Review Article, Int J Cardiovas Res Vol: 6 Issue: 3
Human Pericoronary Adipose Tissue as Storage and Possible Supply Site for Lipoproteins in Atherosclerotic Coronary Plaques
1Japanese Foundation for Cardiovascular Research, Funabashi, Japan
2Department of Cardiology, Tokyo Jikei University School of Medicine, Tokyo, Japan
*Corresponding Author : Yasumi Uchida
Japan Foundation for Cardiovascular Research, 2-30-17, Narashinodai, Funabashi, 274-0063, Japan
Tel: +81-47-462-2159
E-mail: uchida73@ta2.so-net.ne.jp
Received: May 22, 2017 Accepted: June 06, 2017 Published: June 12, 2017
Citation: Uchida Y (2017) Human Pericoronary Adipose Tissue as Storage and Possible Supply Site for Lipoproteins in Atherosclerotic Coronary Plaques. Int J Cardiovasc sRes 6:3. doi: 10.4172/2324-8602.1000313
Abstract
Aim of Review: It is generally believed that low-density lipoprotein (LDL) and monocytes enters the vascular wall from the lumen, and the former becomes oxidized LDL (oxLDL) and the latter macrophages and they participate in atherosclerosis However, definite in vivo clinical evidence is lacking. This review article summarizes our findings on the possible roles of oxLDL and other lipoproteins stored in pericoronary adipose tissue (PCAT), which, when thickened, becomes a risk factor for coronary artery disease.
Findings: Immunohistochemical staining of PCAT and its adjacent coronary arteries obtained from autopsy subjects revealed that oxLDL, high-density lipoprotein (HDL) and apolipoprotein A1 (ApoA1) co-deposited in adipocytes in the majority of PCAT samples. LDL did not deposit in PCAT. The incidence of oxLDL was low in the intima of normal coronary segments, but increased during the growth stage and decreased during the mature stage of plaques, whereas that of HDL and ApoA1 was increased in
the growth stage and increased further in the mature stage. The incidence of LDL in plaques was low and showed no obvious relation to plaque morphology. OxLDL and ApoA1 deposited in either a dotted or diffuse pattern whereas HDL showed a diffuse pattern in both the PCAT and intima. The dotted pattern occurred when oxLDL or ApoA1 was contained in CD68(+) -macrophages that were observed in the PCAT, adventitia, media and intima. A certain group of CD68(+)-macrophages contained both oxLDL and ApoA1. The CD68(+) -macrophages across the border of the PCAT, external and internal elastic laminae were frequently observed, suggesting their traverse from the PCAT toward the intima. The localization of diffusely deposited oxLDL, HDL and ApoA1 coincided with that of intimal vasa vasorum. LDL was deposited diffusely in the intima but its localization did not necessarily coincide with that of vasa vasorum.
Summary: Contrary to the generally believed mechanism, immunohistochemical findings suggested that native oxLDL, HDL and ApoA1 are stored in PCAT and conveyed either by CD68(+) - macrophages or vasa vasorum to coronary plaques. Therefore, therapies targeting the PCAT could be effective in preventing human coronary atherosclerosis.
Keywords: Apolipoprotein A1; High-density lipoprotein; Human coronary
Introduction
It is generally believed that low-density lipoprotein (LDL) enters the vascular wall from the lumen and becomes oxidized low-density lipoprotein (oxLDL) by oxidation, after which it plays a key role in the initiation, progression and destabilization of atherosclerotic plaques. Monocytes also migrate into the vascular wall from the lumen, and become macrophages, which in turn accumulate oxLDL and become foam cells, while producing collagen-degrading enzymes such as metalloproteases and collagenases which destroy collagen fibers, and also hypochlorous acid which damages endothelial cells, resulting in vulnerable plaques [1-6]. It is also generally believed that anti-atherogenic substances such as high-density lipoprotein (HDL) [7,8] and its component apolipoprotein A1 (ApoA1) [9-12] enter the vascular intima from the lumen either directly or via vasa vasorum. Based on these generally believed mechanisms and using the plasma levels of lipoproteins and apolipoproteins as markers, lipid-lowering therapies are administered in the clinical setting, but with limited preventive effects on ischemic cardiovascular events.
Our hypothesis
We considered that there are other hitherto unrecognized storage site(s) and supply route(s) for the pro-atherogenic and anti-atherogenic lipoproteins and their components, which are not reflected in their plasma levels.
OxLDL, HDL and ApoA1 in human pericoronary adipose tissue (PCAT): Pericoronary adipose tissue (PCAT) is a part of epicardial adipose tissue and lies within 3 mm in distance from the adjacent coronary artery [13]. Using immunohistochemical techniques, we demonstrated that oxLDL, HDL and ApoA1 but not LDL are stored in adipocytes of PCAT, both cytoplasm and plasma membrane (Figure 1a, 1b) [13-15]. There are two possibilities for their storage in the adipocytes of PCAT:
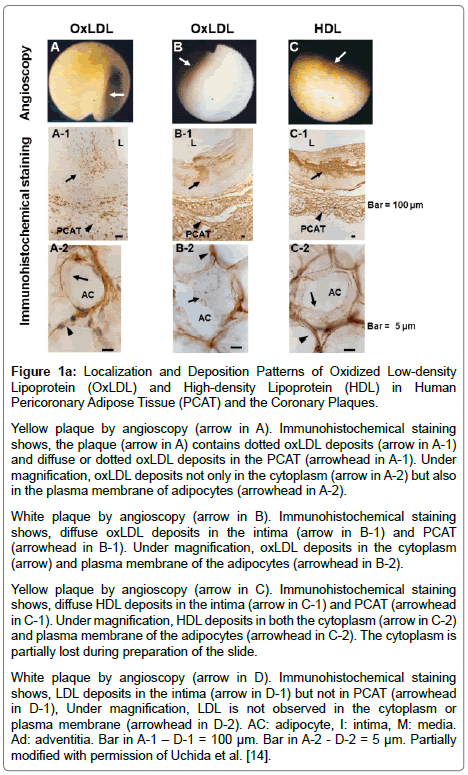
Figure 1a: Localization and Deposition Patterns of Oxidized Low-density Lipoprotein (OxLDL) and High-density Lipoprotein (HDL) in Human Pericoronary Adipose Tissue (PCAT) and the Coronary Plaques.
Yellow plaque by angioscopy (arrow in A). Immunohistochemical staining shows, the plaque (arrow in A) contains dotted oxLDL deposits (arrow in A-1) and diffuse or dotted oxLDL deposits in the PCAT (arrowhead in A-1). Under magnification, oxLDL deposits not only in the cytoplasm (arrow in A-2) but also in the plasma membrane of adipocytes (arrowhead in A-2).
White plaque by angioscopy (arrow in B). Immunohistochemical staining shows, diffuse oxLDL deposits in the intima (arrow in B-1) and PCAT (arrowhead in B-1). Under magnification, oxLDL deposits in the cytoplasm (arrow) and plasma membrane of the adipocytes (arrowhead in B-2).
Yellow plaque by angioscopy (arrow in C). Immunohistochemical staining shows, diffuse HDL deposits in the intima (arrow in C-1) and PCAT (arrowhead in C-1). Under magnification, HDL deposits in both the cytoplasm (arrow in C-2) and plasma membrane of the adipocytes (arrowhead in C-2). The cytoplasm is partially lost during preparation of the slide.
White plaque by angioscopy (arrow in D). Immunohistochemical staining shows, LDL deposits in the intima (arrow in D-1) but not in PCAT (arrowhead in D-1), Under magnification, LDL is not observed in the cytoplasm or plasma membrane (arrowhead in D-2). AC: adipocyte, I: intima, M: media. Ad: adventitia. Bar in A-1 – D-1 = 100 μm. Bar in A-2 - D-2 = 5 μm. Partially modified with permission of Uchida et al. [14].
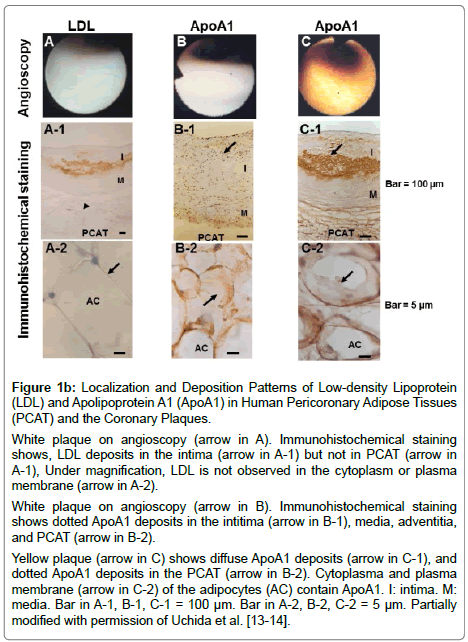
Figure 1b: Localization and Deposition Patterns of Low-density Lipoprotein (LDL) and Apolipoprotein A1 (ApoA1) in Human Pericoronary Adipose Tissues (PCAT) and the Coronary Plaques.
White plaque on angioscopy (arrow in A). Immunohistochemical staining shows, LDL deposits in the intima (arrow in A-1) but not in PCAT (arrow in A-1), Under magnification, LDL is not observed in the cytoplasm or plasma membrane (arrow in A-2).
White plaque on angioscopy (arrow in B). Immunohistochemical staining shows dotted ApoA1 deposits in the intitima (arrow in B-1), media, adventitia, and PCAT (arrow in B-2).
Yellow plaque (arrow in C) shows diffuse ApoA1 deposits (arrow in C-1), and dotted ApoA1 deposits in the PCAT (arrow in B-2). Cytoplasma and plasma membrane (arrow in C-2) of the adipocytes (AC) contain ApoA1. I: intima. M: media. Bar in A-1, B-1, C-1 = 100 μm. Bar in A-2, B-2, C-2 = 5 μm. Partially modified with permission of Uchida et al. [13-14].
(a) They are formed in the adipocytes or
(b) They are conveyed to the adipocytes from the systemic circulation via adventitial vasa vasorum or microvessels connecting the myocardium to PCAT.
Absence of LDL, the precursor of oxLDL, in PCAT supports the latter possibility for oxLDL.
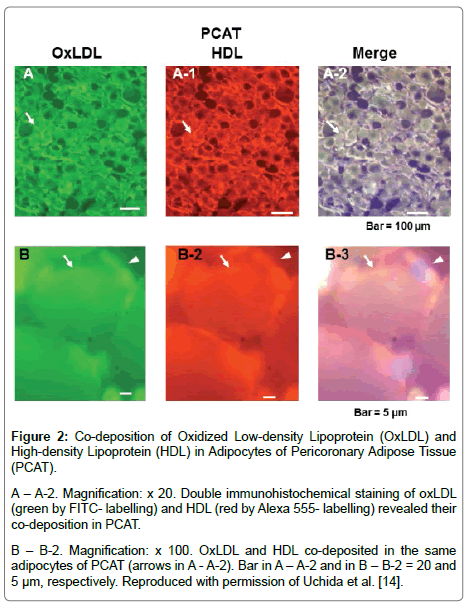
HDL and ApoA1 co-deposit with oxLDL in PCAT: It is also generally believed that HDL and ApoA1 enter the vascular wall from the lumen. Based on this belief, therapies to elevate the plasma levels of HDL are attempted using fibrates or HDL- or ApoA1 -mimetic substances, but their preventive effects on ischemic coronary events are currently inconsistent in the clinical setting [16]. Using immunohistochemical techniques, we found that oxLDL co-deposits with HDL (Figure 2) or ApoA1 in human PCAT [14,15].
Figure 2: Co-deposition of Oxidized Low-density Lipoprotein (OxLDL) and High-density Lipoprotein (HDL) in Adipocytes of Pericoronary Adipose Tissue (PCAT).
A – A-2. Magnification: x 20. Double immunohistochemical staining of oxLDL (green by FITC- labelling) and HDL (red by Alexa 555- labelling) revealed their co-deposition in PCAT.
B – B-2. Magnification: x 100. OxLDL and HDL co-deposited in the same adipocytes of PCAT (arrows in A - A-2). Bar in A – A-2 and in B – B-2 = 20 and 5 μm, respectively. Reproduced with permission of Uchida et al. [14].
Deposition patterns: OxLDL deposited in either a dotted (Figure 1) or diffuse pattern (Figure 1- B-1) in the intima (plaque), but only in a dotted pattern in the adventitia, media and intima13. ApoA1 also showed either dotted or diffuse deposition pattern (Figure 1a – B-1, C-1), whereas HDL and LDL diffuse pattern in the intima (Figure 1a – C-1; 1b – A-1, C-1).
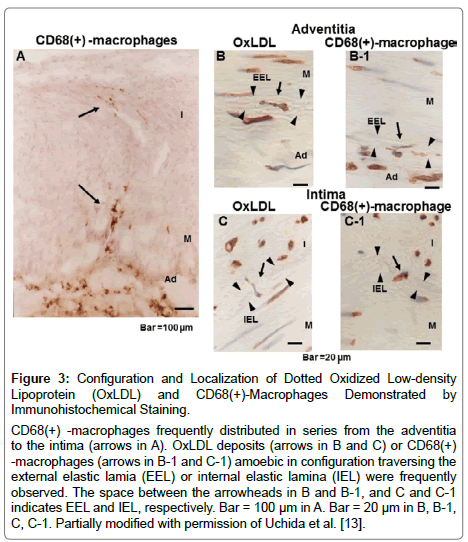
Supply routes of oxLDL and ApoA1 to adjacent coronary intima (plaque): Single immunohistochemical staining revealed that dotted oxLDL and CD68(+) -macrophages frequently showed amoebic configuration, and appeared to traverse the adventitia, and external and internal elastic laminae, suggesting migration from the PCAT to the intima (Figure 3) [13]. Double immunohistochemical staining revealed that dotted oxLDL or ApoA1 deposits were contained in CD68(+) -macrophages (Figure 4). The oxLDL-containing -CD68(+) -macrophages were not found within the adventitial vasa vasorum but were found in the interstitial spaces of PCAT, adventitia, media and intima)[13]. Because the adventitial vasa vasorum did not contain oxLDL-containing CD68(+) -macrophages, it is conceivable that the macrophages which did not contain oxLDL migrated to the PCAT through either the adventitial vasa vasorum or penetrating microvessels that connect the myocardium to the PCAT and thus obtained oxLDL in the PCAT and conveyed it to the intima. It is conceivable that oxLDL was supplied through coronary circulation or from the myocardium and was stored in the PCAT. It seems that ApoA1 is supplied to PCAT via the same routes.
Figure 3: Configuration and Localization of Dotted Oxidized Low-density Lipoprotein (OxLDL) and CD68(+)-Macrophages Demonstrated by Immunohistochemical Staining.
CD68(+) -macrophages frequently distributed in series from the adventitia to the intima (arrows in A). OxLDL deposits (arrows in B and C) or CD68(+) -macrophages (arrows in B-1 and C-1) amoebic in configuration traversing the external elastic lamia (EEL) or internal elastic lamina (IEL) were frequently observed. The space between the arrowheads in B and B-1, and C and C-1 indicates EEL and IEL, respectively. Bar = 100 μm in A. Bar = 20 μm in B, B-1, C, C-1. Partially modified with permission of Uchida et al. [13].
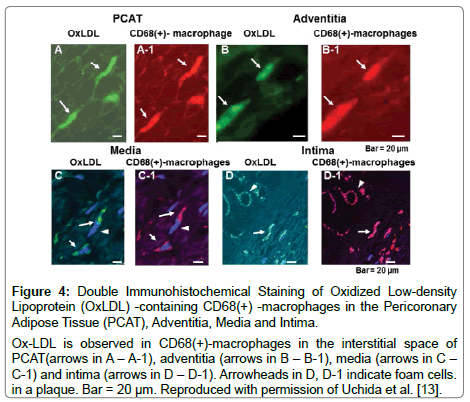
Figure 4: Double Immunohistochemical Staining of Oxidized Low-density Lipoprotein (OxLDL) -containing CD68(+) -macrophages in the Pericoronary Adipose Tissue (PCAT), Adventitia, Media and Intima.
Ox-LDL is observed in CD68(+)-macrophages in the interstitial space of PCAT(arrows in A – A-1), adventitia (arrows in B – B-1), media (arrows in C – C-1) and intima (arrows in D – D-1). Arrowheads in D, D-1 indicate foam cells. in a plaque. Bar = 20 μm. Reproduced with permission of Uchida et al. [13].
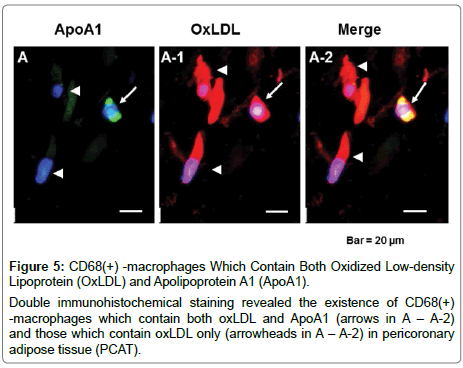
CD68(+) -macrophages contain both oxLDL and ApoA1: Unexpectedly, a certain group of CD68(+) -macrophages in PCAT and intima contained both oxLDL and ApoA1, or oxLDL or ApoA1 only (Figure 5), but to what group of macrophage phenotype this group belongs remains to be elucidated.
Figure 5: CD68(+) -macrophages Which Contain Both Oxidized Low-density Lipoprotein (OxLDL) and Apolipoprotein A1 (ApoA1).
Double immunohistochemical staining revealed the existence of CD68(+) -macrophages which contain both oxLDL and ApoA1 (arrows in A – A-2) and those which contain oxLDL only (arrowheads in A – A-2) in pericoronary adipose tissue (PCAT).
OxLDL, LDL, HDL and ApoA1 in plaques: The incidence of oxLDL was compared between normal coronary segments, white plaques (growth stage of plaque), yellow plaque without a necrotic core (NC) (mature stage) and yellow plaques with a NC (end stage of maturation) that were classified by conventional angioscopy and histology [17]. The incidence was low in normal segments, increased in the growth and mature stages and decreased in the end stage of plaques [13].
Different to oxLDL, the incidence of HDL in the coronary intima increased with plaque growth and increased further with plaque maturation [14]. Especially in plaques with NC, the core was filled with HDL.
The incidence of ApoA1 was low in normal coronary segments, increased with plaque growth and increased further with plaque maturation as with HDL. This finding indicated that, although an anti-atherogenic substance as with HDL, ApoA1 resembled oxLDL in its supply routes to the intima but differed from oxLDL and resembled HDL in its relation to plaque morphology [15].
The incidence of LDL in intima was low and had no obvious relation to plaque morphology [14].
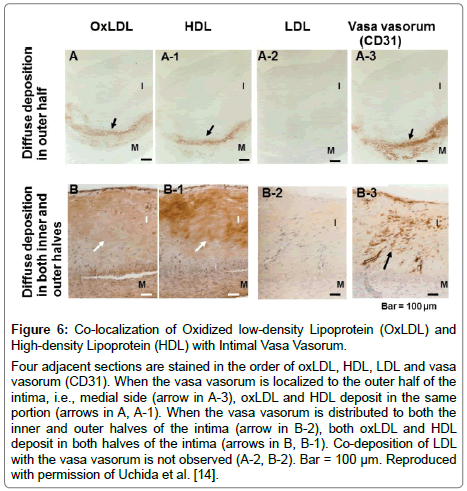
Co-localization of oxLDL, HDL and ApoA1 with intimal vasa vasorum: Co-localization of diffuse oxLDL, HDL or ApoA1 deposits and vasa vasorum was more frequently observed in the outer half (medial side) of the intima in segments with white plaques than in normal segments and those with yellow plaques, and in both the inner (luminal side) and outer halves of the intima more frequently in segments with yellow plaques, indicating that oxLDL spread with the development of vasa vasorum (Figure 6) [13-15]. Deposition of oxLDL in the superficial layer of intima alone was rarely observed. These findings suggested that these proteins were mostly conveyed to the intima by either macrophages or neovascularized vasa vasorum. LDL did not necessarily colocalize with vasa vasorum and therefore its supply route(s) to intima remains to be elucidated.
Figure 6: Co-localization of Oxidized low-density Lipoprotein (OxLDL) and High-density Lipoprotein (HDL) with Intimal Vasa Vasorum.
Four adjacent sections are stained in the order of oxLDL, HDL, LDL and vasa vasorum (CD31). When the vasa vasorum is localized to the outer half of the intima, i.e., medial side (arrow in A-3), oxLDL and HDL deposit in the same portion (arrows in A, A-1). When the vasa vasorum is distributed to both the inner and outer halves of the intima (arrow in B-2), both oxLDL and HDL deposit in both halves of the intima (arrows in B, B-1). Co-deposition of LDL with the vasa vasorum is not observed (A-2, B-2). Bar = 100 μm. Reproduced with permission of Uchida et al. [14].
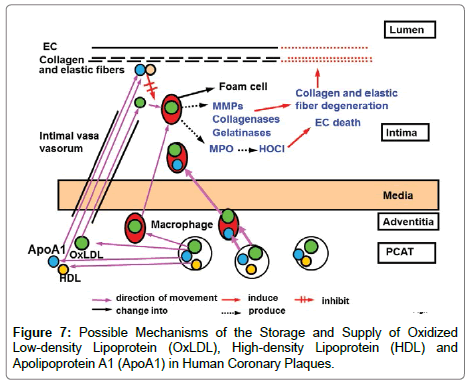
Based on the aforementioned findings, I propose a hypothesis on the storage and supply routes of oxLDL, HDL and ApoA1 (Figure 7). Imbalanced deposition in the PCAT and their supply to the intima may determine the fate of plaques. It remained obscure whether the CD68(+) -macrophages that contain both oxLDL and ApoA1 accelerate or suppress atherosclerosis.
Other reports
Perivascular adipocytes (PVA) produce large numbers of metabolically active substances with both endocrine and paracrine actions [18]; PVA of human internal thoracic artery release NO [19]; PVA releases nicotinamide adenine dinucleotide phosphateoxidase in mice [20]; rat PVA release compliment 23 [21] rat PVA release metylpalmitate ester [22], interleukin-6, plasminogen activator inhibitor-1, monocyte chemoattractant protein-1 genes are overexpressed in epicardial adipose tissue in patients with acute coronary syndrome [23] cultured human epicardial adipose tissues release ApoA1 and glutation S-transferase P [24] But, reports describing storage and release of lipoproteins from human PVA, including PCAT, to the adjacent vessel wall were not found.
Increased volume of epicardial adipose tissue is a risk factor for coronary heart disease [25,26]. Increased supply of pro-atherogenic lipoproteins such as oxLDL from thick PCAT to coronary intima may accelerate coronary atherosclerosis.
Clinical implications
Our findings suggested that molecular therapy targeting lipoproteins in the PCAT could be effective in preventing coronary atherosclerosis, by inhibiting storage of oxLDL or by enhancing storage of HDL and/or ApoA1.
Study limitations
a. Definite evidence of the supply routes of oxLDL, HDL and ApoA1 from the PCAT to the coronary intima (plaque) is lacking because demonstration of real-time movement of these proteins in the clinical setting is beyond the scope of current immunohistochemical techniques. Tracing these native proteins using biomarkers may be one possible method of observing real-time movements of these substances in vivo.
b. It remains to be clarified whether the CD68(+) -macrophages that contain both oxLDL and ApoA1 accelerate or inhibit coronary atherosclerosis [27,28].
Conclusions
Using immunohistochemical techniques as summarized in Table 1, we clarified that an important pro-atherogenic lipoprotein, oxLDL, and anti-atherogenic substances, HDL and ApoA1, are stored in human PCAT. Also, a supply route from the PCAT to coronary plaques, hitherto unrecognized, was strongly suggested by our findings. Imbalance in storage and supply between these proteins may determine the fate of atherosclerotic coronary plaques in patients.
| OxLDL | LDL | HDL | ApoA1 | |
|---|---|---|---|---|
| (A) Deposition sites | ||||
| PCAT | Yes | No | Yes | Yes |
| Intima | Yes | Yes | Yes | Yes |
| (B) Deposition pattern | ||||
| Dotted or diffuse | Diffuse | Diffuse | Dotted or diffuse | |
| (C) Supply routes to intima | ||||
| Macrophages | Yes | No | No | Yes |
| Vasa vasorum | Yes | Unknown | Yes | Yes |
Table 1: Deposition Sites and Patterns and Possible Supply Routes of Oxidized low-density lipoprotein (OxLDL), Low-density lipoprotein (LDL), High-density lipoprotein (HDL) and Apolipoprotein A1(ApoA1) to Coronary Intima.
Disclosure
The authors have no conflicts of interest to declare.
Relationships with Industry
The authors have no relationships with industry.
Funding
No external funding was received for this study.
References
- Shah PK (2009) Inflammation and plaque vulnerability. Cardiovasc Drugs Ther 23: 31-40.
- Chen JH, Riazzy M, Smith EM, Proud CG, Steinbrecher UP, et al. (2009) Oxidized LDL-mediated macrophage survival involves elongation factor-2 kinase. Arterioscler Thromb Vasc Biol 29: 92-98
- Holvoet P (2004) Oxidized LDL and coronary heart disease. Acta Cardiol 59: 479-484
- Takahashi K, Takeya M, Sakashita N (2002) Multifunctional roles of macrophages in the development and progression of atherosclerosis in humans and experimental animals. Med Electron Microsc 35: 179-203.
- Libby P (2015) The vascular biology of atherosclerosis. Elsevier Saunders Ltd, Philadelphia 873-890
- Yoshida H, Kisugi R (2010) Mechanisms of LDL oxidation. Clin Chim Acta 411: 1875-1882
- Assmann G, Schalte H, von Ecckardstein (1996) High-density lipoprotein cholesterol as a predictors of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis 124S: 11-20.
- Toh PP (2010) Davidson MH. High-density lipoproteins: marker ofcardiovascular risk and therapeutic target. J Clin Lipidol 4: 359-364
- Cho KH (2009) Biomedical implications of high-density lipoprotein: its composition, structure, functions, and clinical applications. BMB Rep 42: 393-400
- Boeckold SM, Arsenault BJ, Hovingh GK, Mora S, Pedersen TR, et al. (2013) Levels and changes of HDL cholesterol and apolipoprotein A-1 in relation to risk of cardiovascular events among statin-treated patients: a meta-analysis. Circulation 128:1504-1512
- Emoto T, Sawada T, Morimoto N, Tenjin T, Wakimoto T, et al. (2013) The apolipoprotein B/A1 ratio is associated with reactive oxygen metabolites and endothelial dysfunction in statin-treated patients with coronary artery disease. J Atheroscler Thromb 20: 623-629
- Pan L, Lu G, Chen Z (2014) Combined use of apolipoprotein B/apolipoprotein A1 ratio and non-high-density lipoprotein cholesterol before routine clinical lipid measurement in predicting coronary heart disease. Coron artery Dis 25: 433-438
- Uchida Y, Uchida Y, Shimoyama E, Hiruta N, Kishimoto T, et al. (2016) Pericoronary Adipose Tissue as Storage and Supply Site for Oxidized Low-Density Lipoprotein in Human Coronary Plaques. PLoS One 11: e0150862
- Uchida Y, Uchida Y, Shimoyama E, Hiruta N, Kishimoto T, et al. Human pericoronary adipose tissue as storage and possible supply site for oxidized low-density lipoprotein and high-density lipoprotein in coronary artery. J Cardiol. 69: 236-244
- Uchida Y, Uchida Y, Shimoyama E, Shimoyama E, Hiruta N, et al. (2017) Deposition patterns and localization of apolipoprotein A1 and their relation to plaque morphology in human coronary artery. JSM Atherosclerosis 2: 1025-1034
- Kingwell BA, Chapman MJ, Kontush A, Miller NE (2014) HDL-targeted therapies: progress, failures and future. Nat Rev Drug Discov 13: 445-464
- Uchida Y (2001) Clinical classification of atherosclerotic coronary plaques. Futura Publishing Ltd, Armonk, NY
- Szasz T, Webb RC (2012) Perivascular adipose tissue: more than just structural support. Clin Sci (Lond) 122: 1-12
- Marinowski M, Deja MA, Golba KS, Roleder T, Biernat J, et al. (2008) Perivascular tissue of internal thoracic artery releases potent nitric oxide and prostacyclin-independent anticontractile factor. Eur J Cardiothoracic Surg 33: 225-231
- Ketonen J, Shi J, Mervaala E, Mervaala E (2010) Priadventitial adipose tissue promotes endothelial dysfunction via oxidative stress in diet-induced obese C57BI/6 mice. Circ J 74: 1479-1487
- Ruan CC, Zhu DL, Chen J, Chen Q, Guo SJ, et al. (2010) Perivascular adipose tissue-derived complement 23 is required for adventitial fibroblast functions and adventitial remodeling in deoxycorticosterone acetate-salt hypertensive rats. Arterioscler Thromb Vasc Biol 30: 2568-1574
- Lee YC, Chang HH, Chiang CL, Liu CH, Yeh JI, et al. (2011) Role of perivascular adipose tissue-derived methyl palmitate in vascular tone regulation and pathogenesis of hypertension. Circulation 124: 1160-1171
- Langheim S, Dreas L, Veschini L, Maisano F, Foglieni C, et al. (2010) Increased expression and secretion of restin in epicardial adipose tissue of patients with acute coronary syndrome. Am J Physiol Heart Circ Physiol 298: 746-753
- Somoza AS, Fernandez TE, Fernandez AL, Eiras D, et al. (2012) Changes in lipid transport-involved proteins of epicardial adipose tissue associated with coronary artery disease. Atherosclerosis 224: 492-499
- Verhagen SN, Visseren FL (2011) Perivascular adipose tissue as a cause of atherosclerosis. Atherosclerosis 214: 3-10
- Shimabukuro M, Hirata Y, Tabata M, Dagvasumberel M, Sato H, et al. (2013) Epicardial adipose tissue volume and adipokine imbalance are strongly linked to human coronary atherosclerosis. Arterioscler Thromb Vasc Biol 33: 1077-1084.
- Uchida Y, Uchida Y, Kawai S, Kanamaru R, Sugiyama Y, et al. (2010) Detection of vulnerable coronary plaques by color fluorescent angioscopy. JACC Cardiovasc Imaging 3: 398-408
- Uchida Y, Uchida Y, Hiruta N, Shimoyama E, Sugiyama E (2013) Molecular imaging of native HDL in human coronary plaques by color fluorescent angioscopy. JACC Cardiovasc Imaging 6: 1015-1017
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi