Short Communication, J Infect Dis Immune Ther Vol: 5 Issue: 5
Evaluating Effectiveness of Remdesivir on Intubation Rates in Patients Hospitalized with Severe COVID-19 at Tuba City Regional Health Care Corporation (TCRHCC)
Kyle Nguyen* and Lena Wong
Department of Pharmacy, Tuba City Regional Health Care Corporation, Tuba City, AZ 86045, USA
*Corresponding Author:
Kyle Nguyen, Department of Pharmacy
Tuba City Regional Health Care Corporation, Tuba City, AZ 86045, USA
E-mail: Kyle.nguyen@tchealth.org
Received: August 13, 2021; Accepted: August 27, 2021; Published: September 03, 2021
Citation: Nguyen K, Wong L (2021) Evaluating Effectiveness of Remdesivir on Intubation Rates in Patients Hospitalized with Severe COVID-19 at Tuba City Regional Health Care Corporation (TCRHCC). J Infect Dis Immune Ther 5:5.
Abstract
Background: TCRHCC is a Joint Commission accredited health center providing services to more than 15,000 patients within 6,000 square mile area of the Navajo and Hopi Reservations. As of April 20, 2021, TCRHCC was heavily burdened by COVID-19 with over 5,000 positive cases which are 16.7% of the Navajo Nation COVID-19 positive cases and over 1,000 deaths. On May 21, 2020, TCRHCC Pharmacy and Therapeutics Committee (P&T) added remdesivir on restricted formulary as a treatment option for hospitalized patients with severe COVID-19. This study has been established to evaluate rate of intubation in COVID-19 patients before and after the training, TCRHCC remdesivir criteria compliance, hospitalized COVID-19 patient discharge to home, and transfer out to higher level of care.
Methods: A pre-training retrospective chart review of hospitalized COVID-19 patients eligible to receive remdesivir (n=30) was completed between May 21, 2020 to August 31, 2020 to evaluate initial intubation rates. Training includes education on P&T recommendation of use, appropriate transfer log documentation, and communication among departments was provided to inpatient clinical pharmacists, intensive care and respiratory unit nursing, patient care manager/transfer team from Dec 21 to Dec 31, 2020. Post-training retrospective chart review (n=95) was then completed for the time period between Jan 1 to March 31, 2021 to evaluate the objectives.
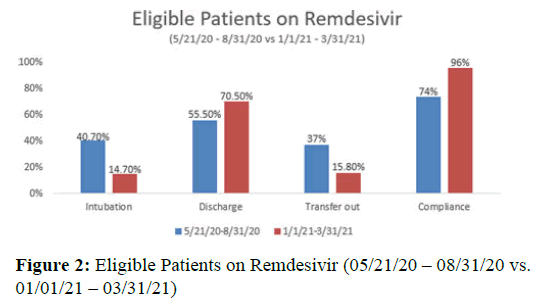
Results: Data between pre and post-training showed: Intubation requirement decreased 26%, TCRHCC remdesivir criteria compliance increased 22%, hospitalized COVID-19 patients discharge to home increase 15%, and transfer out to higher level of care decreased 21%.
Conclusion: Our study showed improvement in intubation requirements in hospitalized COVID- 19 patients using TCRHCC remdesivir restrictions. However, we could not conclude the training and remdesivir utilization improved in hospitalized COVID-19 patients’ clinical outcomes due to vaccine availability, addition of other treatments (dexamethasone, continuous positive airway pressure), study design and improved nursing staff.
Keywords: Remdesivir; Veklury(r); COVID-19; Intubation
Keywords
Remdesivir; Veklury(r); COVID-19; Intubation
Description
Tuba City Regional Health Care Corporation (TCRHCC) is a Joint Commission accredited health center on the Navajo Nation that provides services to more than 15,000 patients within 6,000 square mile area of the Navajo and Hopi Reservations.
As of April 20, 2021, TCRHCC was heavily burdened by COVID-19 with over 5,000 COVID-19 positive cases which are 16.7% of the Navajo Nation COVID-19 positive cases and over 1,000 deaths.
The U.S. Food and Drug Administration (FDA) approved the use of remdesivir in October 2020 for the treatment of hospitalized COVID-19 patients and in December 2020, the Infectious Diseases Society of America (IDSA) released guidelines recommending remdesivir as the treatment for COVID-19.
On May 21, 2020, TCRHCC Pharmacy and Therapeutics Committee (P&T) added remdesivir as a treatment option for hospitalized COVID-19 adult patients (age>18) requiring >5L of O2 by nasal cannula and must start within first 24 hours of admission.
The suggested dose for adults not requiring invasive mechanical ventilation is a single dose of 200 mg infused intravenously over 30 to 120 minutes on Day 1 followed by once-daily maintenance doses of 100 mg infused intravenously over 30 to 120 minutes for 4 days (days 2 through 5) [1]. Patients who do not demonstrate clinical improvement may extend treatment to 5 additional days for a total of 10 days.
Remdesivir use requires daily liver function and eGFR monitoring and cannot be administered concurrently with other IV medications. Adverse drug reactions must be reported in the hospital monitoring system.
Hospitalized COVID-19 patients were observed to require intubation more frequently than other pneumonia. This project has been established to accomplish the following goals:
• Evaluate intubation rate in COVID-19 patients before and after the training
• Evaluate TCRHCC remdesivir criteria compliance rate
• Evaluate hospitalized COVID-19 patient’s discharge to home rate
• Evaluate transfer out rate to higher level of care in COVID-19 patients
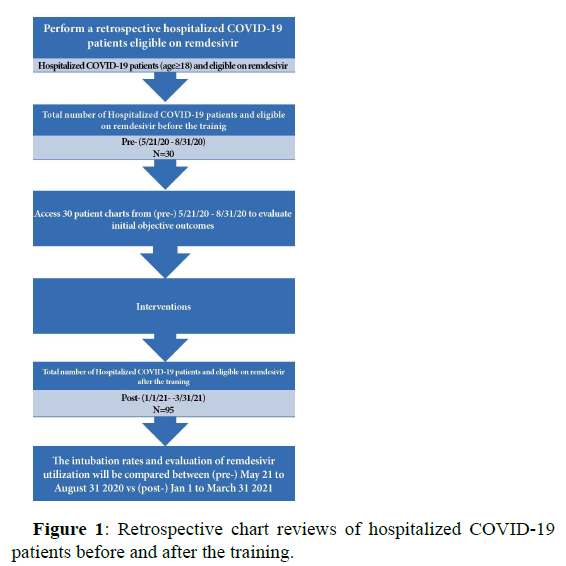
A pre-training retrospective chart review of hospitalized COVID-19 patients eligible to receive remdesivir (n=30) was completed between May 21, 2020 to August 31, 2020 to evaluate initial intubation rates in hospitalized COVID-19 patients [2] (Figures 1 and 2).
Training includes education on remdesivir administration, P&T recommendation of use, appropriate transfer log documentation, and communication among departments was provided to inpatient clinical pharmacists, intensive care and respiratory unit nursing, patient care manager and transfer team from Dec 21 to Dec 31, 2020. Post-training retrospective chart review (n=95) was then completed for the time period between Jan 1 to March 31, 2021 to evaluate the objectives. The pre- and post- evaluation of intubation rates in hospitalized COVID-19 patients will be compared between the time periods to evaluate the outcome objectives [3-6]. In comparing the date between pre and post-training, the requirement of intubation in hospitalized COVID-19 patients who received remdesivir were less than the hospitalized COVID-19 patients prior to the training. Chart review also showed patient’s outcome improvement after the training:
• Intubation rate decreased 26%
• TCRHCC remdesivir criteria compliance rate increased 22%
• Hospitalized COVID-19 patients discharge to home rate increase 15%
• Hospitalized COVID-19 patients transfer out rate to higher level of care decreased 21%
Conclusion
Our study showed that use of remdesivir in hospitalized COVID-19 adult patients (age>18) requiring >5L of O2 by nasal cannula and started within first 24 hours of admission showed a decrease in intubation rate.
However, we could not conclude the interventions and remdesivir utilization improved in hospitalized COVID- 19 patients’ clinical outcomes due to vaccine availability, addition of other treatment (dexamethasone, continuous positive airway pressure), study design and improved nursing staff.
Study Limitation
This retrospective study and the data from the observation enable frequency analysis quantitatively, but it does not permit statistical testing. The study sample group is small and could not be generalized.
Disclosure
The authors have no actual or potential conflicts of interest in relation to this study and did not receive any funding to support the study.
References
- Bhimraj A, Morgan RL, Shumaker AH, Lavergne V, Baden L, et al., (2020) Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Clin Infect Dis.
- Coronavirus 19/COVID19 Dash board. Service Unit Map. TCRHCC: Coronavirus Information Dashboard.
- COVID-19: Navajo Department of Health Navajo Nation.
- U.S.Food & Drug. Remdesivir
- Beigel JH, Tomashek K M, Dodd L E , Mehta A K , Zingman B S , et al., (2020) Remdesivir for the Treatment of Covid-19-Final Report. The New England Journal of Medicine. N Engl J Med. 383:1813-1826
- Tracking Coronavirus in Arizona. The New York Times. Arizona Coronavirus Map and Case Count: The New York Times (nytimes.com). Accessed Oct 30 2018.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi