Research Article, Int J Cardiovasc Res Vol: 8 Issue: 3
In vitro Hemocompatibility Testing of Dyneema Purity® Fibers Patch and Clinically Used Cardiovascular Prostheses
Amir Basir1*, Mark Roest2, Mylène Loncq de Jong1, Wally Müller3, Joost van Herwaarden4, Frans Moll4, Gerard Pasterkamp1, Jolanda Kluin5, Paul Gründeman1 and Philip de Groot2
1Department of Experimental Cardiology, UMC Utrecht, Utrecht, the Netherlands
2Department of Clinical Chemistry and Haematology, UMC Utrecht, Utrecht, the Netherlands
3Department of Chemistry, Cryo-EM, Faculty of Sciences, Utrecht University, Utrecht, the Netherlands
4Department of Vascular Surgery, UMC Utrecht, Utrecht, the Netherlands
5Department of Cardio-Thoracic Surgery, AMC Amsterdam, Amsterdam, the Netherlands
*Corresponding Author: Amir Basir, MD, PhD.
University Medical Center, Utrecht, Heidelberglaan 100 CX Utrecht, The Netherlands
Tel: 0031682005283
E-mail: amirbasir@hotmail.com
Received: February 13, 2019 Accepted: March 09, 2019 Published: March 15, 2019
Citation: Basir A, Roes M, Jong ML, Müller W, Herwaarden JV, et al. (2019) In vitro Hemocompatibility Testing of Dyneema Purity® Fibers Patch and Clinically Used Cardiovascular Prostheses. Int J Cardiovasc Res 8:3. doi:10.4172/2324-8602.1000377
Abstract
Objectives
Currently available materials for vascular- and heart valve prostheses carry drawbacks, including the requirement of strong anticoagulants, moderate durability, and the inapplicability for endovascular treatment. Dyneema Purity® fibers are made from ultra-high molecular weight polyethylene, are very thin, strong, flexible and resistant to fatigue and abrasion. This material might be attractive for use in vascular- and valvular surgery. In this study the hemocompatibility of yarn-composed patches of Dyneema Purity® fibers was assessed.
Methods
Three methods were used to compare the hemocompatibility of yarn-composed patches of Dyneema Purity® fibers with 5 clinically used yarn-composed and
membrane-based patches. First, patches were perfused with human blood in a perfusion chamber, thereafter adhered red blood cells and platelets were assessed by scanning electron microscopy. Second, the platelet activation caused by patches was tested using fluorescence-activated cell sorting assay at 1, 3, 6, 24, and 48 hours. Third, various coagulation parameters activated by patches were measured using enzyme-linked immunosorbent assay.
Results
Perfusion experiment showed no differences between patches of Dyneema Purity® fibers and control patches for single platelet and red blood cell adherence. Less platelet aggregation was visible on Dyneema Purity®, compared with one yarn-composed patch, and aggregates were equal compared with the other 4 controls. The platelet activation experiment showed no differences for Dyneema Purity® and other yarn-composed patches. Compared with membranebased patches, there was more platelet activation at 1 hour and 24 hours, but no significant difference at 48 hours. The coagulation activation experiment showed comparable coagulation activation on all patches.
Conclusion
Patches of Dyneema Purity® fibers are noninferior to the used control patches in terms of red blood cell- and platelet adhesion and platelet- and coagulation activation. Therefore, this material may be attractive for use in cardiovascular applications.
Keywords: Dyneema purity®; UHMWPE; Hemocompatibility testing; Platelet aggregation; Platelet activation; Platelet adhesion; Coagulation activation;Perfusion chamber; Scanning electron microscopy
Introduction
Implantation of vascular and heart valve prostheses is a common procedure for patients with heart valve stenosis or regurgitation and dilated or obstructive vascular disease.
In vascular surgery, the evolution of stent grafts and endovascular delivery systems has substantially improved the application rate and patient outcomes with endovascular aneurysm repair (EVAR) of abdominal aortic aneurysm (AAA). But, the delivery systems still suffer from the inherent drawback of a relatively large diameter. Therefore tortuous, calcified, and small iliac arteries are recognized as important risk factors for less successful EVAR of AAA and are hence considered as a criterion for exclusion of patients who otherwise would be suitable candidates.
The annual global number of patients requiring heart valve replacement is estimated to triple from approximately 290,000 in 2003 to more than 850,000 by 2050 [1]. Two different types of prostheses are currently available for heart valve replacement: mechanical and bio prostheticheart valves. Both carry serious drawbacks. Mechanical prostheses require strong anticoagulant treatment, which increases the risk for bleeding and stroke. Bioprosthetic heart valves have a limited lifespan of ten to fifteen years; thereafter, a reoperation is necessary. Thus far, alternative heart valve grafts, such as decellularized xenografts, have been unsuccessful [2]. The ideal artificial heart valve would have life-long durability, optimal hemocompatibility, and would be suitable for catheter-based implantation.
Currently, polyester (polyethylene terephthalate [PET]), also known as Dacron® fabrics, are used extensively in cardiovascular applications, including vascular prostheses, heart valve sewing cuffs, and annuloplasty rings [3]. Dacron® and Gore-Tex® (expanded polytetrafluoroethylene [ePTFE]) vascular grafts have been successful in bypassing and patching of obstructed blood vessels of large and medium diameters [4].
Here we focus on patches of Dyneema Purity® fibers, which are made from very thin ultra-high molecular weight polyethylene (UHMWPE) fibers. These fibers are approximately twice as strong as steel, are flexible and are resistant to fatigue and abrasion [5]. Prostheses made of these fibers hold promise for use in the minimally invasive treatment of vascular diseases by reducing the profile of the stent graft, including the delivery systems. Heart valves made of Dyneema Purity® fibers could possibly provide a solution for many patients because of the expected prolonged durability and suitability for endovascular implantation. Dyneema Purity® fibers have already been used in cardiovascular applications, such as reinforcement of balloon catheters, and in orthopedics for anterior cruciate ligament repair, and sutures of Dyneema Purity® fibers are the gold standard for rotator cuff repair. But, heart valves and vascular constructs of Dyneema Purity® fibers would be the first applications where implantation with constant contact with blood is considered. Because knowledge about the biocompatibility of Dyneema Purity® fibers for such long-term applications in the blood circulation is limited, their hemocompatibility in blood contact was assessed in this study, as the first step.
In this study, red blood cell and platelet adhesion on, and platelet and coagulation activation of yarn-composed patches of Dyneema Purity® fibers were assessed and compared with clinically used polyester and ePTFE materials in three different experiments.
Materials and Methods
Blood sample preparation
Venous blood was collected in vacuum tubes with 3.2% trisodium citrate from healthy volunteers who had not used medications in the past ten days. The collection of blood conformed to the Declaration of Helsinki. This part of the study was approved by the University Medical Center Utrecht Ethics Review Board, Utrecht, and The Netherlands.
Dyneema Purity® fibers patch preparation
Before testing, patches of Dyneema Purity® fibers were manually cleaned by reversed osmosis water with 10 mL/L alkaline detergent neodisher® MediClean forte (Dr. Weigert Nederland BV, Assen, The Netherlands), rinsed with sterile water, disinfected with 70% ethanol, and sterilized with 100% ethylene oxide at 370 mbar pressure and 50°C for 4 hours with an aeration time of 48 hours.
Control patches
To compare the hemocompatibility of patches of Dyneema Purity® fibers (A), 5 patches commonly used in the human blood circulation were used as controls (B-F): (B) Bard® DeBakey® Knitted Polyester Fabric is a permeable fabric used for patch graft angioplasty and repair of intracardiac defects. (C) Bard® DeBakey® Woven Polyester Fabric is a thin, low-permeability polyester fabric used in outflow tract repairs, patch graft angioplasty, and septal defects. (D) IMPRA® ePTFE Cardiovascular Patch applications include peripheral vascular reconstructions and cardiac and vascular repairs. (E) Gore-Tex® Stretch Vascular Graft Thinwall is tubular shaped and used for different purposes in angiographic access. (F) Gore-Tex® Stretch Vascular Graft Thick wall is also tubular shaped and is approximately 40% thicker than the Thinwall graft (Table 1).
| Material | Specifications | |
|---|---|---|
| A | Dyneema Purity® SGX dtex55 fibers woven patch (twill weave, 152 ends/inch, 86 picks/inch | Yarn-composed |
| B | Bard®DeBakey® Elastic Knitted Polyester Fabric | Yarn-composed |
| C | Bard®DeBakey® Woven Polyester Fabric | Yarn-composed |
| D | Bard Impra® ePTFE Cardiovascular Patch | Membrane-based |
| E | Gore-Tex® Stretch vascular graft thinwall | Membrane-based |
| F | Gore-Tex® Stretch vascular graft thickwall | Membrane-based |
Table 1: Specification of the Dyneema Purity® fibers patch and other yarn-composed and membrane-based patch materials used for the perfusion experiment.
Of these 6 patches, the Dyneema Purity® fibers patch, Bard® DeBakey® Knitted, and Woven Polyester Fabric do not have a smooth surface as they are fabric (e.g. yarn-composed). The other three patches —IMPRA® ePTFE Cardiovascular Patch, and Gore-Tex® Stretch Vascular Graft Thin wall and Thick wall are membrane-based and have a smooth surface.
Perfusion experiment
Perfusion system: Perfusions were performed in a double flat rectangular perfusion chamber, as described by Sakariassen et al. [6] and Nievelstein et al. [7], with some modifications. Briefly, the perfusion chamber was made of polymethyl methacrylate and consisted of 4 separate pieces. The perfusion chambers used in this experiment were longer than the original one, and the central knob was replaced by two knobs (Figure 1). The knobs were located at equal distances from the center of the chamber (2.25 cm), measured from the middle of the knob and had flat surfaces, in contrast to the original knobs. To prevent leakage during the perfusion, the knobs had different diameters depending on the thickness of the patches (Table 2).
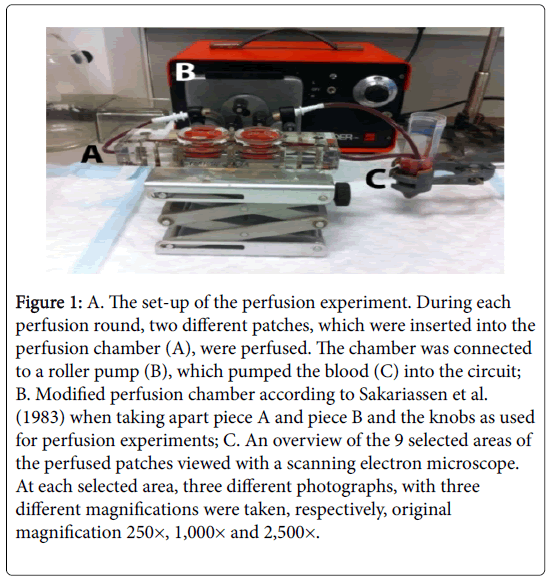
Figure 1: A. The set-up of the perfusion experiment. During each perfusion round, two different patches, which were inserted into the perfusion chamber (A), were perfused. The chamber was connected to a roller pump (B), which pumped the blood (C) into the circuit; B. Modified perfusion chamber according to Sakariassen et al. (1983) when taking apart piece A and piece B and the knobs as used for perfusion experiments; C. An overview of the 9 selected areas of the perfused patches viewed with a scanning electron microscope. At each selected area, three different photographs, with three different magnifications were taken, respectively, original magnification 250×, 1,000× and 2,500×.
| Perfusion round | Upstream chamber | Downstream chamber |
|---|---|---|
| 1 | Patch A, knob Ø29.8 | Patch F, knob Ø29.6 |
| 2 | Patch B, knob Ø29.6 | Patch A, knob Ø29.8 |
| 3 | Patch C, knob Ø29.4 | Patch B, knob Ø29.6 |
| 4 | Patch D, knob Ø29.6 | Patch C, knob Ø29.4 |
| 5 | Patch E, knob Ø29.7 | Patch D, knob Ø29.6 |
| 6 | Patch F, knob Ø29.6 | Patch E, knob Ø29.7 |
Table 2: Sequence of the different patches and knobs used during the perfusion experiment in six perfusion rounds with the six different patches
Patch A: Dyneema Purity® fibers woven patch, Patch B: Bard®DeBakey® Elastic Knitted Polyester Fabric, Patch C: Bard®DeBakey® Woven Polyester Fabric, Patch D: Bard Impra® ePTFE Cardiovascular Patch, Patch E: Gore-Tex® Stretch vascular graft thinwall, Patch F: Gore-Tex® Stretch vascular graft thickwall.
The patches were exposed to the flowing blood by holding them on the knob during the insertion of the knobs into the holes. All knobs with patches fitted into the holes of piece A with some pressure as they always formed a total diameter of 30 mm. Before the patches were inserted into the perfusion chamber, the center area of each patch (exposed to the blood) was marked (Figure 1B). By drawing just ¾ of the rectangle in the center of the patch, the side of the patch that was exposed to the blood was marked as visualized by the scanning electron microscope (SEM). Patches on the knobs served as part of the roof of the flow chamber that was formed when part A and B were joined by ten screws. The dimensions of the two flow channels were 30 × 10 × 1 mm.
The perfusion chamber was connected to Silastic tubing (Ø 3 mm; Dow Corning Corp., Midland, MI, USA) by two inlets oriented at an angle of 20° toward the central hole. At each inlet of the chamber, the rectangular cross-section gradually tapered off to the diameter (Ø 3 mm) of the inlet portals. Leakage of blood between the knobs and piece A was prevented by O rings. A 50-ml Sarstedt plastic centrifuge tube (Sarstedt AG & Co., Nümbrecht, Germany) was modified by removing a 15 × 50 mm rectangle from the top up to the 30 mL blue grading with a razor blade. This modified plastic centrifuge tube was fixed next to the pump with a 4-pronged clamp attached to a stand and accommodated for a perfusion of 15 mL of the donor’s blood. Thereafter, this centrifuge tube with blood was connected to the inlet side of the pump with a 15 cm transparent silicone tube that was cut off obliquely at an angle of 45° and fitted on the bottom of the plastic centrifuge tube. The outlet side of the pump was connected with the inlet side of the flow chamber by the use of a 10 cm transparent silicone tube. Then, a 10 cm transparent silicone tube at the outlet side of the flow chamber was placed into the centrifuge tube with donor blood-about 1 cm above its bottom-and thus completed our perfusion system (Figure 1A).
Perfusion conditions: A non-occlusive peristaltic roller pump (VRE 200, Verder, Düsseldorf, Germany) was used to circulate the blood through the perfusion chamber at a flow rate of 50 mL/min. Each perfusion round was performed with 15 mL of 10 mmol/L HEPES (4- (2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer in saline for three minutes, followed by 15 mL blood for three minutes.
Blood from three randomly chosen donors was used, and six perfusion rounds were performed per donor. During each perfusion, 2 different patches were perfused at the same time (Table 2). The same flow chamber was used for the 6 perfusion rounds and cleaned before each use.
After each perfusion, patches were taken out of the flow chamber, placed in a 100-mL beaker filled with 10 mmol/L HEPES buffer, rinsed, and transferred to petri dishes (Ø 14 cm) filled with 10 mmol/L HEPES buffer. While still submerged in this HEPES buffer, about 8 × 10 mm from the center of each patch was removed with scissors (Robuso Solingen Germany 322/5). The right top corners of the rectangles were cut out to indicate the flow direction.
Scanning electron microscopy
About 8 × 10 mm of the central part of the patches were fixed with 2% (v/v) glutaraldehyde (Agar Scientific Ltd., Essex, UK) and 0.15 mol/L phosphate-buffered saline (PBS; pH 7.4) in 20 mL screw-cap glass vials for at least 1 day at 4°C. After being rinsed 3 times with PBS and 3 times with distilled water, the samples were post-fixed in 2% aqueous osmium tetroxide (Sigma-Aldrich, St. Louis, MO, USA) at 4°C for 16 hours. After being rinsed 3 times with distilled water, the samples were dehydrated in an increasing series of ethanol (Merck, Darmstadt, Germany) of 30%, 50%, 70%, 80%, 90%, 96%, and 100%, with each step lasting at least 15 min at room temperature. The ethanol was changed in 3 steps with anhydrous acetone (50%, 75%, and 100%), which was made according to Conway and Kiernan [8] (1998): 99 mL acetone (Merck) was mixed with 1 mL 1% (v/v) acidified 2,2- dimethoxypropane (DMP; Merck). Acidified DMP was made by adding 50 μL 37% HCL (Merck) to 50 mL DMP. The samples were placed in a CPD-030 Leica critical point drying apparatus (Leica Microsystems, Vienna, Austria) with a CPD chamber that was half filled with anhydrous acetone and contained a sample holder, which was a self-made of aluminium mesh of about 22 × 25 mm. While still submerged in anhydrous acetone in the glass vials, the samples were gently but rapidly placed with tweezers in this sample holder. The CPD chamber was closed and the samples were critical point dried according to the CPD-030 Leica manual with liquid CO2 as the transitional fluid. Depressurizing was done overnight. To this end, a silicon tube was connected with the gas out and the other end was placed in 250 mL cylinder filled with tap water. The gas flow was set so that about five 6-mm-diameter gas bubbles passed from bottom to the top of the cylinder. The dried specimens were mounted on a stub containing a carbon adhesive. Once placed on the stub, the samples were carbon taped at their long sides for best electron conductivity. The samples were coated with 8 nm Pt/Pd by the use of a 208HR sputter coater (Cressington Scientific Instruments Ltd., Chalk Hill Watford, UK). The samples were viewed in a XL30 SEM equipped with a field emission gun (FEI Europe, Eindhoven, The Netherlands) at an acceleration voltage of 5 kV and a WD of about 27 mm. We took photographs from 3 different parts from the middle of the 8 × 10 mm patch, from the top of the patch (containing the notch), and from the bottom of the patch (Figure 1C).
Platelet activation experiment
Platelet activation was assessed after the use of patches of Dyneema Purity® fibers and five control materials as listed in Table 1. During activation of platelets, cell adhesion molecule P-selectin moves from within the platelet to the platelet membrane. Fluorescence Activated Cell Sorting (FACS) was used to measure P-selectin on the cell membrane of the activated platelets. All patches were cut to the same size of 1 cm2. Blood of ten donors was incubated for 1, 3, 6, 24, and 48 hours in a 12-well flat bottom plate (Corning® Costar®). The plate contained the patches submerged in 1 mL blood. One well with blood did not contain any patch and served as a negative control. The plates were kept on a benchtop shaker during the entire incubation period. After incubation, 5 μL of the blood was added to 50 μL of the FACS reaction mix containing 1:25 mouse anti-human CD42b-FITC conjugated (BD Pharmingen, 555472) and 1:25 mouse anti-human CD62p-phycoerythrin (PE) conjugated in HBS (HEPES 10 mmol/L, NaCl 150 mmol/L, MgSO4 1 mmol/L, KCl 5 mmol/L). CD42b (glycoprotein 1b), a Willebrand factor receptor, was used as a platelet marker. Thirty minutes after the reaction started, the reaction mix was fixated with 500 μL fixative (0.4% formaldehyde in physiologic saline). After fixation, the PE signal on the platelets was measured in the BD FACS Canto II using BD FACS Diva 6.1.3 software.
Coagulation activation experiment
All citrated blood samples from every time point, except for 1 donor of the “platelet activation experiment” were centrifugated for ten minutes at 2,000 g. The coagulation parameters thrombin antithrombin complex (TAT), beta-thromboglobulin (beta-TG, NAP-2), soluble P-selectin (sP-selectin) and thrombospondin-1 proteins were measured in the plasma using enzyme-linked immunosorbent assay to assess the coagulation activation caused by patches of Dyneema Purity® fibers and the control patches.
Enzyme-linked immunosorbent assays were performed on a semiautomatic TECAN Freedom EVO robot using Freedom EVOware® 2.3 software. The Maxisorb 384-well plates were coated overnight, at 4°C, with 1 μg/mL anti-human thrombin (Aff.biologicals, SAHT AP), 1 μg/mL anti-hNAP-2 (R&D, MAB393), 1 μg/mL anti-human P-selectin (R&D, DY137), or 0.5 μg/mL anti-human thrombospondin-1 (R&D, DY3074). The next day, the plates were blocked by 1% Bovine Serum Albumin in PBS. The plates were washed and incubated with a dilution of the samples and with a known concentration of the calibration curve.
After incubation and washing, the plates were incubated with a horseradish peroxidase (HRP)-labelled (0.5 μg/mL anti-human antithrombin; Aff.biologicals, SAAT AP HRP) or biotin-labelled (50 ng/mL biotinylated anti-hNAP-2, R&D, BAF393; 100 ng/mL biotinylated anti human sP-selectin, R&D, DY137; 100 ng/mL biotinylated anti-thrombospondin-1, R&D, DY3074) secondary antibody. The plates were again incubated and washed, and substrate was directly added (TAT), or the plates were incubated with 125 ng/mL streptavidin-mono-HRP (DAKO, P0397; betaTG, sP-selectin, thrombospondin-1).
After incubation and washing, detection was performed with SuperSignal West Pico Chemiluminescent substrate (Thermo Scientific, 34080), and read with a luminometer. All wash steps were done 5 times with 0.05% Tween-20 in PBS. All incubation steps were done at room temperature for 2 hours while the samples were being shaken (180 rates/min).
Analysis and statistics
For the perfusion experiment a 10 × 10 raster (10.6 × 7.3 μm) was placed on the SEM photographs (116.7 × 79.8 μm), and the photograph titles were coded automatically by Adobe Photoshop 13.0 software. The photographs were stripped of any kind of information and were assessed at every tangent point (100) of the raster if a single platelet, multiple-clustered platelets, platelet aggregation, or red blood cells were present. This quantification was performed in duplicate by the investigator and by an independent observer who did not know the type of the patches. The data are given as percentage per cm2 and are expressed as mean ± standard deviation.
SPSS 19.0 software (IBM Corp, Armonk, NY USA) was used to analyze adherence on the patches, and randomized block analysis of variance with Dunnett post hoc tests was used.
The interobserver variability was tested with the Interclass Correlation Coefficient. Differences between adherence on patches of Dyneema Purity® fibers and the control patches were analyzed.
For the platelet activation and the coagulation activation experiment, the value at time point 0 was used as the index value to calculate the relative change of activation. The paired t test, calculated with GraphPad Prism 6.0 software, was used to analyze the differences between the different patch materials. A P value of ≤0.05 was considered statistically significant.
Results
Results of the Perfusion Experiment
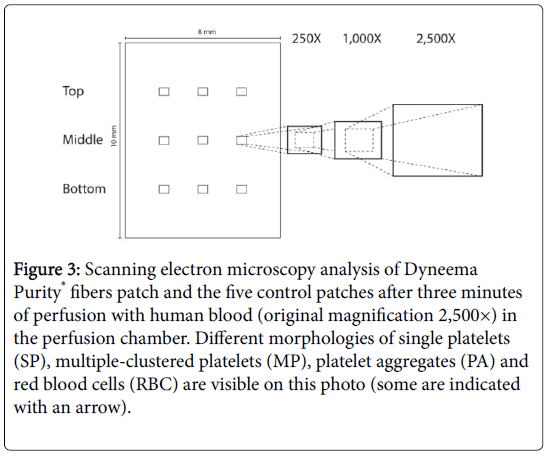
Platelets and red blood cells were by far the most common blood cells that adhered to the different patches as visualized on the SEM photographs. For simplicity, the blood platelets visible on the SEM photos were grouped in 3 categories: (1) 1 blood platelet; (2) multipleclustered platelets (i.e. 2 to 5 clustered blood platelets), and (3) platelet aggregates (i.e., more than 5 blood platelets in a cluster). The number of adhered blood platelets and the number of red blood cells on patches of Dyneema Purity® fibers and 5 control materials are shown in Figure 2.
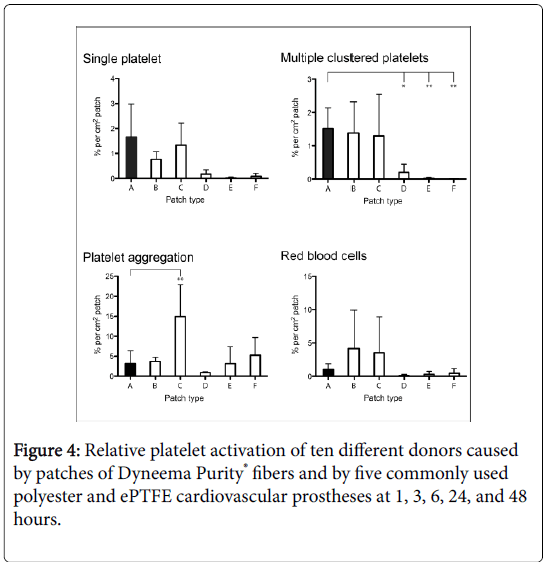
Figure 2: Average number of single platelets, multiple-clustered platelets (two to five platelets grouped together), platelet aggregation (more than five platelets in an aggregate), and red blood cells present on the crossing points (100) of the raster on the scanning electron microscopy photos (original magnification 2,500×).
Patch A: Dyneema Purity® fibers patch, Patch B: Bard®DeBakey® Elastic Knitted Polyester Fabric, Patch C: Bard®DeBakey® Woven Polyester Fabric, Patch D: Bard Impra® ePTFE Cardiovascular Patch, Patch E: Gore-Tex® Stretch vascular graft thinwall, Patch F: Gore-Tex® Stretch vascular graft thickwall.* ≤0.05 **≤0.01
Single blood platelet
Relatively few single platelets were adhered to all six perfused patches. For the yarn-composed group, single platelets were present on 1.7% (per 1 cm2) of patches of Dyneema Purity® fibers, on 0.8% of Bard®DeBakey® Knitted Polyester fabric, and on 1.3% of Bard®DeBakey® Woven Polyester fabric.
For the membrane-based patches, single platelets were adhered on 0.2% (per 1 cm2) of the Bard Impra® ePTFE Cardiovascular Patch, 0% of Gore-Tex® Stretch vascular graft Thinwall, and 0.1% of the Gore- Tex® Stretch vascular graft–Thickwall.
Multiple-clustered platelets
Multiple-clustered platelets adhered on 1.5% (per 1 cm2) of the patches of Dyneema Purity® fibers. This amount was comparable to the other 2 yarn-composed patches Bard®DeBakey® Knitted (1.4%) and woven polyester fabric (1.3%). Significantly more multiple-clustered platelets adhered on patches of Dyneema Purity® fibers compared with the Bard Impra® ePTFE Cardiovascular Patch (0.2%, P=0.011), and the Gore-Tex® Stretch vascular graft Thinwall (0.0%, P=0.005) and Thickwall (0.0%, P=0.007) membrane-based patches.
Platelet aggregation
There was significantly less platelet aggregation on patches of Dyneema Purity® fibers (3.2% per 1 cm2) compared with the yarncomposed Bard®DeBakey® Woven Polyester Fabric (15.0%, P=0.001; Figure 2). Compared with the other yarn-composed patch (Bard®DeBakey® Knitted Polyester Fabric), there was no significant difference (3.7%). In the membrane-based group, 0.9% (per 1 cm2) aggregates adhered to the Bard Impra® ePTFE Cardiovascular Patch, 3.2% to the Gore-Tex® Stretch vascular graft (Thinwall), and 5.2% to the Gore-Tex® Stretch vascular graft (Thickwall), all of which were significantly different from patches of Dyneema Purity® fibers.
Red blood cells
No significant difference was observed in red blood cell adherence to patches of Dyneema Purity® fibers compared with the five control patches. In the yarn-composed group, 1.0% (per 1 cm2) red blood cells adhered to patches of Dyneema Purity® fibers, 4.2% to the Bard®DeBakey® Knitted polyester Fabric, and 3.5% to the Bard®DeBakey® Woven Polyester Fabric.
For the membrane-based patches, 0.1% (per 1 cm2) red blood cells adhered to the Bard Impra® ePTFE Cardiovascular Patch, 0.3% to the Gore-Tex® Stretch vascular graft (Thinwall), and 0.5% to the Gore-Tex® Stretch vascular graft (Thickwall).
Results of platelet activation experiment
Just slight differences in P-selectin activation were measured over time among the six patches (Figure 3). At the 1-hour follow-up (FU), there were no significant differences in P-selectin activation for patches of Dyneema Purity® fibers compared with four of the five control materials. Only the ePTFE cardiovascular patch showed less P-selectin activation (P=0.003). At the 3- and 6-hour FU, there were no significant differences for patches of Dyneema Purity® fibers and the control patches. At the 24-hour FU, there was less platelet activation for the Gore-Tex® Stretch vascular graft Thinwall (P=0.03) and for the ePTFE Cardiovascular Patch (P=0.01) compared with patches of Dyneema Purity® fibers. At the 48 hour FU, there were no differences in P-selectin activation between patches of Dyneema Purity® fibers and the controls.
Figure 3: Scanning electron microscopy analysis of Dyneema Purity® fibers patch and the five control patches after three minutes of perfusion with human blood (original magnification 2,500×) in the perfusion chamber. Different morphologies of single platelets (SP), multiple-clustered platelets (MP), platelet aggregates (PA) and red blood cells (RBC) are visible on this photo (some are indicated with an arrow).
Results of coagulation activation experiment
beta-TG: At the 1-hour FU, less beta-TG was formed by patches of Dyneema Purity® fibers compared with the Bard®DeBakey® Knitted polyester fabric (P=0.004). More beta-TG was measured compared with the ePTFE Cardiovascular Patch (P=0.02). Compared with the other control materials, there were no significant differences at the 1- hour FU. At the 3-hour FU, patches of Dyneema Purity® fibers were comparable to four of the five control patches regarding beta-TG formation. Patches of Dyneema Purity® fibers showed less beta-TG formation compared with the Bard®DeBakey® Woven Polyester fabric (P=0.02). At the 6-hour FU, patches of Dyneema Purity® fibers showed no differences compared with 3 of the 5 patches. Less beta-TG for patches of Dyneema Purity® fibers was measured compared with the Bard®DeBakey® Woven Polyester Fabric (P=0.01) and the Bard®DeBakey® Knitted polyester fabric (P=0.03). At the 24-hour FU, patches of Dyneema Purity® fibers showed no differences compared with four of the five patches. Compared with the ePTFE Cardiovascular Patch, patches of Dyneema Purity® fibers showed more beta-TG (P=0.02). At 48 hours, patches of Dyneema Purity® fibers showed less beta-TG formation compared with Bard®DeBakey® Woven Polyester Fabric (P=0.02), Bard®DeBakey® Knitted polyester fabric (P=0.01) and Gore-Tex® Stretch vascular graft Thickwall (P=0.02). Compared with the other two control patches, there were no significant differences (Figure 4).
sP-selectin: At 1 hour, patches of Dyneema Purity® fibers showed less sP-selectin activation compared with the Bard®DeBakey® Woven Polyester Fabric (P=0.02). More sP-selectin activation was observed compared with the ePTFE Cardiovascular Patch (P=0.003). The ePTFE Cardiovascular patch showed less sP-selectin activation at 3 hours (P=0.05) and 6 hours (P=0.03). At this FU time, no differences were measured for patches of Dyneema Purity® fibers compared with the other four control materials. At 24 hours, less sP-selectin activation was observed for Bard®DeBakey® Knitted polyester fabric (P=0.02), the Gore-Tex® Stretch vascular graft Thinwall (P=0.05) and the ePTFE Cardiovascular Patch (P=0.004). At 48 hours, only the ePTFE cardiovascular patch showed less sP-selectin activation compared with patches of Dyneema Purity® fibers (P=0.007). Compared with the other four control materials, there was no significant difference measured (Figure 4).
Thrombo-1: At 1 hour, patches of Dyneema Purity® fibers showed more thrombo-1 activation than the Gore-Tex® Stretch vascular graft Thinwall (P=0.02) and the ePTFE Cardiovascular Patch (P=0.01). Compared with the other three control materials, no significant differences were measured. At 3 hours, the ePTFE cardiovascular patch showed still less thrombo-1 activation (P=0.001). There was no significant difference compared with the other 4 control materials. At 6, 24, and 48 hours, patches of Dyneema Purity® fibers were noninferior compared with the control patches.
TAT complex: At 1 hour, more TAT complex activation was seen in the Bard®DeBakey® Woven Polyester Fabric and the Bard®DeBakey® Knitted polyester fabric (P=0.002) than the patches of Dyneema Purity® fibers (P=0.05). Compared with the other three control materials, no significant differences were measured. At 3 and 6 hours, there was still more TAT activation for Bard®DeBakey® Woven Polyester Fabric (P=0.005) compared with patches of Dyneema Purity® fibers (P=0.001). At 6 hours, the Bard®DeBakey® Knitted Polyester Fabric also showed more TAT activation (P=0.02). At 24 hours, TAT activation was still more for Bard®DeBakey® Woven Polyester Fabric (P=0.04). At 48 hours, the Bard®DeBakey® Knitted polyester fabric showed more TAT activation (P=0.003). Compared with other control materials, there were no significant differences.
Discussion
In this study red blood cell and platelet adhesion on and platelet and coagulation activation of yarn-composed patches of Dyneema Purity® fibers were assessed and compared with five commonly used yarncomposed and membrane-based control materials in cardiovascular surgery. The main findings of our study were that patches of Dyneema Purity® fibers were noninferior regarding single platelet and red blood cell adherence compared with the five control materials in the acute situation (3 minutes) measured in a perfusion model. However, there was significantly more adherence of multiple-clustered platelets (i.e., two to five platelets per field of interest) on patches of Dyneema Purity® fibers compared with the membrane-based Bard® IMPRA® ePTFE cardiovascular patch (0.2%, P=0.011), and the Gore-Tex® Stretch vascular graft Thinwall (0%, P=0.005) and Thickwall (0%, P=0.007). Significantly less platelet aggregates (i.e., more than five platelets per field of interest), were measured on patches of Dyneema Purity® fibers (3.2%) compared with the yarn-composed Bard®DeBakey® Woven Polyester Fabric (15.0%, P=0.001). No differences were observed in the amount of platelet aggregates compared with the other four control materials.
The aim of our study was to test the acute and the short-term effects of patches of Dyneema Purity® fibers on platelet function and coagulation and to compare these effects with commonly used cardiovascular prostheses. The short term effects were assessed by SEM photographs, after three minutes of blood perfusion with physiologic arterial flow rate of 50 ml/min. Long-term recycling of the same blood with these perfusion conditions would induce apoptosis and would not be reliable. To study the short-term exposure effects of blood to the prostheses on platelet function and coagulation were studied in shaken blood instead of the perfusion model. Hereby we tested the six materials at different exposure times of 1, 3, 6, 24, and 48 hours. The platelet activation of patches of Dyneema Purity® fibers was assessed for 10 donors by measuring amount of P-selectin in time and was comparable and overall noninferior compared with the five clinically used cardiovascular materials. Also, coagulation activation of patches of Dyneema Purity® fibers, tested for nine donors, was noninferior for coagulation parameters beta-TG, sP-selectin, thrombo-1, and TAT complex at up to 48 hours of FU compared with the control materials.
Overall, we conclude that patches of Dyneema Purity® fibers were also noninferior regarding platelet and coagulation activation in shortterm- FU (up to 48 hours). Yarn-composed patches of Dyneema Purity® fibers showed noninferiority or even superiority regarding coagulation activation compared with yarn-composed control materials (Bard®DeBakey® Woven Polyester Fabric and Bard®DeBakey® Knitted Polyester Fabric). Compared with membrane-based patches, patches of Dyneema Purity® fibers showed more coagulation activation at some FU times.
Platelet aggregation may eventually result in thrombus formation and subsequent occlusion. We therefore consider the outcome platelet aggregates as the most relevant outcome. The platelets located in the aggregation differed also in morphology, presumably by activation of the platelets, compared with single platelets and multiple-clustered platelets. Platelets in aggregates were overlapping; thus the individual platelets were no longer clearly recognizable, which was in contrast to the single platelets and the multiple-clustered platelets in which the platelets were recognizable separately.
In the perfusion experiment, the yarn-composed patch of Dyneema Purity® fibers and control patches had relatively more adherent of multiple-clustered platelets compared with the membrane-based patches. This was probably caused by differences in surface roughness, in which platelets might stick between the wires of yarn-composed patches and accumulate. The platelets cannot easily stick to the smooth surface of membrane-based patches to accumulate.
Any type of foreign body material can be thrombogenic by promoting the formation of thrombin and platelet activation. This facilitates platelets to adhere and to express their surface receptors (glycoprotein IIb/IIIa) in an activated phase [9]. Platelet binding then becomes irreversible and can promote more thrombus formation. Therefore, proper testing of the characteristics of graft material is an important issue in modulating blood interaction. The combination of the double flat rectangular perfusion chamber and the roller pump used in this study allowed a standardized way to study the material and the structural properties of the different patches in relation to ex vivo blood [7,8] (Figure 1A and B in our study). Initial experimental blood circulation models with a roller pump appeared efficient, reliable, and cost-effective to assess the hemocompatibility of grafts before their clinical use [10-12]. The modified Chandler loop model is currently most frequently used for these purposes [13,14] but has 2 major disadvantages. First, the continuous blood-air contact induces leukocyte and platelet aggregation and protein denaturation [15] and shear forces on particulate material may result in blood cell detachment [16,17]. The second disadvantage of the Chandler loop is the limitation of blood circulation due to the requirement to keep the air on top of the circuit.
The difference between the roughness of the surfaces of yarncomposed patches (i.e., patches of Dyneema Purity® fibers, and Bard® DeBakey® Knitted Polyester Fabricand Woven Polyester Fabric) and the membrane-based patches (i.e., Bard® IMPRA® ePTFE, Gore-Tex® Stretch Vascular Grafts Thickwall and Thinwall) may affect the adherence of blood cells.
Previously, Van Oeveren et al. showed the woven tubes made of Dyneema Purity® had better properties than the commercially available ePTFE vascular graft, including a lower activation of the inflammatory response and lower hemolysis. In addition, the binding of fibrin and platelets were higher on PET (polyester) tubes compared with tubes of Dyneema Purity® fibers [18].
In our study, we noticed that in general, more adherence occurred on the yarn-composed materials than on membrane-based materials (Figure 3). Within the yarn-composed group, there were also differences in blood cell adhesion between the different patches. This was probably due to differences of the patch structure combined with the chemical properties of the different patch materials. Furthermore solitary red blood cells were often present on the patches, indicating that they were not trapped in a network of platelets but were most likely stuck between the threads of the yarns because of their relatively large size.
To carry out the perfusion, a physiologic arterial shear rate of 350 s-1 (flow rate of 50 mL/min) was chosen because we were interested in the applications of patches of Dyneema Purity® fibers in the blood circulation in areas of high shear rate (arteries and intracardiac). To ensure a leak-proof perfusion, each knob diameter was adapted to the thickness of the different patches to obtain a compound diameter of 30 mm. Because of the high pumping speed, some leakage still occurred during the perfusion, which was mainly after three minutes of perfusion. The leakage before this time was limited and thus did not affect the perfusion. The peristaltic roller pump simulated the peristaltic movements of the blood. The two chambers allowed us to test two different patches in one perfusion experiment, thus reducing the amount of blood needed, and to distribute the perfusion conditions more equally because two different patches were perfused each time. The combination of the two different materials in the same perfusion round was randomly chosen.
Bluestein and Slepian et al. designed a predictive method to test the thrombogenicity mediated by mechanical circulatory support devices. Therefore, the platelet trajectories were extracted and resolved from the numeric simulation of a ventricular assist device. Those were resolved to quantify the stress loading waveforms, which were programmed into a hemodynamic shearing device, where the stress loading conditions within the device were emulated. Also, the thrombin generation rates were assayed using the platelet activity state assay [19] after the samples were removed from the hemodynamic shearing device. This is a good method to test thrombogenicity of mechanical circulatory support devices but is less suitable to test patches because there is no space for patch to be placed. The combination of the perfusion chamber and SEM photographs used in our study made it possible to also test the structure effects of the surfaces of the patches by visualizing the location of platelets and red blood cells and their shape on the patches [20].
Conclusion
The favorable mechanical properties of Dyneema Purity® fibers combined with the noninferiority of these patches in platelet aggregation and platelet- and coagulation activation compared with the currently used cardiovascular prostheses makes patches of Dyneema Purity® fibers attractive for use in (endovascular) cardiovascular applications such as heart valves and vascular prosthesis. In our previous studies, Dyneema Purity® fibers showed to be noninferior compared to currently used cardiovascular prostheses in terms of bacterial- and platelet adhesion [21,22]. Also, vascular- and valvular prostheses made from Dyneema Purity® fibers showed good hemodynamics and intact materials in in vivo studies at six weeks and six months, respectively [23,24]. Further research of Dyneema Purity® fibers is necessary to test the long-term suitability of this material for use in cardiovascular devices and the suitability for endovascular use.
Limitations
In the perfusion experiment, the patch in the upstream perfusion channel might have slightly affected the blood in the downstream perfusion channel. Extensive testing in previous studies confirmed that there was no significant difference in platelet adhesion between the upstream and the downstream perfusion channel. Moreover, there is a possible lack of available information about the coating of the control materials, since the use of coating in patented materials are not always published. Therefore, in this study the effects of coating of the control materials might also have been investigated, aside from the structural and the material properties. This makes the comparison of adhesion to different materials more difficult. On the other hand, the goal of this study was to compare the different materials that are commercially available and already used in the blood circulation of patients.
References
- Yacoub MH, Takkenberg JJM (2005) Will heart valve tissue engineering change the world? Nat Clin Pract Cardiovasc Med 2: 60-1.
- Hopkins RA (2005) Tissue engineering of heart valves: decellularized valve scaffolds. Circulation 111: 2712-2714.
- Joseph R, Shelma R, Rajeev A, Muraleedharan CV (2009) Characterization of surface modified polyester fabric. J Mater Sci Mater Med 1: S153- S159.
- Peck M, Gebhart D, Dusserre N, McAllister TN, L’Heureux N (2012) The evolution of vascular tissue engineering and current state of the art. Cells Tissues Organs 195: 144-158.
- van Dingenen JL (2001) High-performance fibers. Hearle JWS Ed, CRC Press, New York.
- Sakariassen KS, Aarts PA, de Groot PG, Houdijk WP, Sixma JJ (1983) A perfusion chamber developed to investigate platelet interaction in flowing blood with human vessel wall cells, their extracellular matrix, and purified components. J Lab Clin Med 102: 522-535.
- Nievelstein PF, D’Alessio PA, Sixma JJ (1988) Fibronectin in platelet adhesion to human collagen types I and III. Use of nonfibrillar and fibrillar collagen in flowing blood studies. Arteriosclerosis. 8: 200-206.
- Conway K, Kiernan JA (1999) Chemical dehydration of specimens with 2, 2-dimethoxypropane (DMP) for paraffin processing of animal tissues: practical and economic advantages over dehydration in ethanol. Biotech Histochem 74: 20-26.
- Jeong MH, Owen WG, Staab ME, Srivatsa SS, Sangiorgi G, et al. (1996) Porcine model of stent thrombosis: platelets are the primary component of acute stent closure. Cathet Cardiovasc Diagn 38: 38-43.
- Monnink SH, van Boven AJ, Peels HO, Tigchelaar I, de Kam PJ, et al. (1999) Silicon-carbide coated coronary stents have low platelet and leukocyte adhesion during platelet activation. J Investig Med 47: 304-310.
- Amoroso G, van Boven AJ, Volkers C, Crijns HJ, van Oeveren W (2001) Multilink stent promotes less platelet and leukocyte adhesion than a traditional stainless steel stent: an in vitro experimental study. J Investig Med 49: 265-272.
- Mulvihill J, Crost T, Renaux JL, Cazenave JP (1997) Evaluation of haemodialysis membrane biocompatibility by parallel assessment in an ex vivo model in healthy volunteers. Nephrol Dial Transplant 12: 1968-1973.
- Christensen K, Larsson R, Emanuelsson H, Elgue G, Larsson A (2006) Effects on blood compatibility in vitro by combining a direct P2Y12 receptor inhibitor and heparin coating of stents. Platelets 17: 318-327.
- Sinn S, Scheuermann T, Deichelbohrer S, Ziemer G, Wendel HP (2011) A novel in vitro model for preclinical testing of the hemocompatibility of intravascular stents according to ISO 10993-4. J Mater Sci Mater Med. 22: 1521-1528.
- Thorsen T, Klausen H, Lie RT, Holmsen H (1993) Bubble-induced aggregation of platelets: effects of gas species, proteins, and decompression. Undersea Hyperb Med 20: 101-119.
- Culp WC, Porter TR, McCowan TC, Roberson PK, James CA, et al. (2003) Microbubble-augmented ultrasound declotting of thrombosed arteriovenous dialysis grafts in dogs. J Vasc Interv Radiol 14: 343-347.
- Gómez-Suárez C, Busscher HJ, van der Mei HC (2001) Analysis of bacterial detachment from substratum surfaces by the passage of air-liquid interfaces. Appl Environ Microbiol 67: 2531-2537.
- van Oeveren W, Tielliu IF, de Hart J (2012) Comparison of modified chandler, roller pump, and ball valve circulation models for in vitro testing in high blood flow conditions: application in thrombogenicity testing of different materials for vascular applications. Int J Biomater. 2012: 673163.
- Jesty J, Bluestein D (1999) Acetylated prothrombin as a substrate in the measurement of the procoagulant activity of platelets: elimination of the feedback activation of platelets by thrombin. Anal Biochem 272: 64-70.
- Bluestein D, Girdhar G, Einav S, Slepian MJ (2013) Device thrombogenicity emulation: a novel methodology for optimizing the thromboresistance of cardiovascular devices. J Biomech 46: 338-344.
- Basir A, Gründeman P, Moll F, Van Herwaarden J, Pasterkamp G, et al. (2016) Adherence of staphylococcus aureus to dyneema Purity® patches and to clinically used cardiovascular prostheses. PLoS One 11: e0162216.
- Basir A, de Groot P, Gründeman PF, Tersteeg C, Maas C, et al. (2015) In Vitro Hemocompatibility Testing of Dyneema Purity Fibers in Blood Contact. Innov Technol Tech Cardiothorac Vasc Surg 10: 195-201.
- Basir A, Loncq de Jong M, Vink A, Pasterkamp G, Gründeman P, et al. (2018) A Novel Cardiovascular Prosthesis Made from Woven Ultrahigh-Molecular-Weight Polyethylene Fibers, Proof of Concept in a Sheep Model. Ann Vasc Surg 52: 244–254.e1.
- Basir A, Grobben RB, Cramer MJ, van Herwaarden JA, Vink A, et al. (2017) Flexible mechanoprosthesis made from woven ultra-high-molecular-weight polyethylene fibres: proof of concept in a chronic sheep model. Interact Cardiovasc Thorac Surg 25: 942-949.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi