Research Article, J Plant Physiol Pathol Vol: 6 Issue: 1
Interaction Between Strigolactone and Cytokinin on Axillary and Adventitious Bud Development in Zantedeschia
Manandhar S1,2*, Funnell KA1,2, Woolley DJ2 and Cooney JM3
1The New Zealand Institute for Plant & Food Research Limited, Private Bag 11-600, Palmerston North, New Zealand
2Institute of Agriculture and Environment, Massey University, Private Bag 11- 222, Palmerston North, New Zealand
3The New Zealand Institute for Plant & Food Research Limited, Private Bag 3214, Hamilton, New Zealand
*Corresponding Author : Manandhar S
The New Zealand Institute for Plant & Food Research Limited, Private Bag 11-600, Palmerston North, New Zealand
Tel: +64 6953 7700
E-mail: sarina.manandhar@plantandfood.co.nz
Received: December 11, 2017 Accepted: January 05, 2018 Published: January 11, 2018
Citation: Manandhar S, Funnell KA, Woolley DJ, Cooney JM (2018) Interaction Between Strigolactone and Cytokinin on Axillary and Adventitious Bud Development in Zantedeschia. J Plant Physiol Pathol 6:1. doi: 10.4172/2329-955X.1000172
Abstract
Interaction Between Strigolactone and Cytokinin on Axillary and Adventitious Bud Development in Zantedeschia
Strigolactones are known to be involved in the control of branching, particularly axillary bud outgrowth. However, the effect of strigolactone on adventitious bud development is presently unknown, and the interaction of strigolactone with cytokinin on bud outgrowth has not been fully understood. Using a germination assay, high strigolactone levels were detected in a low-branched cultivar (Best Gold) but was less abundant in a high-branched cultivar (Goldilocks), particularly at an early stage of the growth cycle when branching was not visibly evident. In contrast, the concentration of cytokinins directly correlated with branching, suggesting an antagonistic interaction between strigolactones and cytokinins on branching. Supporting this hypothesis, strigolactone reduced the cytokinin stimulation of both axillary and adventitious shoot number in Zantedeschia grown in vitro. The reduced cytokinin concentration in a phenotypically low branched cultivar may have been associated with the inhibition effect of strigolactones. Hence, the fact that the alteration of branching in Zantedeschia or other horticultural species depends on the hormonal balance between these two hormones is quite likely. The idea may be useful for the generation of plants of desired branching at least within the in vitro system.
Keywords: Adventitious buds; Branching; in vitro; Hormones; Bud release
Introduction
Strigolactones (SLs) are a class of hormones; known to control shoot branching [1]. The branching of shoots involves a sequential process of initiation of axillary meristems, formation of axillary buds, bud release and subsequent growth of the axillary shoot [2]. While the interaction between different plant hormones such as auxin, cytokinins (CKs), and gibberellins on bud outgrowth (bud release and/or subsequent growth) have been widely studied, recent work has suggested that interactions between SLs and CKs may also be very important [3,4]. In Pisum sativum (pea), external application of SL inhibited both decapitation-induced [5] or CKinduced [6] axillary shoot length, supporting the hypothesis of the likely interaction between CK and SL on subsequent growth of the released buds. However, the interaction between these two hormones on bud release per se is still unknown. It has been well established that CK is known to stimulate bud release in a range of different plant species. In Zantedeschia spp. K. Spreng, an ornamental plant [7], the CK, 6-benzylaminopurine (BAP), has been reported to stimulate branching in plants both in vitro [8,9] and in vivo [10]. As reported by Subbaraj et al. [10], the effect of CK in stimulating branching was more prominent early in the annual growth cycle of a low branched cultivar, ‘Best Gold’, of Zantedeschia. Hence, it was considered possible that in Zantedeschia, there is an interaction between SL and CK on bud release, at least at an early stage in the annual growth cycle. We therefore hypothesized that SL might act antagonistically with CK on bud release, subsequently affecting branching in Zantedeschia.
As with axillary shoot development, the role of plant hormones such as CKs, gibberellins, auxin and the balance between these hormones in the formation and development of adventitious buds is well known [11]. Hence, the role of SL and its interaction with CK on the formation of adventitious shoots is also reported in this paper. In fact, prior to this study a role for SL in adventitious bud/ shoot formation had not been reported. Since CKs are well known for stimulating adventitious bud/shoot formation; as explored within this paper, it was of interest whether SL influences adventitious shoots as well as axillary shoots and, if so, how SL interacts with CK. In the research reported here, an in vitro technique was applied to study the interaction between SL and CK on both sources of shoot branching (i.e., axillary and adventitious) in Zantedeschia.
In our previous work, within Zantedeschia and some other horticultural species, we found less concentration of SLs in high branched cultivars compared to the low branched cultivars, at the growth stage before branching was visibly evident [12]. In this study, we have investigated the endogenous SLs and CKs within the high and low branched cultivars of Zantedeschia at different stages of annual growth cycle, thus to explore any relationship between the endogenous SLs and CKs on branching of Zantedeschia.
Materials and Methods
Experiment one: Analysis of endogenous strigolactones and cytokinins
Plant growing conditions: The environmental and cultural conditions under which plants were grown were as described by Subbaraj et al. [10]. The flowering sized tubers of two cultivars – ‘Goldilocks’ (phenotypically high branch frequency) and ‘Best Gold’ (low branch frequency) [13] were planted in a heated glasshouse at the Plant Growth Unit, Massey University, Palmerston North (40°22’S 175°37’E) during spring (September). Guttation fluid, a naturally occurring sap flow in a plant due to positive hydrostatic pressure [14] was analysed for endogenous hormonal concentration. As described in Manandhar et al. [12], the fluid was collected from the fully expanded leaves of both cultivars at four different stages of the annual growth cycle i.e., leaf emergence, branching, flowering and foliage senescence, and stored at –20°C until hormonal analysis.
Germination assays for strigolactones analysis: Given the extremely minute quantities of SLs within plant systems [15,16], and the difficulties associated with using LC/MS-based systems to detect these compounds, a germination assay utilising seeds of Orobanche minor was used [12]. While such assays lack chemical identification, they are a useful tool to detect and quantify endogenous SLs. In conducting the assay approximately 30 preconditioned seeds were spread inside a glass Petri dish (5 cm Ø) lined with a double layer of Whatman No.1 filter paper, wetted with 800 μl of guttation fluid. Seeds were then allowed to germinate at 25°C for 8 days in the dark. Microscopic observations of seeds were conducted after 8 days of exposure to treatment solutions.
LC/MS-MS for cytokinin analysis: Cytokinin analysis was carried out using LC/MS-MS following the procedure described in Pilkington et al. [17], with some modifications. Zantedeschia guttation fluid samples, ‘Goldilocks’ and ‘Best Gold’ (stored at –80°C) were thawed and then spiked with 10 ng of a labelled internal standard mix ([2H5] t-Z, [2H5] t-Z9G, [2H6] 2iP, [2H6] iPR, [2H5] t-ZR; OlchemIm Ltd, Olomouc, Czech Republic). Samples were acidified with formic acid to be equivalent to 1 M formic acid and vortexed for 1 min prior to column clean-up on a mixed mode, reverse-phase, cation-exchange cartridge (Oasis MCX 60 mg/3 mL; Waters, ON, Canada). Cartridges were activated using 3 mL acetonitrile and equilibrated using 3 mL 1 M formic acid. After equilibration, the sample was loaded and washed with 3 mL of 1 M formic acid followed by 3 mL of water. Acidic plant hormones were eluted with 4 mL acetonitrile, and the cartridge was then washed with 2 mL of water followed by 4 mL of 0.35 M ammonium hydroxide to elute nucleotide forms of cytokinins. Cytokinin-free base, ribosides and glucosides were then eluted with 3 mL of 0.35 M ammonium hydroxide in 60% acetonitrile (analysis fraction). The analysis fraction eluate was evaporated to dryness using a CentriVap concentrator (Labcon) and stored at –20°C until analysis. Prior to mass spectrometric analysis, the eluted CKs were reconstituted with 200 μL of 10% methanol/water plus 1% acetic acid. LC-MS/MS experiments were performed on a 5500 QTrap triple quadrupole/linear ion trap (QqLIT) mass spectrometer equipped with a TurboIon-SprayTM interface (AB Sciex, ON, Canada) coupled to an Ultimate 3000 UHPLC (Dionex, CA, USA). CKs were separated on a Poroshell 120 SB-C18 2.7 μm 2.1 × 150 mm ID column (Agilent Technologies, CA, USA) maintained at 80°C. Solvents were (A) water + 0.1% formic acid and (B) acetonitrile + 0.1% formic acid and the flow rate was 600 μL-1. The initial mobile phase, 0% B was ramped linearly to 3% B at 9 min, then to 10% B at 15 min and 100% B at 22 min. The column was flushed at 100% B for 1 min before resetting to the original conditions. Injection size was 10 μL. MS data was acquired in the positive mode using a scheduled multiple reaction monitoring (MRM) method (Supplementary Table 1). The operating parameters were as follows: ion spray voltage, 4500 V; temperature, 600°C; curtain gas, 45 psi; ion source gas 1, 60 psi; ion source gas 2, 60 psi.
Experimental design and data analysis: At each of the four stages of the annual growth cycle, guttation fluid was collected from twenty individual plants as subsamples of each cultivar. For hormone analysis, the fluid for each cultivar at each stage of growth was pooled. The percentage germination of O. minor seeds was interpreted as indicating the relative concentration of SLs as being either high or low [12]. Data were analyzed using the GLM procedure of Minitab 16 (Minitab Inc., State College Pennsylvania, USA). Comparison between the means was made at P ≤ 0.05 using Tukey’s method.
Experiment Two: Application of strigolactone and cytokinin
Plant growing conditions and hormone application: Plant material of the Zantedeschia cultivars ‘Best Gold, and, Goldilocks, were propagated in vitro via stem cuttings, which included the stem apex and swollen stem base, but with no roots. Based on preliminary trials to optimize concentration, the hormone treatments were applied to the basal agar medium as described in Manandhar et al. [18].
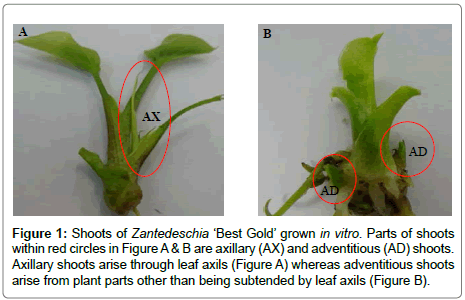
Experimental design and data analysis: The treatments comprised a (2 × 3 × 2) factorial arrangement of each of the two cultivars, three concentrations (0, 0.3 and 0.9 mg L-1) of BAP and two concentrations of (0 and 0.1 mg L-1) of GR24 (a synthetic strigolactone). Each treatment had four replications, represented by four culture vessels with eight stem cuttings per vessel. The number of axillary and adventitious shoots was counted at both four and eight weeks after the treatments were applied. In Zantedeschia, unlike axillary shoots which develop within leaf axils (Figure 1A) adventitious shoots develop from the stem/tuber tissue (Figure 1B). Data were analysed by a three-way ANOVA, using the GLM procedure of Minitab 16 (Minitab Inc., State College PA, USA). Mean separation were conducted at P ≤ 0.05 using the Duncan’s multiple range test method (DMRT).
Figure 1: Shoots of Zantedeschia ‘Best Gold’ grown in vitro. Parts of shoots within red circles in Figure A & B are axillary (AX) and adventitious (AD) shoots. Axillary shoots arise through leaf axils (Figure A) whereas adventitious shoots arise from plant parts other than being subtended by leaf axils (Figure B).
Results
Strigolactones content in Zantedeschia
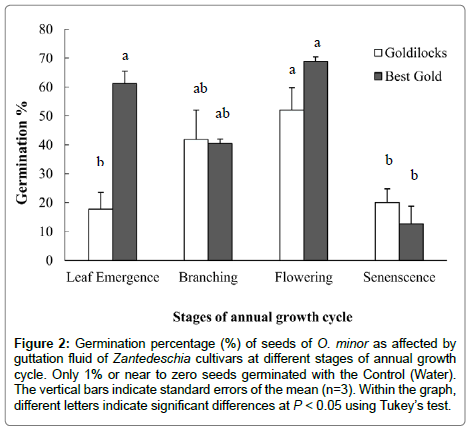
The low-branched cultivar (Best Gold) produced 40% more (P < 0.01) germinated seeds of O. minor than for the high branched cultivar (Goldilocks), during the early stage of growth when leaves on the primary shoot had just started to emerge (Figure 2). However, after branches were fully developed through until the stage of natural senescence, there was no difference between the germination percentages of the seeds within the guttation fluid of high and lowbranched cultivars.
Figure 2: Germination percentage (%) of seeds of O. minor as affected by guttation fluid of Zantedeschia cultivars at different stages of annual growth cycle. Only 1% or near to zero seeds germinated with the Control (Water). The vertical bars indicate standard errors of the mean (n=3). Within the graph, different letters indicate significant differences at P < 0.05 using Tukey’s test.
Cytokinins
Compared with the low branched ‘Best Gold’, ‘Goldilocks’ contained a considerably higher concentration of CKs, particularly trans zeatin riboside (t-ZR) by five hundreds folds and trans zeatin (t-Z) by more than a hundred fold (Table 1). Other forms of CKs such as 2iP, c-Z and c-ZR were detected to some level (1-7 ng mL-1) in ‘Goldilocks’ but not in ‘Best Gold’.
| Cultivars | Cytokinin forms* (ng mL-1) | |||||||
|---|---|---|---|---|---|---|---|---|
| 2iP | t-Z | c-Z | iPR | t-ZR | DHZR | iP9G | c-ZR | |
| ‘Goldilocks’ (high branched) | 1 | 164 | 3 | 6 | 568 | nd | nd | 7 |
| ‘Best Gold’ (low branched) | nd** | 2 | nd | 0.1 | 9 | nd | nd | nd |
**nd means not detected
Table 1: Endogenous cytokinins (ng mL-1) within the guttation fluid of high and low branched cultivars of Zantedeschia during leaf emergence.
Axillary shoots
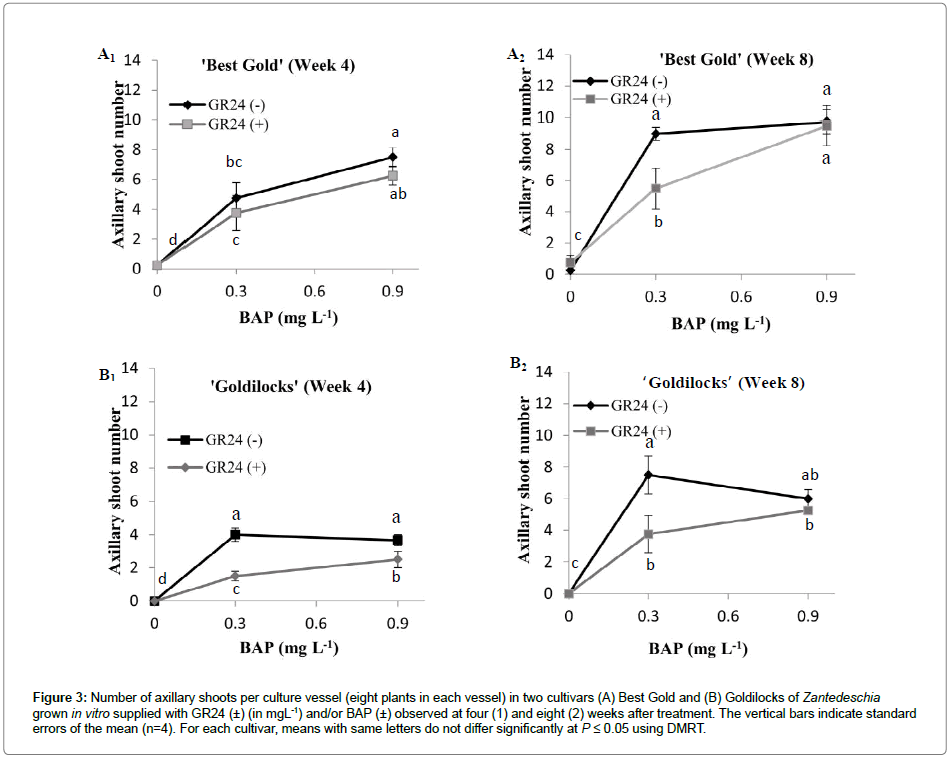
The presence of GR24 greatly reduced (by about half) the number of axillary shoots stimulated by the lower concentration of BAP in the high branched cultivar, but not in the low branched cultivar (Figure 3A) in week 4. However, by week 8, the reduction in the number of axillary shoots produced was only significant at 0.3 mg L-1 of BAP in both cultivars (Figure 3). In terms of changes in the number of axillary shoots, in the absence of GR24, the high branched cultivar (‘Goldilocks’) typically responded positively to the presence of BAP at the low concentration (0.3 mg L-1), and did not produce any additional axillary shoots at the higher concentration (0.9 mg L-1) beyond that produced at 0.3 mg L-1 (Figure 3B). In contrast, shoot numbers in the low branched cultivar (‘Best Gold’) nearly doubled with the increased concentration of BAP at 4 weeks, but the increase was not statistically significant at 8 weeks (Figure 3A). In both cultivars, few if any axillary shoots were produced in the absence of BAP, consequently the effect of GR24 in reducing axillary shoot numbers was only visible when BAP was present.
Figure 3: Number of axillary shoots per culture vessel (eight plants in each vessel) in two cultivars (A) Best Gold and (B) Goldilocks of Zantedeschia grown in vitro supplied with GR24 (±) (in mgL-1) and/or BAP (±) observed at four (1) and eight (2) weeks after treatment. The vertical bars indicate standard errors of the mean (n=4). For each cultivar, means with same letters do not differ significantly at P ≤ 0.05 using DMRT.
Adventitious shoots
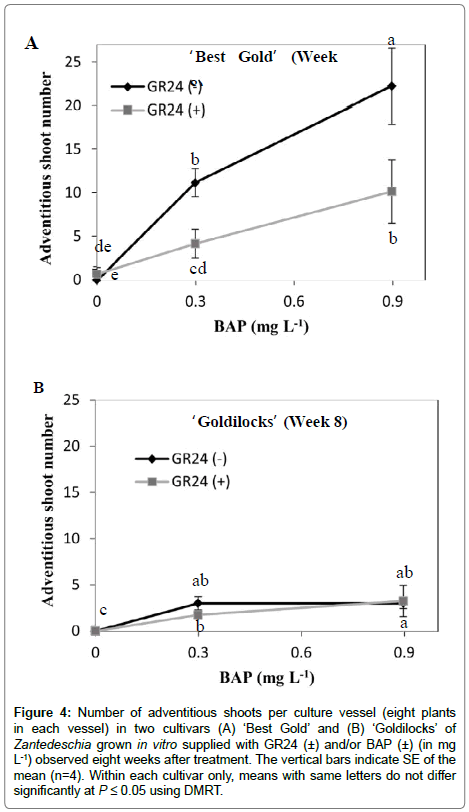
In the presence of BAP, in vitro adventitious shoots arose from the swollen stem base in both cultivars. In the low branched cultivar (‘Best Gold’) after 8 weeks in the absence of GR24, the number of adventitious shoots were nearly double at 0.9 mg L-1 of BAP compared with 0.3 mg L-1 (Figure 4A). However, this effect was not evident with the highly branched ‘Goldilocks’ (Figure 4B), where approximately 80% more adventitious shoots formed in ‘Best Gold’ than in ‘Goldilocks’ (Figure 4). Importantly, GR24 almost halved the formation of BAP-induced adventitious shoots in ‘Best Gold’ (Figure 4A).
Figure 4: Number of adventitious shoots per culture vessel (eight plants in each vessel) in two cultivars (A) ‘Best Gold’ and (B) ‘Goldilocks’ of Zantedeschia grown in vitro supplied with GR24 (±) and/or BAP (±) (in mg L-1) observed eight weeks after treatment. The vertical bars indicate SE of the mean (n=4). Within each cultivar only, means with same letters do not differ significantly at P ≤ 0.05 using DMRT.
Discussion
At the leaf emergence stage, ‘Best Gold’ contained a higher concentration of SLs compared to ‘Goldilocks’ (Figure 2). This difference in concentration of SLs between the high and low-branched cultivars, before the branches had fully developed [19], supports the hypothesis that SLs are involved in inhibiting branching in this genus. At all subsequent stages of the annual growth cycle however, the concentration of SLs was not significantly different between the two cultivars (Figure 2). The presence of SLs in later stages may be due to that there are different forms of SLs [20] preferentially detected by germination assays, any particular form which influences shoot branching might not be present after the shoot branches have been fully developed. Supporting the existence of different forms of SLs at different stages of a growth cycle, Xie et al. [21] reported that the SL, ‘7-oxoorobanchyl acetate’, was found in root exudates of rice during early growth, but not in later stages. It is possible that this form of SL may have a role in controlling the release of axillary buds.
Since CK stimulated more axillary branches in ‘Best Gold’ when applied at the leaf emergence stage [10], it was suggested that reduced shoot branching in Zantedeschia was due to reduced CK, and vice versa. Supporting this hypothesis, guttation fluid of the highly branched ‘Goldilocks’ contained higher concentration of CKs than ‘Best Gold’ (Table 1). It is quite likely that the higher concentration of endogenous SLs of low branched cultivar (Figure 2) may have suppressed the endogenous CKs concentration, resulting in inhibition of release of axillary buds. Since, SL may not inhibit the biosynthesis of CKs [6] it is possible that SL may reduce CK via increasing CK metabolism. To test this hypothesis, studies on the effect of SLs on the degradation of CKs would be useful. Overall, the differences in level of these two hormones might alter branching of Zantedeschia plants grown both in vivo and in vitro.
The reduction in axillary shoot number in response to externally applied SL in Zantedeschia cultivars supports previous reports on the inhibitory influence of GR24 on axillary shoot number in high branched SL-deficient mutants of Arabidopsis and rice [1,4]. Likewise, Gomez-Roldan et al. [1] and Dun et al. [6] also observed inhibition of axillary bud growth by GR24 in pea plants, but only in terms of shoot length rather than bud release. However, in the experiment reported within this paper, in which the number of axillary shoots was considered, the effect of GR24 would be on bud release per se, but not on the further development of the released bud, i.e. the subsequent growth, hence demonstrating the specific important effect GR24 has on shoot branching. Any subsequent effect on length of the shoot is secondary, and probably via a different mechanism.
Interestingly, in ‘Best Gold’, the effect of GR24 on the number of axillary shoots was not evident in the first four weeks (Figure 3A1) though GR24 significantly reduced shoot number after eight weeks (Figure 3A2). Hence, it appears that the response to GR24 by axillary buds of ‘Best Gold’ was slow compared with that evident in the highly branched ‘Goldilocks’, and that the rate of degradation of GR24 may differ between cultivars.
SL reduced CK-induced axillary shoot number (Figure 3), supporting the model of an interaction between SL and CK at the early stage of shoot branching, i.e. during initiation of bud release. Dun et al. [6] also found an interaction in pea seedlings but, in their study, the parameter measured was shoot length rather than bud release, as SL reduced CK-induced shoot length suggesting that the interaction between these two hormones was on the subsequent growth. Hence, it is possible that SL interacts with CK at a different stage of shoot branching in Zantedeschia but, due to the lack of data on SL and/or CK on axillary shoot length of Zantedeschia when grown in vitro, the interaction between these two hormones on axillary shoot length of Zantedeschia remains to be investigated.
In contrast to the inhibition effect of GR24, Crawford et al. [22], Ward et al. [23] and Liang et al. [24] observed no inhibition of axillary shoot length in node/s of stem segments with basally-supplied GR24. In those studies, adding auxin to the cut upper internode, in the presence and/or absence of GR24, inhibited axillary shoot length. Hence, polar auxin transport (PAT) appears as the main cause of axillary bud inhibition via preventing auxin transport out of the buds to the main stem, which is crucial for bud release [25,26]. Contrary to this hypothesis, in the current experiment, despite the presence of auxin from the apical meristem, axillary buds (stimulated by BAP) were not inhibited. Wickson and Thimann [27] and Turnbull et al. [28] showed that CK could release the axillary bud, even in the presence of the apex. Thus, the lack of release was probably due to deficiency of CK rather than lack of auxin transport out of the axillary buds. Since GR24 inhibited the BAP stimulated axillary bud release, it is concluded that an antagonist interaction between CK and SL has occurred on release of buds.
Efforts by Liang et al. [24] and Ward et al. [23] to inhibit axillary shoot length with GR24 failed, possibly because application of GR24 was insufficient to antagonize the effect of a higher level of CKs since effectiveness of GR24 might depend on relative amounts of CK available in axillary buds [6]. Such CKs, after removal of the apex, were contributed by de novo synthesized CK [3] and/or stored CK [29] near buds. On applying auxin to the decapitated stem, CK might be reduced due to the inhibitory effect of auxin on CK biosynthesis [30], thus allowing GR24 to begin to have an effect due to an increased SL/CK ratio [6]. Gomez-Roldan et al. [1] noted that applying GR24 directly to the buds, as compared with via the vascular stream, might differ in their response to bud inhibition, with more effect via direct bud supply. In the current experiment, although GR24 was applied only in the basal medium, it is possible that GR24 was received directly by the axillary buds, as nodes present at the base of the stem cutting were completely immersed in the media. In addition, possibly the level of SL was higher due to the presence of apicallyderived auxin, and less availability of CKs. Supporting this hypothesis, at higher concentrations (0.9 mg L-1) of BAP, GR24 failed to inhibit axillary shoot number in both cultivars (Figure 3).
BAP increased adventitious shoot number, but only in the ‘Best Gold’ (Figure 4). Interestingly, GR24 significantly reduced the number of adventitious shoots induced by BAP. This is the first time the inhibitory effect of SL on adventitious shoot number has been reported, hence adding a new role for SL in plant canopy architecture. Reduction of CK-stimulated adventitious shoot number by externally applied SL in Zantedeschia grown in vitro, suggests these two hormones interact antagonistically with each other in the formation of adventitious buds.
Conclusion
Endogenous SL analysis based on a germination assay of O. minor seeds, supports the hypothesis that in Zantedeschia the presence of SLs reduces shoot branching, at least during the early stage of the annual growth cycle before the visible evidence of branches. Additionally, it is likely that SL may inhibit the branching by reducing the level of cytokinin, suggesting an antagonistic interaction between these two hormones. Supporting this hypothesis, supply of SL in the media of Zantedeschia cuttings grown in vitro can inhibit both CK-stimulated axillary buds and the formation of CK-stimulated adventitious buds. Thus, the antagonistic relationship between CK and SL may explain the phenotypic differences between high branched and low branched cultivars. More importantly, the balance between these two hormones may have horticultural implication in generating plants of desired branching in Zantedeschia or other horticultural species at least within the in vitro system.
Acknowledgements
We acknowledge Massey University Doctoral Scholarship, Plant & Food Research Core funding: 1198 – “Fashionable Plants for the Ornamentals Industry” and 1972 – “Breeding Technology Development” for financial assistance to conduct this research. We also thank Green Harvest Pacific Ltd. and Jim Wilson for providing plant material and manuscript review comments from John Seelye and Ben van Hooijdonk.
References
- Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pages V, Dun EA, et al. (2008) Strigolactone inhibition of shoot branching. Nature 455: 189-194.
- Rameau C, Bertheloot J, Leduc N, Andrieu B, Foucher F, et al. (2015) Multiple pathways regulate shoot branching. Front Plant Sci 5:1-15.
- Shimizu-Sato S, Tanaka M, Mori H (2009) Auxin-cytokinin interactions in the control of shoot branching. Plant Mol Biol 69: 429-435.
- Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, et al. (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455: 195-200.
- Brewer PB, Dun EA, Ferguson BJ, Rameau C, Beveridge CA (2009) Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol 150: 482-493.
- Dun EA, de Saint Germain A, Rameau C, Beveridge CA (2012) Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiol 158: 487-498.
- Funnell KA (1993) Zantedeschia: The physiology of flower bulbs. Elsevier, Amsterdam, Netherland.
- Naor V, Kigel J, Ziv M (2005) The effect of gibberellin and cytokinin on floral development in Zantedeschia spp. in vivo and in vitro. Acta Hort 673: 255-263.
- Ngamau K (2001) Promoting side shoot development in Zantedeschia aethiopica ‘Green Goddess’. Eur J Hortic Sci 66: 85-92.
- Subbaraj AK, Funnell KA, Woolley DJ (2010) Dormancy and flowering are regulated by the reciprocal interaction between cytokinin and gibberellin in Zantedeschia. J Plant Growth Regul 29: 487-499.
- Charrière F, Sotta B, Miginiac É, Hahne G (1999) Induction of adventitious shoots or somatic embryos on in vitro cultured zygotic embryos of Helianthus annuus: variation of endogenous hormone levels. Plant Physiol Biochem 37: 751-757.
- Manandhar S, Woolley DJ, Funnell KA (2014) Crop architecture: Investigating ‘strigolactones’ in different horticultural species having different branching phenotypes. Technological advancement for Vibrant agriculture, Atiner, Athens, Greece.
- D’Arth SM, Simpson SI, Seelye JF, Jameson PE (2007) Bushiness and cytokinin profile in dormant and sprouting tubers of Zantedeschia. Plant Cell Tissue Organ Cult 89: 185-191.
- Singh S, Singh TN (2013) Guttation 1: chemistry, crop husbandry and molecular farming. Phytochem Rev 12: 147-172.
- Boyer FD, Germain AS, Pillot JP, Pouvreau JB, Chen VX, et al. (2012) Structure-activity relationship studies of strigoalctone-related molecules for branching inhibition in garden pea: molecule design for shoot branching. Plant Physiol 159: 1524-1544.
- Matusova R, Rani K, Verstappen FWA, Franssen MCR, Beale MH, et al. (2005) The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiol. 139: 920-934.
- Pilkington SM, Montefiori M, Galer AL, Emery RJN, Allan AC, et al. (2013) Endogenous cytokinin in developing kiwifruit is implicated in maintaining fruit flesh chlorophyll levels. Annals of Botany 112: 57-68.
- Manandhar S, Woolley DJ, Funnell KA (2015) Investing the role of strigolactones in control of shoot branching in cultivars of Zantedschia. Acta Hort 1104: 165-170.
- Halligan EA, Brooking IR, Funnell KA, Catley JL (2004) Vegetative and floral shoot development of Zantedeschia ‘Black Magic’. Sci Hortic 99: 55-65.
- Yoneyama K, Awad AA, Xie X, Yoneyama K, Takeuchi Y (2010) Strigolactones as germination stimulants for root parasitic plants. Plant Cell Physiol 51: 1095-1103.
- Xie X, Yoneyama K, Kisugi T, Uchida K, Ito S, et al. (2013) Confirming stereochemical structures of strigolactones produced by rice and tobacco. Mol Plant 6: 153-163.
- Crawford S, Shinohara N, Sieberer T, Williamson L, George G, et al. (2010) Strigolactones enhance competition between shoot branches by dampening auxin transport. Development 137: 2905-2913.
- Ward SP, Salmon J, Hanley SJ, Karp A, Leyser O (2013) Using Arabidopsis to study shoot branching in biomass willow. Plant Physiol 162: 800-811.
- Liang J, Zhao L, Challis R, Leyser O (2010) Strigolactone regulation of shoot branching in chrysanthemum (Dendranthema grandiflorum). J Exp Bot 61: 3069-3078.
- Domagalska MA, Leyser O (2011) Signal integration in the control of shoot branching. Nat Rev Mol Cell Biol 12: 211-221.
- Prusinkiewicz P, Crawford S, Smith RS, Ljung K, Bennett T, et al. (2009) Control of bud activation by an auxin transport switch. Proc Natl Acad Sci USA106: 17431-17436.
- Wickson M, Thimann KV (1958) The Antagonism of Auxin and Kinetin in Apical Dominance. Physiol Plant 11: 62-74.
- Turnbull MH, Murthy R, Griffin KL (2002) The relative impacts of daytime and night-time warming on photosynthetic capacity in Populus deltoides. Plant Cell Environ 25: 1729-1737.
- Woolley DJ, Wareing PF (1972) The role of roots, cytokinins and apical dominance in the control of lateral shoot form in Solanum andigena. Planta 105: 33-42.
- Nordström A, Tarkowski P, Tarkowska D, Norbaek R, Åstot C, et al. (2004) Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: A factor of potential importance for auxin-cytokinin-regulated development. Proc Natl Acad Sci USA 101: 8039-8044.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi