Research Article, J Plant Physiol Pathol Vol: 9 Issue: 8
Impact of Shade Intensities on Rhizome Yield and Quality of Costus speciosus: A Viable Option as an Intercrop
Narendra A Gajbhiye, Kuldeepsingh A Kalariya, Ram P Meena, V Thondaiman and Parmeshwar L Saran**
Department of Biosciences and Biotechnology, Fakir Mohan University, Vyasa Vihar, Balasore-756089, Odisha, India
*Corresponding Author:
Parmeshwar L Saran
ICAR-Directorate of Medicinal and Aromatic Plants Research, Boriavi 387310, Anand, Gujarat, India
Tel: +91-2692-271605
E-mail: plsdehradun@gmail.com
Received Date: July 27, 2021; Accepted Date: August 18, 2021; Published Date: August 25, 2021
Citation: Gajbhiye NA, Kalariya KA, Meena RP, Thondaiman V, Saran PL (2021) Impact of Shade Intensities on Rhizome Yield and Quality of Costus speciosus: A Viable Option as an Intercrop. J Plant Physiol Pathol 9:8. 260.
Copyright: © All articles published in Dental Health: Current Research are the property of SciTechnol, and is protected by copyright laws. “Copyright © 2021, SciTechnol, All Rights Reserved.
Abstract
The maximum number of stems/plants, stem weight, root length, rhizome spread, fresh rhizome weight and dry rhizome weight were observed under 75% Shade Intensity (SI) followed by 50% natural SI. The light response study revealed that net Photosynthetic Rate (PN) was maximum at 1000 μ mol (photons) m-2 s-1 and further increase in light intensity decreased the PN. The NPQ increased steadily and this kind of PAR requirement (?750 μ mol) makes the plant suitable for partially shade area with very low light transmission. Diosgenin content in rhizome was low in all shaded plants as compare to 0% SI (open field conditions) in which it was maximum (826 mg g-1). Over all rhizome and diosgenin yield content were maximum under 75% natural SI as an intercrop.
Keywords: Costus; Diosgenin; Rhizome yield; Shade intensity
Introduction
Costus specious Koen (keu) belongs to the family Costaceae is an important ornamental as well as medicinal plant extensively used in indigenous system of medicines for cure of several illnesses. It is commonly grown as ornamental herb in kitchen garden or backyards. Sometimes also grown as pot plant for Indore beautification in India. It is locally known as keu and crape ginger in country and erect, perennial, succulent, ornamental herb arising directly from rhizome [1]. The rhizome is tubular, branched having circular nodal scars and few wiry rootlets (yellowish brown in colour) and fibrous. Plants bears large (15-35 and 6-10 cm), thick leaves spirally arranged on stem are elliptical to oblong in shapes. It produces whitish, thick, large flowers and cone like terminal spikes with bright red brackets and yellowish throat of lips. The fruits are globosely trigonous, red capsules type (2 cm) containing black seed with white aril [2]. The rhizome which is the commercial part of plant is used as an alternative source for diosgenin. Rhizome extract exhibit cardiotonic, hydrocholeretic, diuretic depressant activities [3]. It is mostly used to control diabetes and blood sugar levels. Its traditional uses include remedial of pneumonia, rheumatism, dropsy, urinary, gout rheumatism, bronchial asthma and jaundice diseases. Juice of rhizome also applied to head for cooling and relief from head-ache, bruised leaves are applied in fever, decoction of stem is used in fever and dysentery [4]. In general, the diosgenin content in rhizome was reported up to 3.37% on dry weight basis [5].
It is widely distributed in tropical to subtropical parts of the country up to 1200 m above mean sea level. It favours moist tropical evergreen forest but commonly grown along roadside, near water streams and waste land. It is naturally found in plenty from Assam, Meghalaya, Bihar, Orissa, Madhya Pradesh, West Bengal, Western Ghats and Sub Himalayan parts of Uttarakhand, Himachal etc., [1]. It is cultivated well in rich moist clay loam soils in shady area under forest. Best time for cultivation is rainy season which offering high humidity and low temperature type climate in south Indian forests [6, 7]. Tolerance of plant under shade condition sustained the growth due to increase in leaf area, photosynthetic capacity per unit of light and sugar content [8]. The leaves under shade condition made an efficient use of the less intense irradiation reaching up to them like safed musli, rauvolfia, patchouli, turmeric, shatavari and brahmi [9]. Such medicinal plants have been successfully intercropped with fuel wood trees like parkia, chandan (white), Ashoka tree, bael, aonla, sugar apple, Moringa, neem, Indian soapberry, arjun, black myrobalan, in the early years until the tree canopy closes. Therefore, South Asian countries have been a tradition of practicing mixed farming system depending upon the spacing and nature of trees [10]. Systematic efforts to know the exact light requirement in non-traditional area to harvest maximum quantity of Keu rhizomes along with good quality are still not standardized. Thus, identification of suitable SI requirements was very much essential to ensure premium produce and its quality for sustainable utilization of natural resources in the era of changing global environment.
Materials and Methods
Experimental site
The research experiment was conducted at the ICAR-Directorate of Medicinal and Aromatic Plants Research (DMAPR), Boriavi, Anand, Gujarat (India) during 2018-2019. The experimental farm is located at an altitude of 45.1 m above mean sea level and at 22°35’N and 72°55’E. It has a semi-arid, subtropical type climate with hot dry summers and mild winters.
Plant materials
Rhizomes were transplanted at a spacing of 50 × 50 cm in natural shade intensities under 28 years old trees of Azadarichta indica (neem) during second week of July, 2018. The natural shade intensity under the tree canopy was measured at about 12.00 hours. The average natural shade intensity just under the tree was nearly 75% (0-4 m adjacent trunk), 50% (4.1-8 m) and 25% (8.1-12 m). The open field was considered as zero shade intensity. Each plot of 405 × 315 cm size was replicated five times. One irrigation in every twenty days interval except rainy season were given. The experiment was layout following the standard horticultural practices. The observations were recorded for plant height, number of stems per plants, stem diameter, number of nodes per plant, number of leaves per stem, leaf length, leaf width, leaf area, rhizome length, rhizome spread, rhizome weight and number of feeder roots at harvesting stage. These observations were averaged out to get the mean value of three replication from each treatment after 18 months from the date of planting or second week of December, 2019. Plant height was recorded between the collar region and shoot tip of the main axis. Rhizome were separated from the plant for measuring fresh weight and dried properly after peeling for dry weight until constant moisture content at room temperature.
Photosynthetic light response studies
Chlorophyll fluorescence and photosynthetic performance to different light intensities was studied with portable photosynthesis system, LICOR 6400 XT (LI-COR Inc. Lincon, Nebraska, USA). To measure maximum efficiency of photosynthesis (Fv/Fm), leaves were adapted to dark period of minimum 30 minutes followed by a saturating flash light of 3,000 μmol (photon) m-2s-1 to achieve the maximum fluorescence. Subsequently, actual efficiency of photosynthesis (Fv’/Fm’) was measured [11]. The net Photosynthetic Rate (PN), Stomatal Conductance (gs) and Transpiration Rate (E) were recorded between 08:00 and 11:00 h. A completely mature leaf was kept in the chamber by ensuring the thermocouple touching it from the underside. Temperature (ambient) was set at giving a stable leaf temperature observation. Based on the light curve studies, the artificial light inside the cuvette was given at 1000 PAR including 10% of blue light. The CO2 was supplied at the flow rate of 500 m mol s-1 to supply 400 ppm CO2 inside the chamber. Stable reading of flow, CO2 and H2O were considered for logging the final reading of gaseous exchange parameters.
Determination of diosgenin content in dry rhizome
Diosgenin is major chemical constituent of Costus speciosus, for its determination the root sample dried and ground in cyclone mill (M/s Uday Corporation). The reference standard was purchased for M/s Sigma, India and prepared 100 ppm stock solution in methanol and stored at -20°C in freeze. One gram of dried root powder was extracted by 100 mL methanol three times (3 × 100 mL) by reflux on water bath at temperature 95 ± 5°C. The extracts were combined, filtered and evaporated up to dried by using rotatory evaporator (M/s Hidolph, Germany). The dried extract was hydrolysed by adding 40 mL of 10% HCL on water bath for 30 minutes. The cool down sample solution was partitioned by separating funnel three times with chloroform (3×100 ml). The pooled chloroform extract evaporated on rotary evaporator and residue dissolved in HPLC grade methanol. The sample solution filter through 0.45 μm nylon syringe filter and used for analysis. The chemical analysis was performed on Ultra- Fast Performance Liquid Chromatography (UFLC M/s Shimadzu Corporation, Kyoto, Japan) system. It consists of two L20AD pumps, DGU-20A3 Degasser, SIL-20AC HT auto sampler CTO-10ASvp column oven, CBM-20 a communication bus module and LC Solution software. The analysis performed by using RP-C18 Altima column 100 × 4.6 mm, 3 μm and mobile phase consist of acetonitrile and 0.1% formic acid in water in ratio of 82:18 v/v in isocratic condition. The flow rate was 1 ml/min and total run time 20 min. The peaks were monitor at 190-400 nm using Photodiode Array Detector, and integration and quantification was done at 203 nm. The calibration curve was plotted by loading different concentrations of reference standard against peak area (Figure 1A). The peak of diosgenin in sample was identified based on retention time and quantification by regression equation Y=4.2603e05+0.4745 [12]. The resolved peak chromatogram of reference standard and root extracted sample shown in (Figure 1B).
Statistical analysis
The analysis of variance was done in Randomized Block Design (RBD) for various observations observed during experiment by using statistical software SAS 9.2. DMRT comparisons among the different shade-net intensities. The results were presented at 5% level of significance and Critical Difference (CD) values were calculated to compare the various treatment means.
Results and Discussion
Keu was observed under different shade intensities (0, 25, 50 and 75%) for plant growth parameters including rhizome yield and quality parameters (Table 1). The growth parameters like plant height, number of stems, stem diameter, number of nodes per plant, number of leaves, leaf length, leaf width, leaf area, rhizome length, rhizome spread, rhizome weight and number of feeder roots were observed maximum under 75% shade intensity (145.09 cm, 4.75, 2.09 cm, 20.50, 15.79, 24.32 cm, 10.93 cm, 246.49 cm2, 31.12 cm, 37.72 cm, 1450 g and 151.17, respectively), whereas minimum (66.72 cm, 3.72, 1.32 cm, 15.03, 11.58, 17.70 cm, 7.28 cm, 101.21 cm2, 19.90 cm, 31.47 cm, 560 g and 58.17 respectively) in 0% shade intensity or open field. Light is an important environmental factor that impacts phenological growth parameters [13]. Similarly, rhizomatous crop like turmeric considered as partial shade-tolerant crop and the total shoot-root biomass was markedly higher in plants raised under 48, 52 and 68% Relative Light Intensity (RLI) as compared to the 100% RLI [14]. Shatavari in arecanut orchard produced fresh root yield (10,666 kg ha-1) and subsidized to maximum kernel equivalent yield (1524 kg ha-1) among sixteen medicinal and aromatic plants as an intercrop [15]. Overall, maximum survival rate, as an intercrop in mulberry orchard was reported in A. racemosus, while Acorus calamus, Aloe barbadensis, Cyperus scariosus, Plumbago zeylanica, Rauvolfia serpentina also suggested that among these medicinal plants, could be grown as an intercrop [16].
Table 1: Effect of shade net intensity on rhizome weight and contributing parameters in Costus speciosus.
Number of nodes per plantNumber of leaves per tillerLeaf length (cm)Leaf width (cm)Leaf area (cm2)Rhizome length (cm)Rhizome spread (cm)Number of feeder rootsRhizome weight15.03c11.58c17.70c7.28c101.21d19.90b31.47b58.17c560d15.73c14.53 ab18.36bc8.88b136.44c19.13b31.88b60.83b760c17.01b14.76 ab22.64ab10.54a178.91b19.62b36.35ab69.17b1050b20.50a15.79 a24.32a10.93a246.49a31.12a37.72a151.17a1450aMeans with the same letter (superscript) in the columns do not showing significantly different (P = 0.05) - (Duncan Multiple Range Test)The fresh rhizome yield was highest in 75% SI (55.06 t ha-1) followed by 50% SI (39.71 t ha-1), whereas it was lowest in 0% SI (21.39 t ha-1). Rhizome yield performance was more than double under 75 % shade intensity as compared to 0% SI (Figure 2). It was recorded that maximum weight gain of rhizome taken place with increasing shade intensity. This might be due to medicinal plants prefer to grow in forests under limited shade, organic matter rich moist soils with high Relative Humidity (RH) and trivial temperatures [17].
The light response curve studies performed in costus genotypes at a fixed reference of 400 μ mol CO2 mol-1 indicated that plant can initiate photosynthesis with photosynthetic active radiation (PAR) of 250 μ mol (photons) m-2 s-1 only (Figure 3). Increasing the light intensity, the PN gradually increased till 500 μ mol (photons) m-2 s-1. The PN was maximum at 1000 μ mol (photons) m-2 s-1 and further increase in light intensity decreased the PN. Increasing PAR from 250 to 1000 had resulted in 17% higher PN. The gs become steady after 500 μ mol (photons) m-2 s-1. It is interesting to note that the non-photochemical quenching increased steadily with increase in light intensity. This kind of PAR requirement makes the plant suitable for very low light transmission under partially shaded area. The stomatal limitations showed positive relationship with increase in light intensities. It is also worth to note that the ΦPSII was maximum at 100 μ mol (photons) m-2 s-1 and continuously decreased with higher light intensities. The actual efficiency of photosynthesis was maximum at 100 μ mol (photons) m-2 s-1 and decreased thereafter. The ETR, E and NPQ increased with increase in light intensities. The Keu plant can photosynthetically perform well even below PAR light of 750 μ mol (photons) m-2 s-1. Costus spiralis and C. longebracteolatus plants perform significantly for growth parameters especially pseudo stem length under 50% shading by red and blue nets. This might be due to plant growth and development were repressed under full sun exposure (PAR light >1000 μ mol) along with early leaf necrosis of these species as compared to shade intensities [18].
Dried rhizome (commercial part) was analysed for diosgenin content and average maximum diosgenin content (0.826 mg g-1) was observed under 0% SI followed by 25% and 75% (0.772 mg g-1 and 0.764 mg g-1, respectively) SI (Figure 1A and 4). Similarly, the curcumin content was distinctly higher in turmeric plants raised under 59% RLI as compared to the 100% RLI [13]. Although shade-grown leaves are not directly exposed to sunlight, they produce additional chlorophyll to capture diffuse radiation to produce the carbohydrates needed for plant growth. While, curcumin (5.42%), oleoresin (10.15%) and essential oil (5.60%) content increased under shaded conditions in turmeric [19]. Shatavarin IV content in Shatavari was enhanced under different shade intensities [9] Graphical Presentation.
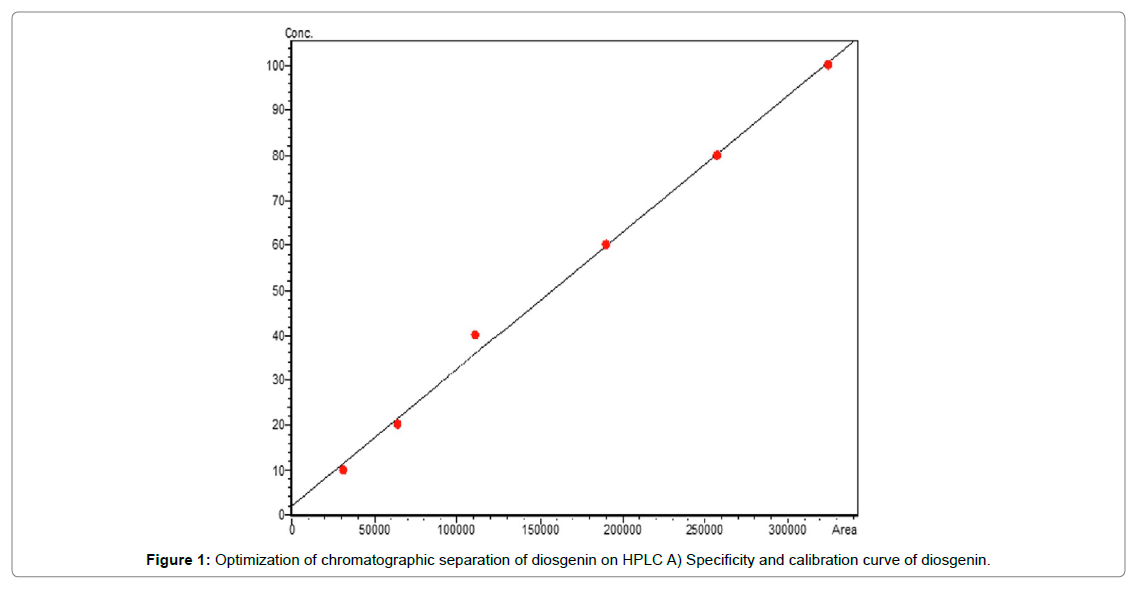
Figure 1: Optimiza tion of chromatographic separation of diosgenin on HPLC A) Specificity and calibration curve of diosgenin.
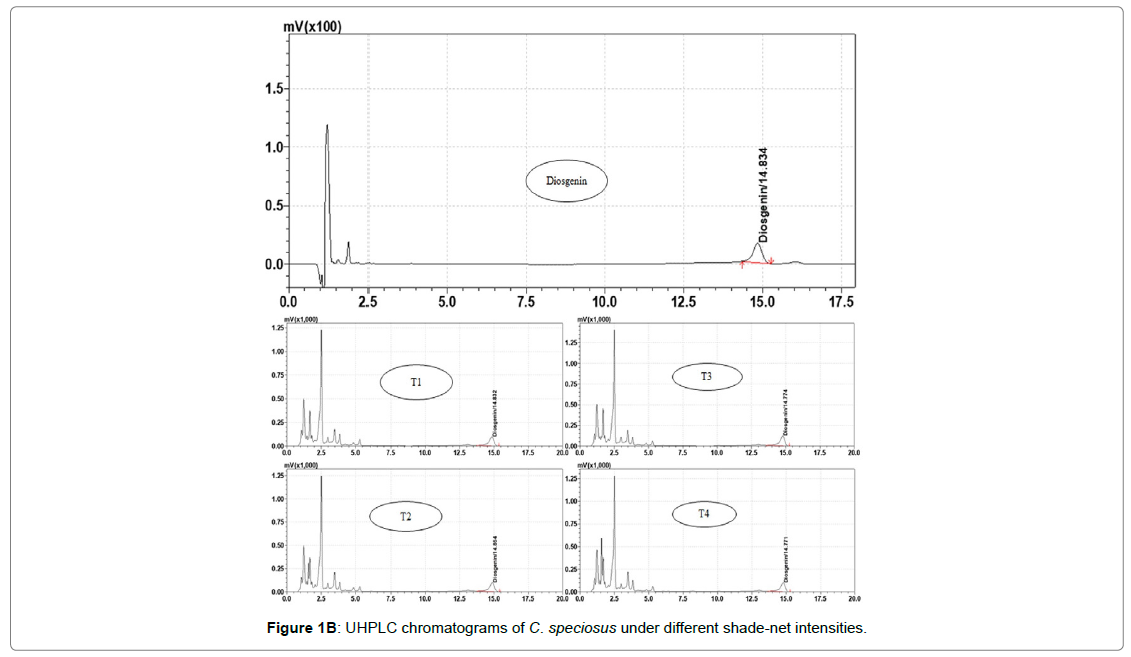
Figure 1B: UHPLC chromatograms of C. speciosus under different shade-net intensities.

Figure 2: Performance of rhizome yield in C. speciosus under different natural shade intensities.
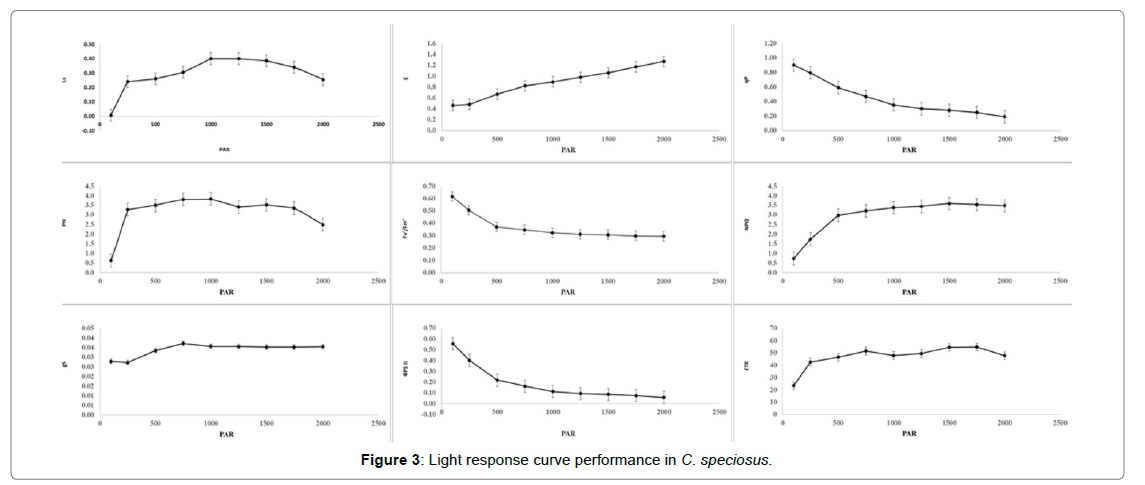
Figure 3: Light response curve performance in C. speciosus.
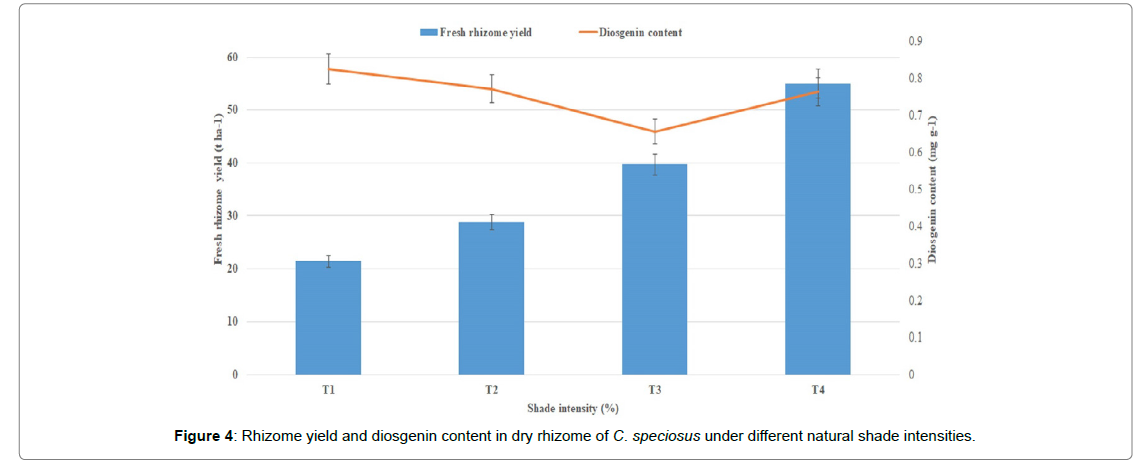
Figure 4: Rhizome yield and diosgenin content in dry rhizome of C. speciosus under different natural shade intensities.

Conclusion
In conclusion about double rhizome yield was observed under 75% SI followed by 50% SI. At the same time diosgenin content was not significantly higher in open field condition as compared with shade intensities. For harnessing of rhizome yield and diosgenin content, 75% SI as an intercrop was found most suitable compared with rest SI including open field condition. Over all this crop prefers partial shade condition, therefore, costus is highly suitable as an intercrop under perennial trees like neem for sustainable cultivation.
Acknowledgments
The authors are grateful to the Indian Council of Agricultural Research for facilities and NASF, New Delhi for providing financial support.
References
- Gupta RK (2010) Medicinal and Aromatic Plants, CBS Publishers and Distributors 234: 499.
- Gupta AK, Tondon N, Sharma M (2008) Quality Standards of Indian Medicinal Plants, Medicinal Plants Unit, Published by Indian Council of Medical Research 8: 48.
- TWI (2007) The Wealth of India: First Supplement Series (Raw Materials), National Institute of Science Communication and Information Resources, CSIR 2: 211-213.
- Srivastava S, Singh P, Mishra G, Jha KK, Khosa RL (2011) Costus specious (keukand): a review. Der Pharmacia Sinica 2: 118-128.
- Singh I, Gautam YK, Vimala Y (2013) Detection and Isolation of Diosgenin from Costus speciosus Callus Raised from Non-Germinal Seeds. International Journal of Chemical Life Science 2: 1240-1242.
- Prasad PN (1982) “Studies on Costus speciosus (Koen) sm. and Malortieanus C, Wendl H Ph.D Thesis, University of Madras, Tamil Nadu, India.
- Muniyandi SK, Nandanan AT, Veeti SC, Narayanan A, Ganesan B (2013) "Studies on Costus speciosus Koen Alcoholic Extract for Larvicidal Activity," International Journal of Pharmacognosy and Phytochemistry Research 5: 328-329.
- Ginting J, Sengli B, Damanik J, Sitanggang JM, Muluk C (2015) Effect of Shade, Organic Materials and Varieties on Growth and Production of Upland Rice. International Journal of Science and Technology Research4: 68-74.
- Saran PL, Singh S, Solanki VH, Kalariya KA Meena RP, et al. (2019) Impact of shade-net intensities on root yield and quality of Asparagus racemosus: A viable option as an intercrop. Industrial Crops and Products 141: 111740.
- Kikon N, Angami T (2018) Medicinal and aromatic plants for climate change adaptation: Scope and prospects with special reference to North Eastern Himalayas. Journal of Medicinal Plants 6: 150-155.
- Maxwell K, Johnson GN (2000) Chlorophyll fluorescence-a practical guide. Journal of Experimental Botany 51: 659-668.
- Li P, Mou Y, Liu S, Sun W, Lou J, et al. (2012) Quantitative determination of diosgenin in Dioscorea zingiberensis cell cultures by Micro plates spectrophotometry and H PLC. African Journal of Pharmacy pharmacology 6: 1186-1193.
- Kasajima S, Inoue N, Mahmud R, Fujita K, Kato M (2007) Effect of light quality on developmental rate of wheat under continuous light at a constant temperature. Plant Production and Science10: 286-291.
- Hossain MA, Akamine H, Ishimine Y, Teruya R, Aniya Y, Yamawaki K (2009) Effects of Relative Light Intensity on the Growth, Yield and Curcumin Content of Turmeric (Curcuma longa L.) in Okinawa, Japan.Plant Production and Science 12: 29-36.
- Sujatha S, Bhat R, Kannan C, Balasimha D (2011) Impact of intercropping of medicinal and aromatic plants with organic farming approach on resource use efficiency in arecanut (Areca catechu L.) plantation in India. Industrial Crops and Products 33: 78-83.
- Shashi K (2015) Intercropping of some Medicinal Plants with Mulberry. CIB Tech J Bio-Protoco 4: 27-30.
- Venugopal CK, Mokashi AN, Jholgiker P (2008) Studies on comparative performance of patchouli (Pogostemon patchouli Benth.) under open and partial shade ecosystem. Journal of Medicinal and Aromatic Plant Science30: 22-26.
- Mazzini-Guedes RB, Pivetta KFL, Gimenes R, Romani GN, Batista de Souza, et al. (2016) Initial growth of Costus longebracteolatus and Costus spiralis ‘French Kiss’ under different light conditions. Ornamental Horticulture22: 326-334.
- Padmapriya S, Chezhiyan N (2009) Effect of shade, organic, inorganic and biofertilizers on morphology, yield and quality of turmeric. Indian Journal of Horticulture 66: 333-339.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 
