela NC, Phanthumchinda K, Likitiaroen Y (2006) Thrombolytic therapy in acute ischemic stroke in Asia: The first prospective evaluation. Clin Neurol Neurosurg 108:549-552.
- Overgaard K, Sperling B, Bovsen G, Pedersen H, Gam J, et al. (1993) Thrombolytic therapy in acute ischemic stroke. A Danish pilot study. Stroke 24:1439-1446.
- Padma V, Fisher M, Moonis M (2005) Thrombolytic therapy for acute ischemic stroke: 3h and beyond. Expert Rev Neurother 5:223-233.
- Wardlaw JM, Murray V, Berge E, del Zoppo GJ (2014) Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev 2014:213.
- Tsivgoulis G, Kargiotis O, Alexandrov AV (2017) Intravenous thrombolysis for acute ischemic stroke: a bridge between two centuries. Expert Rev Neurother 17:819-37.
- Kellert L, Rocco A, Sykora M, Hacke W, Ringleb PA (2011) Frequency of increased blood pressure levels during systemic thrombolysis and risk of intracerebral haemorrhage. Stroke 42:1702-1706.
- Endo K, Kario K, Koga M, Nakagawara J, Shiokawa Y, et al. (2013) Impact of early blood pressure variability on stroke outcomes after thrombolysis. The SAMURAI rt-PA registry. Stroke 44:816-818.
- Aiyagari V, Gujjar A, Zazuvlia AR, Diringer MN (2004) Hourly blood pressure monitoring after intravenous tissue plasminogen activator for ischemic stroke. Does everyone need it? Stroke 35:2326-2330.
- Canadian Association of Emergency Physicians Committee on Thrombolytic Therapy for Acute Ischemic Stroke (2001) Thrombolytic therapy for acute ischemic stroke. CJEM 3:8-12.
- Leigh R, Zaidat OO, Suri MF, Lynch G, Sundararajan S, et al. (2004) Predictors of hyperacute clinical worsening in ischemic stroke patients receiving thrombolytic therapy. Stroke 35:1903-1907.
- Hafez S, Coucha M, Bruno A, Fagan SC, Ergul A (2014) Hyperglycemia, acute ischemic stroke, and thrombolytic therapy. Transl Stroke Res 5:442-453.
- Roje-Bedekovic M, Vargek-Solter V, Coric L, Sabolek K, Breitendeld T, et al.(2009) Thrombolysis for acute ischemic stroke-our experiences as part of SITS-MOST. Acta Clin Croat 48:287-293.
- Hill MD, Buchan AM (2005) Canadian Alteplase for Stroke Effectiveness Study (CASES) Investigators. Thrombolysis for acute ischemic stroke: results of the Canadian Alteplase for Stroke Effectiveness Study. CMAJ 172:1307-1312.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi