Research Article, Int J Cardiovasc Res Vol: 6 Issue: 5
Investigation of Serum oxLDL, anti-oxLDL Antibody, MMP-9 and hsCRP Levels in Patients with Angiographically Defined Ruptured of Coronary Plaques
Sumiya Tserendavaa1*, Odkhuu Enkhtaivan1, Tsogtsaikhan Sandag1, Zorigoo Shagdar2 and Munkhzol Malchinkhuu1
1Mongolian National University of Medical Sciences, Mongolia
2Ulaanbaatar Songdo Hospital, Mongolia
*Corresponding Author : Tserendavaa Sumiya
Mongolian National University of Medical Sciences, Zorig Street, Sukhbaatar district, Ulaanbaatar, Mongolia
Tel: +976-99053929, +976-11-321249
E-mail: sumiyamgl@gmail.com
Received: August 18, 2017 Accepted: September 05, 2017 Published: September 11, 2017
Citation: Tserendavaa S, Enkhtaivan O, Sandag T, Shagdar Z, Malchinkhuu M (2017) Investigation of Serum oxLDL, anti-oxLDL Antibody, MMP-9 and hsCRP Levels in Patients with Angiographically Defined Ruptured of Coronary Plaques. Int J Cardiovasc Res 6:5. doi: 10.4172/2324-8602.1000324
Abstract
Background
When the coronary atherosclerotic plaque becomes vulnerable, it easily ruptures with subsequent thrombus formation, leading to acute myocardial infarction. Prior studies indicate that oxLDL, antioxLDL antibody, MMP-9, hsCRP play a key role in pathogenesis of plaque rupture. To study the involvement of oxLDL, anti-oxLDL antibody, MMP-9 and hsCRP in the pathogenesis of unstable coronary plaque
Methods: The study enrolled 80 consecutive patients with coronary artery disease who underwent PCI. The case group (n=40) should have a unstable coronary plaque, confirmed by conventional
angiography, whereas control group (n=40) should have stable coronary atherosclerosis. Serum oxLDL, anti-oxLDL antibody, MMP-9 levels was determined by ELISA. The hsCRP detected method on the automated analyzer. Gensini and SYNTAX score were also utilized for assessing the severity of coronary artery disease.
Results: Serum oxLDL (p=0.01), anti-oxLDL antibody (p<0.001), MMP-9 (p<0.001), hsCRP (p=0.009) in the case group were more than in the control group. The binary logistic regression analysis shows that MMP-9 (β=0.985, p<0.001), anti-oxLDL antibody (β=0.892, p<0.001), hsCRP (β=0.041, p=0.005), oxLDL (β=0.011, p=0.016) may play a role in the unstable coronary plaque. ROC Curve analysis shows that MMP-9 (area=0.87, p<0.001) variance is more than anti-oxLDL antibody (area=0.78, p<0.001), hsCRP (area=0.73, p<0.001), oxLDL (area=0.63, p=0.038) making it a diagnostically beneficial for the vulnerable plaque. Gensini score correlated with anti-oxLDL antibody (r=0.25, p=0.026), MMP-9
(r=0.42, p<0.001). But SYNTAX score correlated with anti-oxLDL antibody (r=0.41, p<0.001), MMP-9 (r=0.20, p<0.001)
Conclusion: The serum oxLDL, anti-oxLDL antibody, MMP-9 and hsCRP are significantly involved in the unstable coronary plaque.
Keywords: Oxidized LDL; Anti-oxLDL antibody; Matrix Metalloproteinase 9; CRP; Coronary atherosclerosis; Unstable coronary plaque
Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide [1]. In the last 20 years, CVD has also been the leading cause of all death in the Mongolian population that occupies one of three cases. Mortality from acute myocardial infarction (AMI) occupies 69.6% of CVD [2].
Thrombosis and vulnerable plaque of coronary atherosclerosis is important pathogenesis mechanism of AMI [3]. Myocardial perfusion suddenly deficit from atherothrombosis in the unstable coronary plaques, leading to unstable angina, AMI and sudden cardiac death [4].
Over the last years the researchers try to explain the vulnerable plaque regarding to the matrix metalloproteinase 9 (MMP-9). Macrophages are a potent source of MMP-9 [5]. Fang and colleagues showed that differentiated macrophages from circulating monocytes isolated from patients with AMI or stable angina had a twofold increase in mRNA and protein levels of MMP-9 compared with the control groups [6]. When the total antioxidant capacity decreases the oxidation is intensified, and that oxidative stress develops the oxidized LDL (oxLDL). Later it forms the oxLDL and anti-oxLDL complex [7].
The serum MMP-9 levels were highly correlated with incidence of vulnerable plaque. An increase in systemic MMP-9 levels is highly correlated with cardiovascular mortality in patients with atherosclerosis [8]. In the process of immune and inflammatory responses the MMP-9 that involved in the breakdown of extracellular matrix collapses the elastin, gelatin and collagen-IV [6]. It also helps to form an immune complex binding the anti-oxLDL with oxLDL. Some researchers consider that parallel reactions of antibodydependent immune, complement and hypersensitivity III leads to the vulnerable plaque and as a result cause the unstable coronary plaque [9]. Also there were determined that the serum oxLDL, antioxLDL antibody and MMP-9 levels are directly correlated with the coronary atherosclerotic plaque complications [10]. Moreover, the oxLDL, anti-oxLDL antibody, MMP-9 and high sensitivity C-reactive protein (hsCRP) levels can be the evaluation marks for in the unstable coronary plaque, especially AMI, even the death diagnosing indicators for them [11,12].
To understand the pathogenesis of unstable coronary plaque is a need to study the thrombus formation regarding to the serum oxLDL, anti-oxLDL antibody, MMP-9, and hsCRP levels. Therefore, we are eager to study the serum oxLDL, anti-oxLDL antibody, MMP-9 and hsCRP levels regarding the pathogenesis of unstable coronary plaque. Consequently the study can be helpful for the diagnosis, treatment and complication assessment of the AMI.
Materials and Methods
The study was conducted using the case-control design. Initially, we have chosen the 80 patients who had coronary angiography in the departments of cardiovascular medicine of Ulaanbaatar Songdo Hospital and Shastin’s National Hospital, respectively. The main inclusion criterion for the case group are that the patients (n=40) should have a unstable coronary plaque, whereas control group (n=40) should have stable coronary atherosclerosis, confirmed by a conventional coronary angiography. The level of serum oxLDL, anti oxLDL antibody, MMP-9 was the enzyme-linked immunosorbent assay (ELISA). We used SYNTAX and Gensini score by quantitative coronary angiography for assessing the severity of coronary heart disease. Each group was selected according to the following criteria.
Case group
Case group involved participants who had type 1 of AMI according by ESC Guidelines for the management of AMI in patients presenting with ST-segment elevation [13]. Type 1 of AMI is characterized by atherosclerotic plaque rupture, ulceration, fissure, erosion or dissection with resulting intraluminal thrombus in one or more coronary arteries leading to decreased myocardial blood flow and/or distal embolization and subsequent myocardial necrosis. Type 1 of AMI have met the following criteria: 1) Symptoms of acute coronary syndromes occurred suddenly and lasted for more than 2 hours, 2) Acute myocardial ischemic syndrome was detected on new or presumed new significant ST-T wave changes on 12 leads ECG, 3) The serum troponin I is increased (>0.01 ng/ml) and 4) Intracoronary thrombus that related unstable coronary plaque detected by conventional coronary angiography in epicardial arteries.
Control group
This group includes the patients that had the stable coronary atherosclerosis according by 2013 ESC guidelines on the management of stable coronary artery disease [14]. The patients were collected by the following criteria: 1) The patient has symptoms of stable angina, 2) ECG reading is no signs of acute coronary syndrome, 3) The serum troponin I is normal (<0.01 ng/ml) and 4) Stable atherosclerotic stenosis (no plaque rupture and new thrombus formation) are detected at conventional coronary angiography [15].
Coronary angiographic findings
Multiple views were obtained in all patients, with visualization of the left anterior descending (LAD) and left circumflex coronary (LCx) arteries in at least four views and the right coronary artery (RCA) in at least two views. Based on the results of coronary angiography, all scores were calculated in a blinded fashion by three cardiologists. Gensini score equals the sum of all segment scores (each segment score equals a segment weighting factor multiplied by a severity score), as previously described [16]. Segment weighting factors range from 0.5 to 5.0. Severity scores reflecting the specific percentage luminal diameter reduction of the coronary artery segment are 32 for 100%, 16 for 99%, 8 for 90%, 4 for 75%, 2 for 50%, and 1 for 25%. Thus, segments supplying a larger area of myocardium are more heavily weighted and multiple severe proximal lesions gain the highest score. We calculated Gensini score after primary percutaneous coronary intervention (PCI) without incorporating culprit lesion.
Each coronary lesion that produced a luminal narrowing ≥ 50% in vessels ≥ 1.5 mm was separately scored using the SYNTAX score calculator, and this was then added up to provide an overall SYNTAX score. The online latest updated version (2.28) was used for the calculation of the SYNTAX scores (http://www.SYNTAXscore. com) [17]. Angiographic coronary thrombus burden was scored based on the TIMI thrombus grading scale ranging from grade 0 (no thrombus), to grade 5 (very large thrombus content that completely occludes vessel flow) [18]. TIMI thrombus grading was based on the initial diagnostic coronary angiography. After restoring ante grade flow through guide wiring or small balloon dilatation in patients with TIMI thrombus grade 5, a coronary angiography enabled rest ratification of an underlying residual thrombus defined by final TIMI thrombus grade [19]. Multivessel disease was defined as the presence of a stenosis greater than 50% in three major epicardial coronary arteries based on coronary angiography.
The serum oxLDL, anti-oxLDL antibody, ÃÂœÃψ-9 and hsCRP
We took about 5 ml peripheral venous blood for the coronary angiography inside 12 hours. The blood was centrifuged for 15 minutes at 1000 rpm; the serum was stored at - 70°Ã¡ until it was used. The serum oxLDL (Cell Biolabs, Inc. USA), anti-oxLDL antibody (Elab Science Biotechnology, Inc. USA) and MMP-9 (R and D Systems, Inc. USA) titers were determined and analyzed by ELISA according to the manufacturer’s recommended protocol. The hsCRP detected method on the biochemistry-automated analyzer (Abbott’s automated analyzer, MIC Group, Inc. USA)
Statistical analysis
Quantitative variables were expressed as mean ± standard deviation, and qualitative variables were expressed as percentages. A comparison of parametric values between two groups was made using a two-tailed Student t-test. Pearson tests were used for correlation analysis. Binary logistic regression analysis was used to evaluate the independent association between serum markers and plaque rupture. A p value of < 0.05 was considered statistically significant. All statistical analyses were carried out using SPSS (SPSS 21 software, IBM, Inc. USA).
Ethical aspects
The study was approved by the Ethics Committee of Mongolian National University of Medical Sciences (ID: 6/3/201506, approved on Jan 01, 2015).
Results
The variable of major risk factors, Gensini, SYNTAX score and serum oxLDL, anti-oxLDL antibody, MMP-9, hsCRP marker in patients with AMI (case group, n=40) and stable angina pectoris (control group, n=40) are presented in Table 1. There was no statistically significant difference in terms of age of cases and controls, 58 ± 1.77 and 58 ± 1.62 years, respectively (p=0.81). There were not any significant differences (p>0.05) for the smoking index, body mass index, heart rate, blood pressure, white blood cell and neutrophil cell. The serum oxLDL (p=0.01), anti-oxLDL antibody (p<0.001), ÃÂœÃψ-9 (p<0.001) and hsCRP (p=0.009) levels of case group were correspondingly more than the control group (Table 1).
| Variable | Case group (n=40) | Control group (n=40) | p value |
|---|---|---|---|
| Age | 58.7 ± 1.77 | 58.1 ± 1.62 | p=0.81 |
| Smoking index, pack/day | 32.8 ± 15.5 | 30.2 ± 17.1 | p=0.12 |
| Body index, kg/m2 | 27.3 ± 3.95 | 27.1 ± 4.7 | p=0.76 |
| Heart rate, /min | 75.3 ± 12.7 | 71.1 ± 11.1 | p=0.12 |
| Systolic pressure, mmHg | 124.4 ± 19.2 | 126.5 ± 19.8 | p=0.71 |
| Diastolic pressure, mmHg | 77.3 ± 14.3 | 77.3 ± 14.3 | p=0.39 |
| White blood cell, 1x106 | 5.9 ± 1.13 | 5.7 ± 0.87 | p=0.47 |
| Neutrophil (seg), % | 57.2 ± 3.11 | 52 ± 1.01 | p=0.27 |
| Gensini score | 46.3 ± 27.7 | 11.9 ± 8.03 | p<0.001 |
| SYNTAX score | 23.5 ± 10.39 | 10.2 ± 8.37 | p<0.001 |
| OxLDL, µg/ml | 1.41 ± 0.016 | 1.33 ± 0.026 | p=0.01 |
| anti-oxLDL antibody, mU/ml | 71.0 ± 2.06 | 60.2 ± 1.37 | p<0.001 |
| MMP-9, ng/ml | 396 ± 18.23 | 223 ± 13.80 | p<0.001 |
| hsCRP, mg/dl | 2.96 ± 0.296 | 0.17 ± 0.031 | p=0.009 |
Table 1: Patient’s characteristics.
The Gensini scores had direct correlation with the serum oxLDL (r=0.076, p=0.502), anti-oxLDL antibody (r=0.248, p=0.026), MMP-9 (r=0.424, p<0.001) and hsCRP (r=0.215, p=0.055) levels. But SYNTAX score correlated with serum oxLDL (r=0.083, p=0.462), anti-oxLDL antibody (r=0.414, p<0.001), MMP-9 (r=0.205, p=0.048) and hsCRP (r=0.185, p=0.101). To calculate the SYNTAX scores we involved 54 patients with low score (score ≤ 22), 18 patients intermediate score (score 23-32), 8 patients high score (score ≥ 33), respectively. All these 3 groups had serum MMP-9 (F=14.1, p<0.001), anti-oxLDL antibody (F=4.37, p=0.016) elevations. But the serum oxLDL and hsCRP levels did not have any changes (р>0.05).
Coronary atherosclerosis changes are divided into four main groups. These are: (1) coronary arteries with no stenosis or unchanged (left main, LAD, LCx and RCA no significant severe stenosis or <75% stenosis); (2) coronary artery has one significant severe stenosis (1 vessel has ≥75% stenosis); (3) coronary artery has two significant severe stenosis (2 vessels have ≥75% stenosis); (4) coronary artery has three significant severe stenosis (3 vessels has ≥75% stenosis). As the number of arteries with the severe stenosis increased, the serum oxLDL, anti-oxLDL antibody and MMP-9 levels increased along with it and was significant p<0.05 (oneway ANOVA test, Table 2).
| Variable | No severe stenosis | 1 vessel | 2 vessels | 3 vessels | One-way ANOVA | |
|---|---|---|---|---|---|---|
| (n=23) | (n=32) | (n=19) | (n=6) | F | p-value | |
| OxLDL, ug/ml | 1.31 ± 0.176 | 1.36 ± 0.126 | 1.44 ± 0.076 | 1.45 ± 0.085 | 3.87 | 0.007 |
| Anti-oxLDL antibody, mU/ml | 60.6 ± 1.83 | 67.4 ± 1.71 | 68.6 ± 2.5 | 75.6 ± 11.12 | 3.62 | 0.033 |
| MMP-9, pg/ml | 231 ± 43.5 | 255 ± 27.5 | 350 ± 21.2 | 341 ± 28.2 | 4.176 | 0.017 |
| HsCRP, mg/dl | 0.77 ± 0.264 | 0.76 ± 0.330 | 0.12 ± 0.016 | 0.12 ± 0.040 | 0.427 | 0.291 |
Table 2: The one-way ANOVA test of serum oxLDL, anti-oxLDL antibody, MMP-9, hsCRP enzyme in number of coronary atherosclerotic severe stenosis.
The Binary logistic regression analysis was performed to determine if the serum oxLDL, anti-oxLDL antibody, ÃÂœÃψ-9 and hsCRP level modifications could be risk for the coronary atherosclerotic plaque rupture. The serum oxLDL, anti-oxLDL antibody, ÃÂœÃψ-9 and hsCRP contents were accelerated and it served as the factor for the plaque rupture and thrombus complication formation (oxLDL β=0.011, p=0.016; anti-oxLDL antibody β=0.892, p<0.001; MMP-9 β=0.985, p<0.001; hsCRP β=0.041, p=0.005).
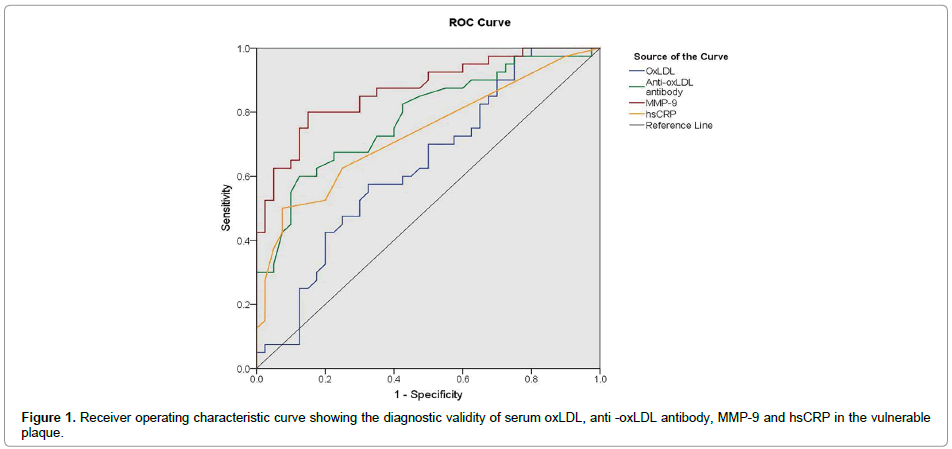
The ROC curve analysis demonstrated that MMP-9 (area=0.870, p<0.001) enzyme level elevation is more than other biomarkers (oxLDL area=0.635, p=0.038; anti-oxLDL antibody area=0.783, p<0.001; hsCRP area=0.733, p<0.001) making it diagnostically beneficial for the coronary atherosclerotic plaque rupture (Figure 1).
Discussion
The mean age with the coronary disorders was 58.5 ± 1.96, whereas the patients’ in the study of Yamashita [20] and Huang [21] et al. was 65 ± 10 years and 63.5 ± 9.2 years, respectively. Our patients were younger than those patients in 5-7 years. It can be explained by food with saturated fat; especially excessive fat usage can cause the early oxidation of LDL. Moreover it also can be the reason of coronary atherosclerosis complications for the Mongolian people.
There were not noticed any significant differences when we compared the case and control groups regarding to some risk factors of coronary disease as smoking, arterial hypertension, body mass index, age and sex. It is because that the patients’ coronary artery structure and function were already modified. The above-mentioned risk factors can only be the reason for the coronary atherosclerotic pathogenesis, but not for the thrombosis due to plaque rupture.
Our study, the anti-oxLDL antibody level was significantly higher (p<0.01) in subjects with case group (involves the thrombosis due to plaque rupture) than in control group (with stable coronary atherosclerosis). It is similar to the results of the Fiotti [22], Yamashita [20] and Medeiros [23] et al. studies. The present result shows that the serum oxLDL (β=0.011, p=0.016), anti-oxLDL antibody (β=0.892, p<0.001), MMP-9 (β=0.985, p<0.001) and hsCRP (β=0.041, p=0.005) affect to rupture of fibrotic cap in the coronary atherosclerotic plaque. Several studies have observed an association between raised levels of anti-oxLDL antibody, MMP-9 and plaque rupture [24-26]. This study shows an association between anti-oxLDL antibody and serum MMP-9 concentration in vivo. Since anti-oxLDL antibody could be expected to reflect the presence of oxLDL in the artery wall [27] and serum MMP-9 concentration reflects vascular inflammation [28], our results suggest that oxLDL may be associated with MMP- 9 secretion by inflammatory cells in atherosclerotic plaques [8]. As a consequence, same with the others [20,28] hypotheses, the thrombosis due to plaque rupture.
The serum oxLDL, anti-oxLDL antibody, ÃÂœÃψ-9 and hsCRP levels were analyzed by ROC curve. It shows serum markers may be diagnostic possibilities of complication of plaque rupture and thrombosis. Particularly the elevated ÃÂœÃψ-9 levels indicated the complications (MMP-9 area=0.870, p<0.001; oxLDL area=0.635, p=0.038; anti-oxLDL antibody area=0.783, p<0.001; hsCRP area=0.733, p<0.001). The studies of Yamashita [20], Zhang [9] and Popović et al. [29] have the analogous results. These findings indicate a pivotal role of anti-oxLDL antibody, MMP-9 in atherothrombosis in patients after AMI, whereby anti-oxLDL antibody and MMP-9 might act as a circulating biomarker, reflecting a proinflammatory state associated with poorer survival and acting as a causative agent with a local effect on plaque destabilization and progression.
In this study, there were involved 23 patients without any arteries changes (LM, LAD, LCx, RCA have no severe stenosis <75%), 32 patients with a modified artery (an artery ≥75% severe stenosis), 32 patients with two modified arteries (two arteries ≥75% severe stenosis), 6 patients with 3 modified arteries (3 arteries ≥75% severe stenosis) (Kalela et al. [30] classification). The serum anti-oxLDL antibody and MMP-9 levels elevates (p<0.05) correspondingly to numbers of damaged arteries and their complications. This is similar to the Kalela [30] and Moohebati et al. [24] results.
Taken together, the data of the present study strongly support the hypothesis that local inflammatory stimuli might play a role as a transient risk factor for acute ischemic events in patients with AMI, destabilizing pre-existing atherosclerotic plaque. However, further studies are required to explore the mechanisms that underlie atherosclerotic plaque rupture with increases in oxLDL, anti-oxLDL antibody, MMP-9 and hsCRP plasma levels.
In conclusion, the serum oxLDL, anti-oxLDL antibody, MMP-9 and hsCRP levels are significantly involved in the unstable coronary plaque.
Limitations of the Study
The major limitation of this study is the small sample size. Therefore the ability to generalise this correlation might be limited. In addition, the use of coronary angiography to visually quantify atherosclerosis is limited because remodelling may obscure substantial disease burden in arterial walls that can be detected by intravascular ultrasound.
Acknowledgments
This research was supported by the Mongolian National University of Medical Sciences and Ulaanbaatar Songdo Hospital.
Conflict of Interest
The authors have no financial conflicts of interest.
References
- Mendis S, Puska P, Norrving B (2011) Global Atlas on Cardiovascular Disease Prevention and Control. Geneva.
- Bat-Erdene C, Zultsetseg C, Davaajargal S, Narantuya K, Boldbaatar B (2015) Health Indicators 2015. Ulaanbaatar.
- Crea F, Liuzzo G (2013) Pathogenesis of acute coronary syndromes. J Am Coll Cardiol 61: 1-11.
- Bentzon JF, Otsuka F, Virmani R, Falk E (2014) Mechanisms of plaque formation and rupture. Circ Res 114: 1852-1866.
- Moore KJ, Tabas I (2011) Macrophages in the pathogenesis of atherosclerosis. Cell 145: 341-355.
- Yabluchanskiy A, Ma Y, Iyer RP, Hall ME, Lindsey ML (2013) Matrix Metalloproteinase-9?: Many Shades of Function in Cardiovascular Disease. Physiology 28: 391-403.
- Lopes-Virella MF, Virella G (2013) Pathogenic role of modified LDL antibodies and immune complexes in atherosclerosis. J Atheroscler Thromb 20: 743-754
- Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, et al. (2000) Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest 106: 55-62.
- Zhang J, Wang D, He S (2015) Roles of antibody against oxygenized low density lipoprotein in atherosclerosis: recent advances. Int J Clin Exp Med 8: 11922-11929
- Kalela A, Koivu TA, Höyhtyä M, Jaakkola O, Lehtimäki T, et al. (2002) Association of serum MMP-9 with autoantibodies against oxidized LDL. Atherosclerosis 160: 161-165.
- Yousuf O, Mohanty BD, Martin SS, Joshi PH, Blaha MJ, et al. (2013) High-sensitivity C-reactive protein and cardiovascular disease: a resolute belief or an elusive link? J Am Coll Cardiol 62: 397-408
- Raposeiras Roubín S, Barreiro Pardal C, Roubín-Camiña F, Ocaranza Sanchez R, Alvarez Castro E, et al. (2013) High-sensitivity C-reactive protein predicts adverse outcomes after non-ST-segment elevation acute coronary syndrome regardless of GRACE risk score, but not after ST-segment elevation myocardial infarction. Port J Cardiol. 32: 117-122.
- Steg PG, James SK, Atar D, Badano LP, et al. (2012) ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 33: 2569-2619
- Montalescot G, Sechtem U, Achenbach S, Andreotti F, et al. (2013) 2013 ESC guidelines on the management of stable coronary artery disease: Eur Heart J 34: 2949-3003.
- Abrams J (2016) Chronic Stable Angina. N Engl J Med 12: 2524-2533.
- Gensini GG (1983) A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 51: 606.
- Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, et al. (2005) The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention 1: 219-227.
- Tanboga IH, Topcu S, Aksakal E, Kalkan K, Sevimli S, et al. (2014) Determinants of angiographic thrombus burden in patients with ST-segment elevation myocardial infarction. Clin Appl Thromb Hemost 20: 716-722.
- Gibson CM, Lemos JA de, Murphy SA, Marble SJ, McCabe CH, et al. (2001) Combination therapy with abciximab reduces angiographically evident thrombus in acute myocardial infarction. Circulation. 103: 2550-2554.
- Yamashita H, Ehara S, Yoshiyama M, Yoshiyama M, Naruko T, et al. (2007) Elevated Plasma Levels of Oxidized Low-Density Lipoprotein Relate to the Presence of Angiographically Detected Complex and Thrombotic Coronary Artery Lesion Morphology in Patients With Unstable Angina. Circ Res 71: 681-687.
- Huang H, Mai W, Liu D, Hao Y, Tao J, et al. (2008) The oxidation ratio of LDL: a predictor for coronary artery disease. Dis Markers 24: 341-349.
- Fiotti N, Altamura N, Orlando C, Simi L, Reimers B, et al. (2008) Metalloproteinases-2, -9 and TIMP-1 expression in stable and unstable coronary plaques undergoing PCI. Int J Cardiol 127: 350-357.
- Medeiros AM, von Mühlen CA, Gidlund MA, Bodanese R, Gottlieb MG, et al. (2010) Antibodies against oxLDL and acute coronary syndrome. Arq Bras Cardiol 95: 47-54.
- Moohebati M, Kabirirad V, Ghayour-Mobarhan M, Esmaily H, Tavallaie S, et al. (2014) Investigation of Serum Oxidized Low-Density Lipoprotein IgG Levels in Patients with Angiographically Defined Coronary Artery Disease. Int J Vasc Med 2014: 1-9
- Newby AC (2006) Do metalloproteinases destabilize vulnerable atherosclerotic plaques? Curr Opin Lipidol 17: 556-561.
- Dave T, Ezhilan J, Vasnawala H, Somani V (2013) Plaque regression and plaque stabilisation in cardiovascular diseases. Indian J Endocrinol Metab 17: 983-989
- Friedman P, Horkko S, Steinberg D, Witztum JL, Dennis EA (2002) Correlation of Antiphospholipid Antibody Recognition with the Structure of Synthetic Oxidized Phospholipids. J Biol Chem 277: 7010-7020.
- Blankenberg S, Rupprecht HJ, Poirier O, Bickel C, Smieja M, et al. (2003) Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation 107: 1579-1585.
- Popović S, Canović F, Ilić M, Rafajlovski S, Dimitrijević-Srećović V, et al. (2015) Matrix metalloproteinase-9 index as a possible parameter for predicting acute coronary syndrome in diabetics. Vojnosanit Pregl 72: 421-426.
- Kalela A, Koivu TA, Sisto T, Kanervisto J, Höyhtyä M, et al. (2002) Serum matrix metalloproteinase-9 concentration in angiographically assessed coronary artery disease. Scand J Clin Lab Invest 62: 337-342.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi