Research Article, J Plant Physiol Pathol Vol: 9 Issue: 8
High Salinity Induced Alternations of Protein Profile and Antioxidant Activity in Rice (Oryza sativa L.)
Abhaya Kumar Sahu, Punam Kumari and Bhabatosh Mittra**
Department of Biosciences and Biotechnology, Fakir Mohan University, Vyasa Vihar, Balasore-756089, Odisha, India
- *Corresponding Author:
- Dr. Bhabatosh Mittra
Department of Biosciences and Biotechnology, Fakir Mohan University, Vyasa Vihar, Balasore-756089, Odisha, India
Tel: +91 9938984421
E-mail: bhaba_mit@yahoo.co.uk
Received Date: July 03, 2021; Accepted Date: August 11, 2021; Published Date: August 18, 2021
Citation: Sahu AK, Kumari P, Mittra B (2021) High Salinity Induced Alternations of Protein Profile and Antioxidant Activity in Rice (Oryza sativa L.). J Plant Physiol Pathol 9:8. 259.
Copyright: © All articles published in Dental Health: Current Research are the property of SciTechnol, and is protected by copyright laws. “Copyright © 2021, SciTechnol, All Rights Reserved.
Abstract
Indicator of oxidative stress such as H2O2 content was elevated steadily in shoot tissues of rice seedlings with the linear increment of salinity concentrations, the increase was significant in both control and salt-stressed seedlings. Interestingly, the oxidants were increased slightly in desalinized seedlings (400mM NaCl experienced seedlings transferred to 0mM NaCl concentration). Twenty-one days old seedlings of rice (Oryza sativa L.) treated with different concentrations of NaCl (100, 200, and 400mM) under hydroponic culture. A significant amount of total soluble proteins were drawn out from different seedlings after 7d of treatment and partially characterized by SDS-PAGE. The comparative protein profile analyzed on SDS-PAGE indicated an apparent Salt- Sensitive Protein (SSP)-23-kDa protein which was decreased with proportionally increased NaCl concentrations whereas reappeared in desalinized condition. The antioxidant enzymes viz. SOD and APX were significantly higher in shoot tissues of salt-stressed as compared to the control seedlings. Salt-stressed also induces the high content of GSSG significantly in seedlings. The nonenzymatic antioxidant molecules such as reduced GSH content were decreased in salt-stressed seedlings as compared to control, interestingly it was decreased again in desalinized seedlings. This suggests that all oxidants, enzymatic antioxidants, and antioxidants were changed in cellular level collinearly with perturbation of protein profile in both salinized and desalinized shoot tissues of seedlings.
Keywords: Oryza sativa; Salt stress; Oxidative stress; Oxidants; Antioxidants; Antioxidant enzymes; Salt-sensitive protein
Introduction
A continuous encroachment of sea to terrestrial land has converted normal soils to salinity possibly because of salt accumulation, where inexperienced soil dwellers including plant species, animals, and below-ground species are perturbed and struggle to survive because of the induced salt stress. Specifically, salt stress is one of the most detrimental environmental threats to crop production worldwide [1].
Adverse environmental conditions caused by salt limit crop growth and productivity [2]. Most plants including crop species withstand a limited concentration of NaCl but at higher concentrations, their growth and development are retarded [3]. Salinity stress leads to a variety of fundamental changes in physiological-biochemical, and molecular pathways either make some species susceptible or tolerant depending on their genotypes. High salinity stands responsible for hyper ionic and hyperosmotic effects, which ultimately trigger the demise of the plant [3]. Salt stress generated a high amount of Active Oxygen Species (AOS) divides into radicals such as superoxide anion (O2-), Hydroxyl Radical (OH-) and non-radicals Hydrogen Peroxide (H2O2), and Singlet Oxygen (1O2) which further triggers the oxidative stress. Plant cells mitigate ROS by up regulation of enzymatic antioxidants such as Superoxide Dismutase (SOD), Ascorbate Peroxidase (APX), Peroxidase (POX), Catalase (CAT), and Glutathione Reductase (GR) while non-enzymatic antioxidants such as ascorbic acid, glutathione, and α-tocopherol. Many reports about antioxidant activity and NaCl stressed are already published in variety of plant species [4,5]. Although adequate reports are available in this regard, still salt stress-induced oxidative status and their role in the maintenance of redox state is still awaited. The present work unravels and reports on the mechanism of salt-sensitive evaluated on the basis of their oxidants and antioxidant machinery with the modulation of protein changes in case of a rice species (Oryza sativa L. Niranjan) coped to saline habitat. Further, it has been investigated if any macromolecules specifically protein gets perturbed concerning variation in NaCl concentrations and indicative of a salt stress marker.
Materials and Methods
NaCl treatment and germination
Healthy rice seeds obtained from local cultivars (Oryza sativa L. Niranjan) were surface sterilized with 0.1 percent HgCl2 with an incubation time of 2-3 minutes. Seeds were then isolated and washed with distilled autoclaved water at an interval of 5 minutes each. In the pre-UV-treated Laminar airflow chamber, seeds were then transferred to autoclaved Petri plates precoated with cleaned muslin cloths. Then plates containing seeds were kept and maintained under the dark condition for 48h. Under the laminar air flow chamber, germinated seedlings were aseptically transferred to autoclaved test tubes and For seven days, the plants were kept in a development room at 26°C and 80% absolute humidity as during 14-hour photoperiod and 22°C and 70% relative humidity during the 10-hour dark period, until they reached the two-leaf stage. Four sets of experimental set-up were made considering fifty seedlings in each group. An experimental group consisting of fifty seedlings was treated with 100, 200 and 400mM NaCl concentrations except for one group which was kept untreated and served as control. After the treatment, seedlings were kept under observation in a growth room for another seven days. Seedlings experienced to 400 m M NaCl concentration for twentyone days were carefully transferred to a hydroponic solution without any salt concentration and Over the next seven days, the plant was allowed to flourish.
Assay of H2O2
The H2O2 content was determined using the method [6]. Healthy tissues in both the shoot and the root were homogenised in 0.1 percent (w/v) Trichloro Acetic Acid (TCA) and stirred for 15 minutes at 12,000 rpm. The supernatants were obtained in sterile Eppendorf tubes, as well as a chemical combination of 0.5 ml supernatant and 0.5 ml 10 mM phosphate buffer was created (pH 7.0), and 1 ml of KI was added, the absorbance was taken in 390 nm and using UVVIS spectrophotometer. Using an extinction value of 0.28M-1cm-1, the amount of H2O2 was calculated and represented as M g-1f.w.
Extraction of total Soluble Protein (TSP)
According to [7] the TSP was isolated from different fresh shoot tissues and prepared extracts using chilled 10mM sodium phosphate buffer (pH 7.5) followed by centrifugation at 10,000rpm (2,500g) for 10 mins. Refined TSP was obtained after the purified supernatants were processed and PEG treated.
Partial characterization of total soluble protein by SDS-PAGE
The individual TSP were characterized on 10% SDS-PAGE accordingly [8] and were silver stained. The developed gel was photographed and the banding pattern was comparatively analyzed.
Assay of Superoxide Dismutase (SOD)
The activity of superoxide dismutase (SOD) was calculated using Beyer and Fridovich’s method [9]. The lysates were produced from tissues using 2 ml of 50 mM phosphate buffer (pH 7.0) containing 1 mM EDTA (w/v) and 2 percent Polyvinyl pyrrolidine (w/v), centrifuged at 13,000 rpm for 20 minutes at 40°C, as well as the supernatant was utilised for the enzyme analysis. The reaction mixture consisted 1.5 ml of 50 mM phosphate buffer(pH 7.0), 0.3 ml of 130 mM L-methionine (w/v), 0.3 ml of 750 μM NBT(w/v),0.3 ml of 100 μM EDTA (w/v), 0.3 ml of 20 μM Riboflavin (w/v), 0.25 ml of distilled water and 50 μl enzyme extract and measured at 580nm. Each unit of SOD was determined as the photo-inhibition of NBT by 50%, and the enzyme activity was represented as Unit g-1 fresh weight.
Assay of Ascorbate Peroxidase (APX)
The activity of Ascorbate Peroxidase (APX) was determined utilising Nakano and Asada technique [10]. Fresh tissues (0.5g) were homogenised in 2 ml of 50mM phosphate buffer (pH 7.0) containing 1mM EDTA (w/v) and 2% Polyvinylpyrrolidine (w/v), and the lysates were centrifuged at 13,000 rpm for 20 minutes at 40°C, following which the supernatant was used for enzyme testing. The reaction mixture contained 600 μl of 50 mM phosphate buffer (pH 7.0), 100 μl of 1mM EDTA (w/v), 100 μl of 5 mM ascorbic acid (w/v),100 μl of 1mM H2O2 (w/v) and 100μl of enzyme extract. The ascorbate peroxidase content was estimated at 290nm and expressed as unit g-1 fresh weight.
Assay of oxidized GSH (GSSG) and reduced GSH content
According to Anderson ME [11] the reduced glutathione (GSH) and GSSG contents were assayed. Fresh tissues 0.5g from each sample were homogenized separately with 2.5 ml of 5% 5-Sulfo Salicylic Acid (SSA) and centrifuged at 10,000 rpm for 10 min at 2-4°C. To determine the total GSH content of supernatants by the 5,5’-Di Thiobis-2-Nitro Benzoic acid (DTNB)-GSSG reductase method. The rate of production of 5-Thio-2-Nitro Benzoate (TNB) at 412nm was used to boost the accuracy of the GSH standard curve. To trap the GSH, 2 μl of 4-vinyl pyridine(w/v) was used for estimation of GSSG, which present in the 5-sulfosalicylic acid supernatant solution. The GSSG content was then determined once again using the DTNBGSSG reductase technique. Reduced GSH and GSSG content were expressed as Unit g-1 fresh weight.
Results
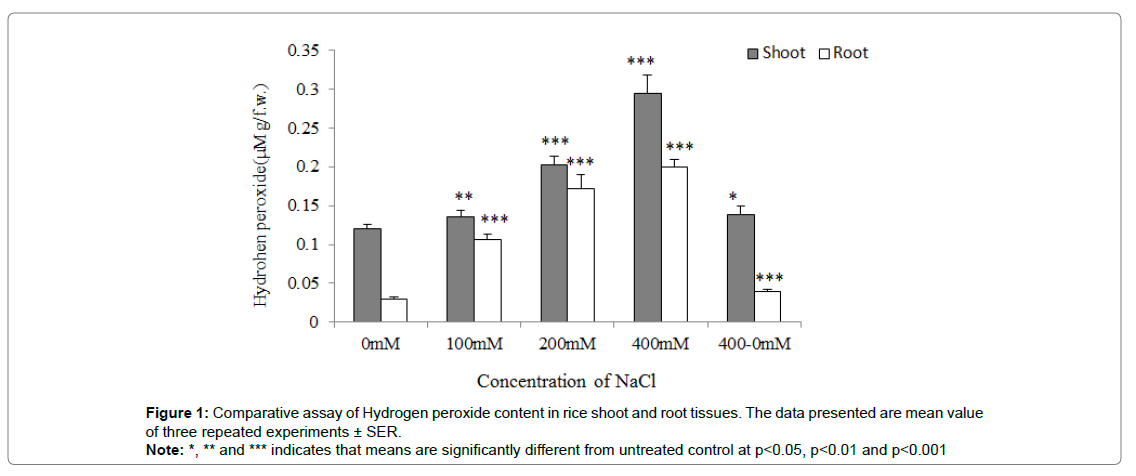
Estimation of Hydrogen Peroxide (H2O2) content
H2O2 level was observed to be minimum at untreated control seedlings as compared to the seedlings grown at 400mM NaCl concentration where H2O2 concentration was observed to be maximum as significantly (p˂0.001). The H2O2 concentration was observed to be gradually increased in case of seedlings experienced to 100 and 200mM NaCl concentration as compared to untreated control at p<0.01 and p<0.001. Interestingly, seedlings pre-experienced to 400mM NaCl allowed to grow at 0mM NaCl for another 7d showed a marginally significant decrease in H2O2 content (p˂0.05). The H2O2 content was estimated marginal in root tissues as compared to shoot tissues significantly (Figure 1).
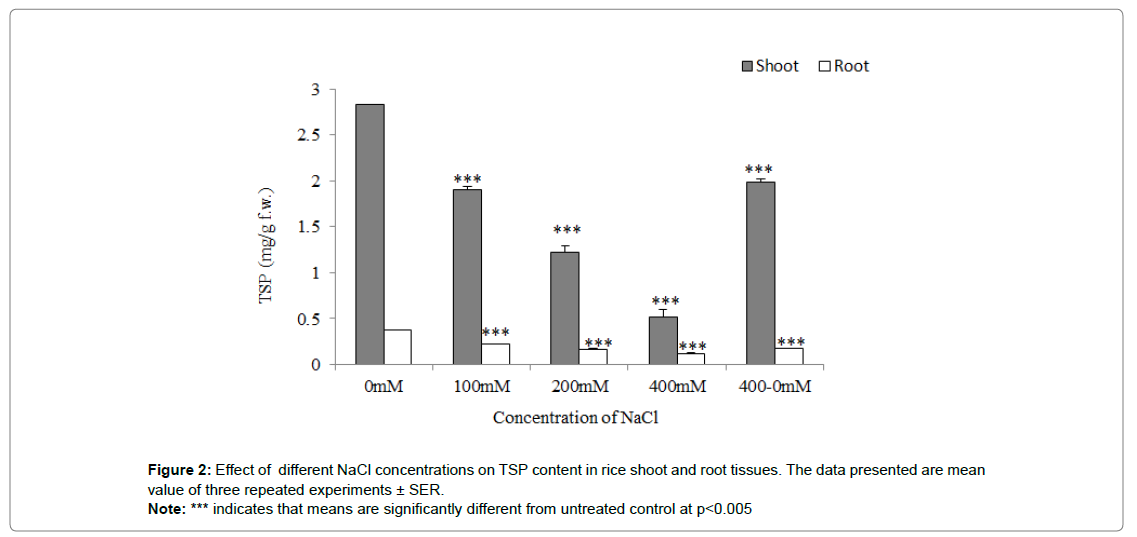
Total Soluble Protein (TSP) content
The concentration of TSP was determined in 400mM NaCl treated seedlings was estimated low significantly as compared to control (0mM NaCl-treated) at p˂0.001. The 100 and 200mM NaCl treated shoot was also found to be significantly reduced TSP content as compared to the control at p˂0.001. The TSP content was estimated marginal in root tissues as compared to shoot tissues significantly (Figure 2).
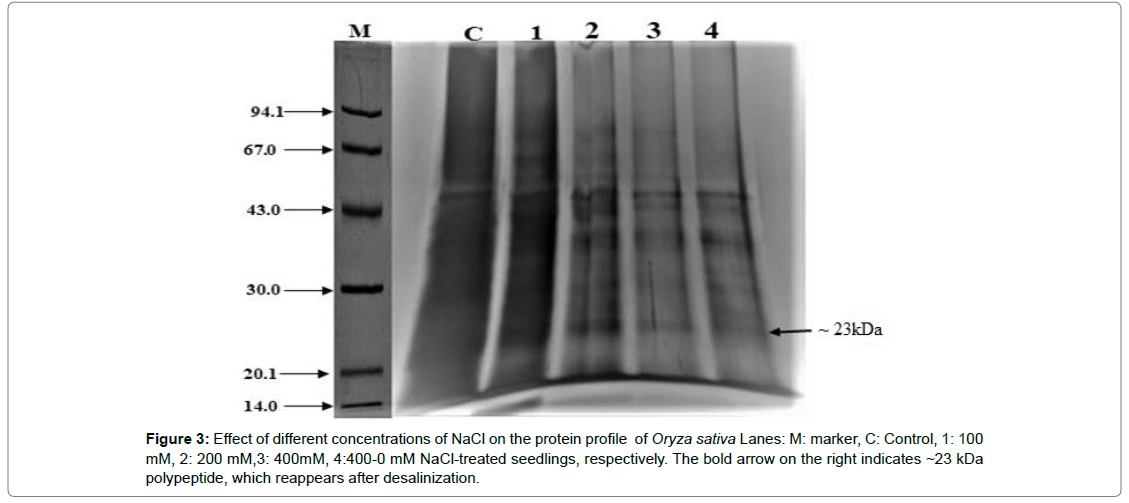
Analysis of protein profile by SDS-PAGE
Comparative SDS-PAGE analysis showed approximately 10-11 nos. of protein bands in the case of seedlings grown in hydroponic medium containing 0mM NaCl concentration as compared to seedlings experienced to 400 mM NaCl, which yielded 9-10nos. of protein bands. Seedlings experienced to 100 and 200 mM NaCl concentration showed 12-13 nos. of protein bands and 10-11 nos. of protein bands respectively. Interestingly seedlings pre-experienced to 400mM NaCl concentration for 7d, when allowed to grow further in a hydroponic medium having 0mM NaCl concentration, showed 7-8 nos. of proteins. A few no. of bands detected and observed to be up and down-regulated based on their expression pattern while experiencing a gradient of salt concentration (Figure 3).
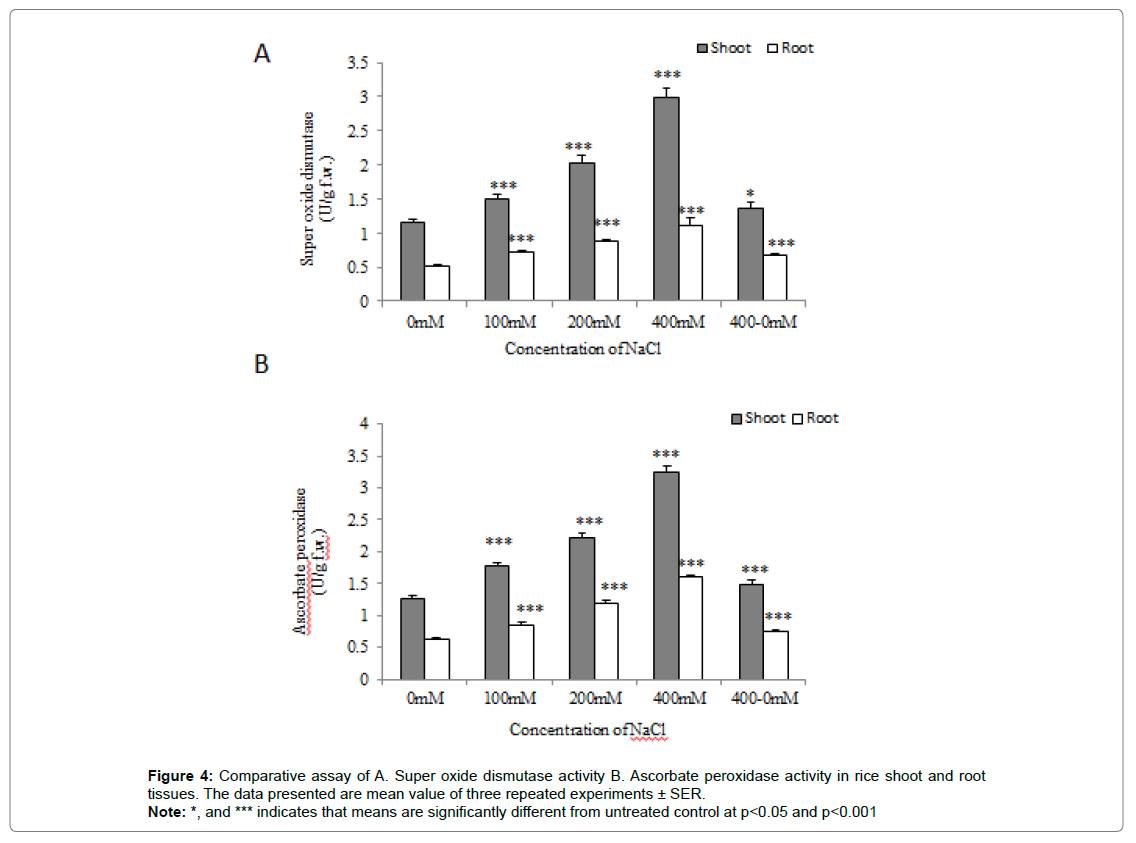
Estimation of Superoxide Dismutase (SOD) activity
A comparative analysis of the estimated SOD content in the case of 28d old rice seedling showed maximum SOD concentration in case of seedlings grown at 400mM NaCl concentration as compared to seedlings experienced to untreated control significantly at (p˂0.001).
The SOD activity was observed to be increased in 100 and 200 mM NaCl treated seedlings as compared to control. Interestingly, seedlings pre-experienced to 400 mM NaCl when allowed to grow at 0 mM NaCl for another 7d showed a marginal enhanced activity of SOD (Figure 4A).
Estimation of Ascorbate Peroxidase (APX) activity
APX activity in the case of seedlings grown at 400 mM NaCl concentration was significantly maximum as compared to seedlings grown at 0 mM NaCl supplemented hydroponic culture at p˂0.001. The APX activity was gradually icreased in the case of seedlings grown at 100 and 200 mM NaCl supplemented hydroponic culture as compared to control. In the case of seedlings pre-experienced to 400 mM NaCl, a small decrease in APX activity was observed and further allows for another 7d to grow at 0 mM NaCl concentration (Figure 4B).
Figure 4:Comparative assay of A. Super oxide dismutase activity B. Ascorbate peroxidase activity in rice shoot and root tissues. The data presented are mean value of three repeated experiments ± SER.
Note: *, and *** indicates that means are significantly different from untreated control at p<0.05 and p<0.001
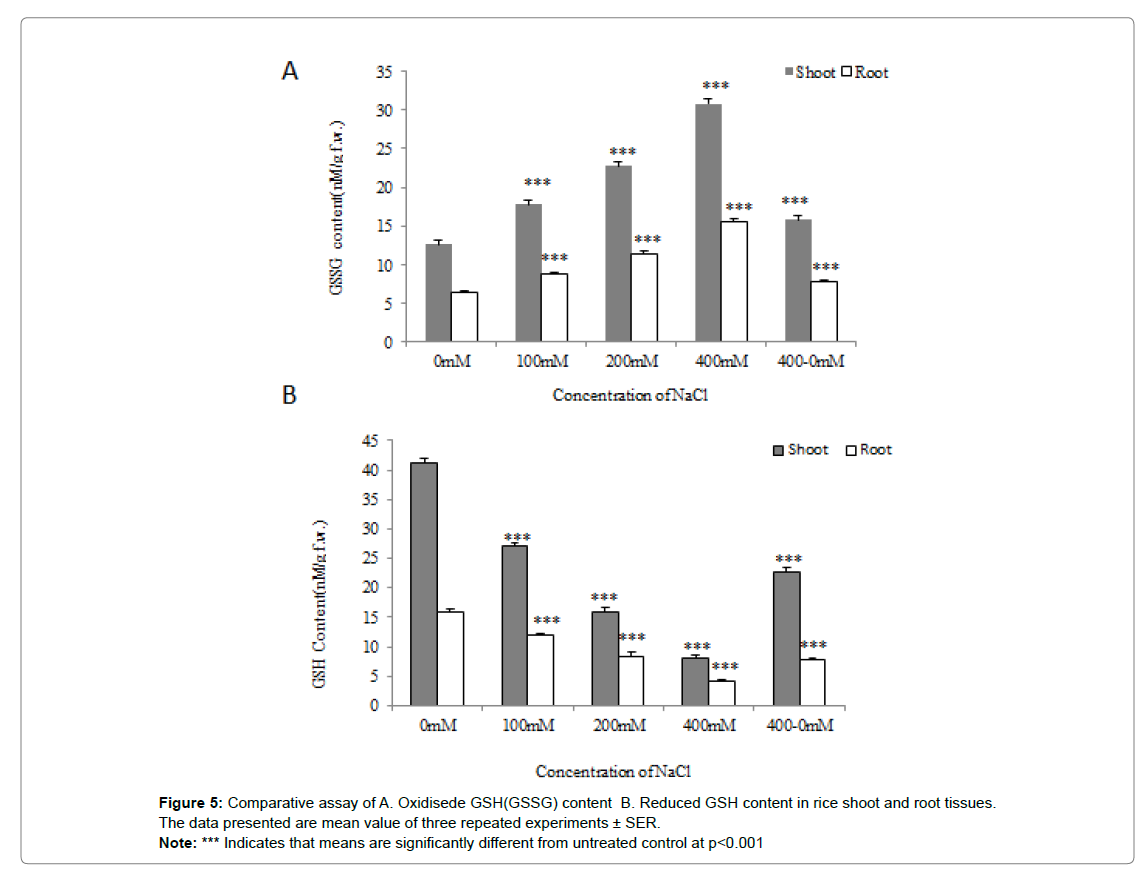
Oxidized GSH (GSSG) content
The maximum content of GSSG was observed in the case of seedlings grown at 400mM NaCl supplemented hydroponic culture as compared to control seedlings with significantly (p˂0.001). Not much difference in GSSG was observed in the case of seedlings grown at 100 and 200mM NaCl supplemented hydroponic culture.
A marginal decrease in the content of GSSG was observed in the case of seedlings pre-experienced to 400mM NaCl and allowed to grow at 0mM NaCl for another 7d (Figure 5A).
Reduced GSH content
The reduced GSH content was observed maximum in case of seedlings grown at 0mM NaCl supplemented hydroponic culture as compared to seedlings experienced to 400mM NaCl with significantly (p˂0.001). A marginal difference in reduced GSH was observed in the case of seedlings experienced to 100 and 200mM NaCl. Interestingly, a slight reduction in reduced GSH content was observed in the case of seedlings pre-experienced to 400mM NaCl and allowed to grow at 0mM NaCl concentration for another 7d (Figure 5B).
Discussion
Salt stress is a possible environmental risk that decreases crop yield worldwide [1]. Plants produce antioxidants and antioxidant enzymes to offer protection against oxidative stress at the cellular level induced by any abiotic stressors [12]. It is also known that stress-induced dysfunction of plant metabolic activity generates an enhanced ROS content [13,14]. A comparative study on oxidants, antioxidants and antioxidant enzymes of Oryza sativa grown under a range of salinity (100,200 and 400mM NaCl) showed up and down-regulation during their incubation condition. The H2O2 content was increased linearly with the increase in salinity from 100 to 400mM NaCl. Interestingly, a marginal decrease in H2O2 content was noticed in the case of seedlings pre-experienced to 400mM and further allow to grow at 0mM NaCl for 7d. An earlier report on H2O2 content was observed to be lower in unstressed barley plants as compared to the salt-stressed situation [15,16]. The other reports stated that H2O2 induced oxidative stress in plant tissues by the increase of salt concentration [17-19]. However, the present investigation showed the enhanced rate of H2O2 content in 400mM salt-treated seedlings as compared to control seedlings where H2O2 content was observed to be minimum which indicates the elevation of oxidative status which directly reduces the growth of rice species during treatment of different salt concentrations. It was suggested that high concentrations of H2O2 induced lipid peroxidation with leading to programmed cell death [20].
With increasing NaCl concentrations, the TSP content in shoot tissues has decreased steadily. Earlier reports showed that the content of free amino acids increased with steadily increasing levels of NaCl [21]. Our findings revealed that the TSP content was reduced in 400mM NaCl treated seedlings as compared to control seedlings which might be an acceleration of H2O2 degrades the proteins by elevated protease enzymes [22] who concluded that various salt concentrations decreased the total proteins in leaves of Mangrove Bruguiera parviflora and different NaCl concentrations supplemented plants might be showed less amount of TSP. A comparative protein profile revealed the overexpression of an apparent 23-kDa protein in the case of seedlings experiencing to 0mM NaCl supplemented hydroponic medium as compared to seedlings experienced to 400mM NaCl, In which the expression of such a protein/peptide was found to below. A gradual decrease in the expression and intensity of such protein/ peptide suggest the protein possibly offer a tolerance against 400mM NaCl. A slightly enhanced expression could be observed in the case of seedlings pre-experienced to 400mM NaCl and allowed to grow at 0mM NaCl for another 7d. Our result is more or less concomitant with an earlier finding where such up and down-regulation was envisaged in case if Bruguiera parviflora (Mangrove species) showing the perturbance of 23-kDa protein (Figure 2) during the investigation incubated with a gradient of salt concentration ranging from 100 to 400mM NaCl [23]. The up and down-regulation of proteins might be assisted the cellular system of the plant from osmotic adjustment during salt adaptation, protect from free radicals, and in physiological responses. Most plant species survive at low or moderate salinity, but their growth and development are seriously restricted above 200mM NaCl [3]. However, the present study reports that the rice species showed detrimental growth even at 400mM NaCl and suggestive of a salt-sensitive species.
It is known that plants produce antioxidant enzymes as a secondary protectant to fight against ROS induced by any stresscausing agent at the cellular level [24]. The enzymatic antioxidants such as Super Oxide Dismutase (SOD), Catalase (CAT), Ascorbate Peroxidase (APX), Glutathione Peroxidase (GPX) are known to catalyze the composition of oxidants and free radicals [25]. In an earlier report, it has been suggested that the detoxification of ROS toxicity is countered by scavenging cellular ROS mediated by a defense system in plants to cope with oxidative stress via the known enzymatic and non-enzymatic system [12]. Superoxide dismutase (SOD) is known to catalyze the superoxide anion (O2 -) to hydrogen peroxide (H2O2) transformation which is detrimental to macromolecules, as well as to cell status. The results showed enhanced activity of SOD in 400mM NaCl supplemented seedlings as compared to the seedlings grown at 0mM NaCl, possibly the accumulation of maximum H2O2 generation would have been there in seedlings grown at 400mM NaCl concentration. The declining activity of SOD in control seedlings which controlled by the lower generation of H2O2. The SOD activity was increased in salt-stressed peas, beets, corn, and tomatoes [18,26,27] in matured green plants, respectively. Our present result is concomitant with the earlier finding in this regard.
In larger plants, algae, and other species, APX plays the most important function in scavenging ROS and defending cells. During various stress conditions, enhanced expression of APX in plants has been demonstrated. In the current study, APX activity was found to be more prevalent in seedlings grown at 400mM NaCl as compared to the control seedlings where the APX activity was observed to be marginal. A gradual increase in APX activity from 100 to 400mM NaCl experienced seedlings imply that a rise in APX activity possibly because of the high accumulation of H2O2. Increased activity of the leaf APX under Cd stress has according to T. aestivum [28]. A marginal elevation of APX activity observed in 400mM NaCl preexperienced seedlings allow to grow at 0mM NaCl for 7d, indicates up-regulation of H2O2 content. As similar to high APX activity was also found in salt-stressed A. doliolum [29].
Both ROS and antioxidants such as ascorbate and glutathione are actively involved in the signaling process in plants during the oxidative load [30] and act as a stress marker. Tripeptide glutathione (Ƴ-Glu-Cys-Gly; GSH) is one of the key plant metabolites considered to be the most effective intracellular protection against oxidative damage caused by ROS. It happens extensively in reduced form (GSH) in plant tissues [31]. In comparison to control seedlings, the oxidised GSH (GSSG) content gradually increases as the salinity level rises. A slight elevation in GSSG content was observed in seedlings pre-experienced to 400mM NaCl and further allowed to grow at hydroponic medium for another 7d. The above results are consistent with [32] reported that GSSG content was observed low in leaves of rice salt-sensitive as compared to salt-tolerant cultivars. The nonenzymatic antioxidant molecule, glutathione was functionalized as a regulator of cellular activities [33]. GSH can act directly with free radicals, minimizing enzyme deactivation owing to thiol group oxidation [34]. The reduced GSH content was estimated to be maximum in control seedlings and less indifferent salt supplemented seedlings. A steady decrease in the reduced GSH content was observed as the NaCl concentration increased in seedlings. In similar to [35] recorded a substantial decrease in the content of GSH in plants of salt-stressed Withania somnifera. Interestingly, a marginal elevation of reduced GSH content was observed in 400mM NaCl recovered seedlings. Our results concomitant to [36-40] reported that GSH content was increased in salt-stressed rice cultivar but decreased with an increase of NaCl concentration in salt-sensitive rice cultivars. The above results showed that salt-stressed induced oxidative stress indicates the enhanced generation of H2O2 in different salttreated seedlings. The basal levels of SOD and APX activity were higher in salt incubated seedlings. The GSSG content was also higher in saline conditions. From the present study, the antioxidant enzymes and antioxidant system might provide the defense system during salt stress and the perturbation of 23-kDa protein maybe regulate the osmotic adjustments in the cytosol of Oryza sativa L. under desalinized condition. The N-terminal amino acid sequencing of 23-kDa protein provides the novel idea of its nature of protein and its role in salt-stressed.
Acknowledgments
The authors highly acknowledge Fakir Mohan University, Balasore, Odisha, India for the necessary and laboratory facility to carry out the study. Nevertheless, the authors are very glad to the National Rice Research Center (NRRI), Cuttack for providing the seeds of Rice (Niranjan) plant.
Conflict of Interest
All authors have equal contribution in preparing the current manuscript.
References
- Ashraf M, Ali Q (2008) Relative membrane permeability and activities of some antioxidant enzymes as the key determinants of salt tolerance in canola (Brassica napus L.). Environ Exp Bot 63: 266-273.
- Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annual Rev Plant Biol 59: 651-681.
- Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annual review of Plant Biol 51: 463-499.
- Broetto F, Luttge U, Ratajczak R (2002) Influence of light intensity and salt-treatment on mode of photosynthesis and enzymes of the antioxidative response system of Mesembryanthemum crystallinum. Funct Plant Biol 29: 13-23.
- Agarwal S, Pandey V (2004) Antioxidant enzyme responses to NaCl stress in Cassia angustifolia. Biol Plant 48: 555-560.
- Noren Z, Ashraf M (2009) Change in antioxidant enzymes and some key metabolites in some genetically diverse cultivars of radish (Raphanus sativus L.). Environmental and Experimental Botany 67: 395-402.
- Mittra B, Sharma S, Das AB, Henry SL, Das TK, et al. (2008) A novel cadmium induced protein in wheat: characterization and localization in root tissue. Biol. Plant 52: 343-346.
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680-685.
- Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Analytical Biochemistry 161: 559-566.
- Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant cell physiology 22: 867-880.
- Anderson ME (1985) Determination of glutathione and glutathione disulfide in biological samples. Methods in enzymol 113: 548-555.
- Noctor G, Arisi ACM, Jouanin L, Kunert KJ, Rennenberg H et al. (1998) Glutathione: Biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J Exp Bot 49: 623-647.
- Beak KH, Skinner DZ (2003) Alteration of antioxidant enzyme gene expression during cold acclimation of near-isogenic wheat lines. Plant science 165: 1221-1227.
- Adams WW, Zarter CR, Ebbert V, Demmig-Adams B (2004) Photoprotective strategies of overwintering evergreens. Bioscience 54: 41-49.
- Sairam RK, Rao KV, Sri vastava GC (2002) Differential response of wheat genotypes to long-term salinity stress in relation to oxidative stress, antioxidant activity and osmolytes concentration. Plant Sci 163: 1037-1046.
- Fedina IS, Grigorova ID, Georgieva KM (2003) Response of barley seedlings to UV-B radiation as affected by NaCl. J Plant Physiol 160: 205-208.
- Gomez JM, Hernendez JA, Jimenez A, del Rio LA, Sevilla F (1999) Differential response of antioxidative enzymes of chloroplasts and mitochondria to long term NaCl stress of pea plants. Free Rad Res 31: S11-S18.
- Hernandez JA, Jimenez J, Mullineaux P, Sevilla F (2000) Tolerance of pea (Pisum sativum L.) to long term stress is associated with induction of antioxidant defences. Plant Cell Environ 23: 853-862.
- Mandhania S, Madan S, Sawhney V (2006) Antioxidant defense mechanism under salt stress in wheat seedlings. Biol Plant 227:227-231.
- Quan LJ, Zhang B, Shi WW, Li HY (2008) Hydrogen peroxide in plants: a versatile molecule of the reactive oxygen species network, J Integrat Plant Biol 50: 2-18.
- Parida A, Das AB, Das P (2002) NaCl stress Biocauses changes in photosynthetic pigments, proteins and other metabolic components in the leaves of a mangrove, Bruguiera parviflora, in hydroponic cultures. J Plant Biol 45: 28-36.
- Parida AK, Mittra B, Das AB, Mohanty P (2004) Salt-stress Induced Alterations in Protein Profile and Protease Activity in the Mangrove Bruguiera parviflora. Z Naturforsch 59: 408-414.
- Parida AK, Mittra B, Das AB, Das TK, Mohanty P (2005) High salinity reduces the content of a highly abundant 23-kDa protein of the mangrove Bruguiera parviflora. Planta 221: 135-140.
- Mittova V, Tal M, Volokita M, Guy M (2003) Up-regulation of the leaf mitochondrial and peroxisomal antioxidative systems in response to salt induced oxidative stress in the wild salt-tolerant tomato species Lycopersicon pennellii. Plant Cell Environ 26: 845-856.
- Saikachout S, Hamza JK, Bouraoui NK, Leclerc JC, Ouerghi Z (2013) Salt-induced changes in antioxidative enzymes activities in shoot tissues of two Atriplex varieties. Not Bot Horti Agrobo 41: 115-121.
- Bor M, Ozdemir F, Türkan I (2003) The effect of salt stress on lipid peroxidation and antioxidants in leaves of sugar beet Beta vulgaris L. and wild beet Beta maritima L. Plant Sci 164:77-84.
- Azevedo-Neto AD, Prisco JT, Eneas-Filho J, de Abreu CEB, et al. (2005) Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt tolerant and alt sensitive maize genotypes. Environ Exp Bot 56: 87-94.
- Khan NA, Samiullah S, Singh R, Nazar A (2007) Activities of antioxidative enzymes, sulphur assimilation, photosynthetic activity and growth of wheat (Triticum aestivum) cultivars differing in yield potential under cadmium stress, J Agro Crop Sci 193: 435-444.
- Srivastava AK, Bhargava P, Rai LC (2005) Salinity and copper-induced oxidative damage and changes in antioxidative defense system of Anabaena doliolum, W J Microb Biotechnol 22: 1291-1298.
- Noctor G, Veljovic-Jovanovic S, Driscoll S, Novitskaya L, Foyer CH (2002) Drought and oxidative load in the leaves of C-3 plants: a predominant role for photorespiration ? Annals of Bot 89: 841-850.
- Jimenez A, Hernandez JA, Pastori G, del Rio LA, Sevilla F (1998) Role of the ascorbate-glutathione cycle of mitochondria and peroxisomes in the senescence of pea leaves. Plant Physiol 118: 1327-1335.
- Chawla S, Jain S, Jain V (2012) Salinity induced oxidative stress and antioxidant system in salt-tolerant and salt-sensitive cultivars of rice (Oryza sativa L.). Plant Biochem Biotechnol 22: 27-34.
- Renenberg H, Lamoureux GL (1990) Physiological processes that modulate the concentration of glutathione in plant cells. In: Rennenberg H, Brunda CH, de Kok LJ, Sluten I (eds) Sulfur nutrition and sulfur assimilation in higher plants. SPB Academic Publishers, The Hague, pp 53-66.
- Wang SY, Jiac HJ, Faust M (1991) Changes in ascorbate, glutathione and related enzyme activities during thiadiazuren-induced bud break of apple. Physiol Plant 82: 231-236.
- Jaleel CA, Lakshmanan GMA, Gomathinayagam M, Panneerselvam R (2008) Triadimefon induced salt stress tolerance in Withania somnifera and its relationship to antioxidant defense system. S Afr J Bot 74: 126-132.
- Khan MH, Panda SK (2008) Alterations in root lipid peroxidation and antioxidative responses in two rice cultivars under NaCl-salinity stress. Acta Physiol Plant 30: 81-89.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265-275.
- Panda SK, Khan MH (2003) Salt stress influences lipid peroxidation and antioxidants in the leaf of an indica rice (Oryza sativa L). Physiol Mol Biol Plants 9: 273-278.
- Raskin I, Skubatz H, Tang W, Meeuse BJD (1990) Salicylic acid levels in thermogenic and non-thermogenic plants. Ann Bot 66: 369-373.
- Warrier RR, Paul M, Vineetha MV (2013) Estimation of salicylic acid in eucalyptus leaves using spectrophotometric methods. Genetics and Plant Physiology 3: 90-97.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi