Research Article, Int J Cardiovasc Res Vol: 8 Issue: 2
Left Atrial Function and Volume an Independent Markers of Cardiovascular Involvement in Early Chronic Kidney Disease
Wafaa S El-Sherbeny* and Suzan B Elhefnawy
Faculty of Medicine, Tanta University, Gharbia Governorate, Egypt
*Corresponding Author : Wafaa S El-Sherbeny, MD
Lecturer of Cardiovascular Medicine, Faculty of Medicine, Tanta University, Gharbia Governorate, Egypt
Tel: 00201224214937
E-mail: Wfelsherbeny@gmail.com
Received: September 10, 2018 Accepted: February 19, 2019 Published: February 25, 2019
Citation: El-Sherbeny WS, Elhefnawy SB (2019) Left Atrial Function and Volume an Independent Markers of Cardiovascular Involvement in Early Chronic Kidney Disease. Int J Cardiovasc Res 8:2. doi: 10.417/2324-8602.1000373
Abstract
Aim: value of left atrium (LA) volume and function evaluated by strain analysis in the detection of myocardial involvement in early chronic kidney disease (CKD) patients with coexistent of hypertension.
Methods: 33 patients with CKD stage 2 or stage 3, with mild hypertension compared with 32 age matched hypertensive patients with normal kidney function and 30 healthy control subjects, all participants underwent trans thoracic echocardiography to assess left ventricular (LV) systolic and diastolic function, LV mass and LA volume index (LAVI) by 2D and pulsed wave Doppler imaging, and LA segmental strain measured by tissue Doppler imaging (TDI).
Results: Stage 3 CKD had the most reduced diastolic function compared with other groups. (LAVI) was higher significantly in both CKD group (36.2+8.4 ml/m2) and HT group (34.31+4.75 ml/m2) compared to control group (22.18+3 ml/m2) (p=0.001), LAVI was significantly higher in stage 3 compared with stage 2 CKD (p=0.001), Global systolic strain (GS)was significantly reduced in CKD group (17.48+4.3%) compared to both HT group (27.92+5.17%) and controls (31.75+6.8%) (p=0.001), GS was significantly lower in HT group compared with controls (p=0.014), systolic strain of four left atrial walls were different significantly between CKD group and both HT and control groups. there was high significant difference between stage 2 and stage 3 CKD as regard left atrial lateral and anterior walls systolic strain (p=0.001), inferior and septal wall systolic strain were significantly lower in stage 3 compared to stage 2 CKD.
Conclusion: LA dysfunction and enlargement are evident in patients with early CKD, LA systolic strain was reduced in CKD earlier than LA enlargement which occur later, hence LA systolic strain and volume index could be used to detect myocardial involvement in early CKD.
Keywords: CKD; LA function; LAVI; Strain imaging
Abbreviations
LA: Left Atrium; CKD: Chronic Kidney Disease; LV: Left Ventricular; LAVI: Left Atrium Volume Index; TDI: Tissue Doppler Imaging; HT: Hypertensive; GFR: Glomerular Filtration Rate; CVD: Cardiovascular Disease; ESRD: End Stage Renal Disease; RAAS: Renin–Angiotensin-Aldosterone System; S: Systolic Strain; SPSS: Statistical Program for Social Science; SD: Standard Deviation; ANOVA: A One-Way Analysis Of Variance; LVMI: Left Ventricular Mass Index; BMI: Body Mass Index; LVEF: Left Ventricular Ejection Fraction; GS: Global Systolic Strain; ACE: Angiotensin Converting Enzyme
Introduction
Chronic kidney disease (CKD) includes either glomerular filtration rate (GFR) ≤ 60 ml/min/1.73 m for more than 3 months, or other pathological abnormalities or markers of kidney damage [1].
Cardiovascular mortality and morbidities are high in CKD, and the cardiovascular disease (CVD) outcomes worsens in the presence of CKD [2,3] both CKD and CVD share the common traditional risk factors. The traditional (Framingham risk score) underestimate cardiovascular risk in patients with CKD [4], hence a need for non-invasive cardiac markers to predict future adverse cardiac events in CKD patients.
The modified national kidney foundation classification for CKD [5] all stages have high risk for adverse CVD, however ≥ 11% risk for adverse cardiovascular events reported in stage 3 CKD [6].
The left atrium (LA) has multiple functions, during ventricular systole diastole [7].
LA enlargement and changes in LA volume has been reported to predict cardiovascular event in dialysis patients [8], however hypertrophy of left ventricle and systolic dysfunction in end stage renal disease (ESRD) may alter LA size and function.
There are limited data regarding LA involvement in early CKD, hypertension and diabetes mellitus commonly present with CKD, both of them may alter LA function and volume independently [9,10]. Activation of renin –angiotensin-aldosterone system (RAAS) in CKD caused atrial fibrosis [11,12] which leads to LA enlargement and dysfunction. Evaluation of LA function by conventional echocardiography is challenging, strain imaging is semi-automated technique and an angle-independent may provide a simple, quantitative assessment of atrial function [13].
The present study investigates the value of LA volume and function evaluated by strain analysis in the detection of myocardial involvement in early CKD patients with coexistent of hypertension.
Patients and Methods
The study was performed at cardiovascular department at Tanta University Hospital in the period from May 2016 to June 2017. 95 subjects had been included in the study divided into three groups.
Group 1: which include 33 patients with CKD stage 2 or stage 3, 15 stage 2 (eGFR 60-89 ml/min per 1.73 m) and 18 stage 3 (eGFR 30-59 ml/min per 1.73 m2 by the modified diet in renal disease formula) with mild hypertension on one or more antihypertensive medication, patients were identified from the renal outpatient department.
CKD staging was done according to standard criteria [5] the cause of CKD was hypertension (25), chronic glomerulonephritis (3), polycystic kidney (1), renal calculus (2), drug induced (1) and systemic lupus erythromatosis (1).
Patients had previous cardiovascular, peripheral vascular or cerebrovascular disease were Excluded, patients with valvular heart disease, and history of atrial fibrillation or congestive heart failure all were excluded from the study.
Group 2 includes 32 age and gender matched patients with mild hypertension controlled on antihypertensive medication, with normal kidney function and had no other cardiac risk factors, no history of cerebrovascular or peripheral vascular disease.
Group 3 includes 30 Healthy normal control subjects with same age.
All CKD patients underwent transthoracic echocardiography to assess LV function, mass and valvular abnormality; stress echocardiography was done to rule out occult ischemia.
Written informed consent was obtained from all participants in this study
Echocardiography: Trans thoracic echocardiographic examination was done using vivid 9,General Electric, Horten, Norway with 2.5 MHZ frequency transducer, 2D,color and pulsed wave Doppler and tissue Doppler imaging (TDI) were obtained from slandered echocardiographic views and according to standard practice (14).
Left ventricular measurement: LVEF was measured by M –mode of LV walls and internal dimensions in the parasternal long axis view. LV diastolic function pulsed Doppler of Trans mitral flow velocities were obtained with the sample volume placed at the tips of mitral leaflet in the apical four chamber view. Peak E and A velocity, and E/A ratio was calculated, TDI to measure early (E`) and late (A`) diastolic velocity by placing sample volume at septal and lateral mitral annulus [14,15], E/E` ratio was calculated. LV mass and LV indexed to body surface area was calculated using the LV diameter and wall thickness at end-diastole by M-mode in the parasternal long axis view [16].
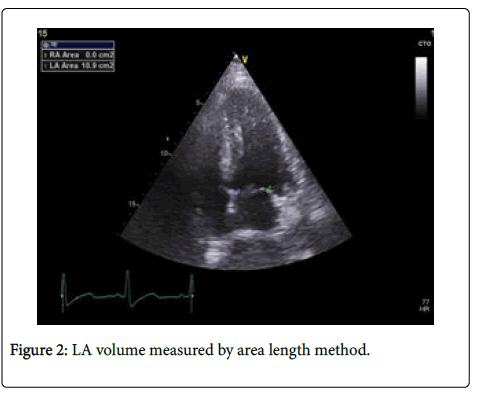
Left atrium measurement: -LA volume, was obtained using biplane area length method where: LA volume=(0.85 × Area 4 ch × Area 2 ch)/ (Longest LA length). Apical four chamber and two chamber views were used to measure the LA area and long axis at end of ventricular systole LA area was measured by tracing the endocardial border of LA. While LA long axis dimension was measured as a line perpendicular to the mitral annular plane extending to the back wall of LA. These LA volumes obtained for each subject were indexed to his/her body surface area. BSA was calculated by a simple and commonly used formula "Mosteller formula": BSA (m2)=(Height (cm) × Weight (kg)/ 3600)1/2.
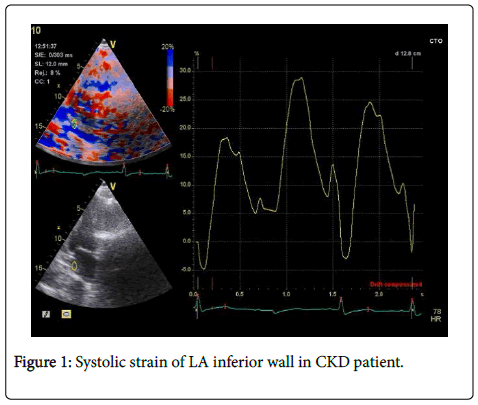
-LA systolic strain, LA systolic strain (S) was measured using colorcoded tissue Doppler imaging (TDI) using standard apical views, a sample volume (2 mm because of thin atrial walls) was placed at mid segment of septal and lateral walls of LA (from the apical 4 chamber view), LA inferior and anterior walls (from apical 2 chamber view) and tracked frame by frame to maintain its position within LA wall. Off line analysis was done using available software. Peak systolic strain was measured in each segment and global strain for final analysis was calculated by averaging values from the four LA walls.
Statistical Analysis
Analysis of data was done using Statistical Program for Social Science (SPSS) version 20.0 Quantitative data were expressed as mean ± standard deviation (SD).
The following tests were done: Independent-samples t-test was performed when comparing between two means, a one-way analysis of variance (ANOVA) when comparing between more than two means and Post Hoc test was used for multiple comparisons between different variables.
Results
95 patients and control subjects were enrolled in this study, patients characteristic: mean age was 59.7+8 years in CKD group, 58.6+9 years in hypertensive group and 57.7+9.1 years in control group with no significant difference between three groups, nineteen patients (57.5%) in CKD group and 18 (56.2%) patients in HT group were male compared with 13 (43.3%) in control group. CKD group and HT group had higher Body mass index (BMI) compared with control group (p=0.001) (Table 1) (Figure 1).
| Control (n=33) | CKD (n=30) | HT(n=32) | P1 | P2 | P3 | ||
|---|---|---|---|---|---|---|---|
| LVEF (%) | Range | 57.2 – 69.4 | 58.3 – 69.9 | 58.6 – 68.7 | 0.107 | 0.124 | 0.917 |
| Mean ± SD | 62.15 ± 4.62 | 63.96 ± 4.12 | 63.85 ± 4.14 | ||||
| LVMI | Range | 55.1 – 112.9 | 66.8 – 127.8 | 84 – 130 | 0.001* | 0.001* | 0.016* |
| g/m2 | Mean ± SD | 80.85 ± 16.8 | 97.74 ± 7.56 | 108.03 ± 15.11 | |||
| LAVI | Range | 16.9 – 27.1 | 25.1 – 47.9 | 22.4 – 39 | 0.001* | 0.001* | 0.147 |
| ml/m2 | Mean ± SD | 22.48 ± 3.1 | 36.82 ± 8.32 | 34.31 ± 4.75 | |||
| BMI | Range | 24.5 – 31.4 | 26.6 – 34.7 | 26.5 – 35 | 0.001* | 0.001* | 0.931 |
| Kg/m2 | Mean ± SD | 28.12 ± 2.02 | 30.25 ± 2.14 | 30.31 ± 3.17 | |||
Table 1: Comparison between the studied groups regarding LVEF, LVMI, LAVI and BMI
P1: between control and CKD groups. P2: between control and HT groups, P3: between CKD and HT groups.
LV Function
LVEF was normal in the studied groups with no significant difference between the three groups. LVMI was significantly high in both hypertensive and CKD groups compared to controls and significantly higher in HT group (mean 108.03 +15.1 g/m2) compared to CKD group (mean 97.74+17.56 g/m2) (p=0.01) (Table 1).
Peak A velocity was significantly higher in the CKD (mean 0.77+0.1 m/s) compared to both HT (mean 0.65+0.1 m/s) and controls group (p=0.001), there was no significant in E wave velocity between the three groups. E/A ratio was significantly lower in the CKD group compared to HT group (p=0.001) and to controls (p=0.005). LV diastolic function was reduced in early stages of CKD, in CKD group there were 4 patients (12%) with normal LV diastolic function, 14 patients (42.4%) with grade 1 (impaired relaxation), 12 patients (36.3%) with grade 2 (pseudo normal relaxation) and 3 patients (9%) with grade 3(restrictive pattern), in contrast, 19% of patients in HT group had normal diastolic function,46% had impaired relaxation (grade 2), 35% had grade 3 diastolic dysfunction and none had restrictive pattern. Subjects in control group either had normal function or grade 1 of diastolic dysfunction.
Stage 3 CKD had the most reduced diastolic function, 8 patients with impaired relaxation, 7 patients with pseudo normal relaxation and 3 patients with restrictive pattern. E` velocity was significantly lower in the CKD and HT groups compared to controls (p=0.001) and E/E` ratio was higher in CKD and HT group compared to controls (p=0.001) (Table 2).
| Control (n=30) | CKD (n=33) | HT(n=32) | P1 | P2 | P3 | ||
|---|---|---|---|---|---|---|---|
| A velocity | Range | 0.46 – 0.81 | 0.59 – 0.95 | 0.48 – 0.86 | 0.001* | 0.451 | 0.001* |
| m/s | Mean ± SD | 0.63 ± 0.11 | 0.77 ± 0.11 | 0.65 ± 0.11 | |||
| E/A ratio | Range | 0.78 – 1.32 | 0.61 – 2.14 | 0.8 – 1.4 | 0.004* | 0.688 | 0.001* |
| Mean ± SD | 1.12 ± 0.19 | 0.86 ± 0.33 | 1.10 ± 0.2 | ||||
| È cm/s | Range | 6.38 – 10.8 | 6.41 – 11.2 | 6.5 – 9.0 | 0.001* | 0.001* | 0.303 |
| Mean ± SD | 9.73 ± 1.02 | 7.73 ± 1.27 | 8.0 ± 0.75 | ||||
| E/E` ratio | Range | 6.58 – 8.1 | 7.49 – 15.56 | 6.7 – 12 | 0.001* | 0.001* | 0.631 |
| Mean ± SD | 7.31 ± 0.40 | 9.62 ± 2.48 | 9.36 ± 1.79 | ||||
Table 2: Comparison between the studied groups regarding diastolic function. P1: between control and CKD groups. P2: between control and HT groups, P3: between CKD and HT groups.
LA Parameters
-LA volume index (LAVI) was significantly larger in both CKD group (mean 36.82+8.32 ml/m2) and HT group (mean 34.31+4.75 ml/m2) compared to control group (mean 22.48 + 3.1 ml/m2) (p=0.001), (Table 1) (Figure 2). Mean LAVI in stage 2 CKD was 34.50+5.18 ml/m2 and in stage 3 CKD was 45.53+4.27 ml/m2, LAVI was significantly higher in stage 3 compared with stage 2 CKD (p=0.001) (Table 3).
| CKD group | P-value | ||
|---|---|---|---|
| Stage 2 (n=15) | Stage 3 (n=18) | ||
| LAVI (ml/m2) | |||
| Mean ± SD | 34.50 ± 5.1 8 | 45.53 ± 4.27 | <0.001 |
| Range | 27.1-39.2 | 37.2-49.3 | |
Table 3: LAVI compared between the stages of CKD.
Atrial systolic strain
Global systolic strain (GS) derived as an average of 4 segments (lateral, septal, anterior and inferior walls) was significantly reduced in CKD group (mean 17.48+4.3%) compared to both HT group (mean 27.92+5.17%) and controls (mean 31.75+6.8%) (p=0.001) (Table 4), GS was significantly lower in HT group compared with controls (p=0.014), systolic strain of four left atrial walls were different significantly between CKD group and both HT and control groups (Table 4) (Figure 1). There was high significant difference between stage 2 and stage 3 CKD as regard left atrial lateral and anterior walls systolic strain (p=0.001), inferior and septal wall systolic strain were significantly lower in stage 3 compared to stage 2 CKD (Table 5).
| Control (n=30) | CKD (n=33) | HT(n=32) | P1 | P2 | P3 | ||
|---|---|---|---|---|---|---|---|
| Lateral S (%) | Range | 23.22 – 52.23 | 8.12 – 28.51 | 20 – 38.5 | 0.001* | 0.001* | 0.001* |
| Mean ± SD | 38.92 ± 9.28 | 16.31 ± 6.34 | 28.95 ± 4.85 | ||||
| Septal S (%) | Range | 13.93 – 47.44 | 7.62 – 20.69 | 13.5 – 30.8 | 0.001* | 0.002* | 0.001* |
| Mean ± SD | 26.73 ± 9.77 | 14.35 ± 3.71 | 20.48 ± 3.98 | ||||
| Anterior S (%) | Range | 17.82 – 47.84 | 5.83 – 34.78 | 16.8 – 40.5 | 0.001* | 0.047* | 0.001* |
| Mean ± SD | 32.32 ± 7.87 | 17.28 ± 8.43 | 28.51 ± 6.92 | ||||
| Inferior S (%) | Range | 33.92 – 74.74 | 8.52 – 33.28 | 30.5 – 64.2 | 0.001* | 0.002* | 0.001* |
| Mean ± SD | 47.51 ± 11.28 | 19.69 ± 7.61 | 39.47 ± 8.15 | ||||
| GS | Range | 25.9 – 39.7 | 12.7 – 23.8 | 19.5 – 35.5 | 0.001* | 0.014* | 0.001* |
| Mean ± SD | 31.75 ± 6.87 | 17.48 ± 4.32 | 27.92 ± 5.17 | ||||
Table 4: Comparison between the three groups regarding left atrial walls systolic strain and global strain. P1: between control and CKD groups. P2: between control and HT groups, P3: between CKD and HT groups.
| CKD group | P. value | ||||
|---|---|---|---|---|---|
| Stage 2 (n=15) | Stage 3 (n=18) | ||||
| Lateral S (%) | |||||
| Mean ± SD | 15.23 ± 2.92 | 9.76 ± 1.31 | <0.001 | ||
| Range | 11.52-20.52 | 7.82-11.83 | |||
| Septal S (%) | |||||
| Mean ±SD | 13.25 ± 2.09 | 11.07 ± 1.92 | 0.004 | ||
| Range | 11.32-17.24 | 7.62-13.81 | |||
| Anterior S (%) | |||||
| Mean ±SD | 18.24 ± 7.05 | 8.65 ± 1.66 | 0.001 | ||
| Range | 11.62-34.69 | 5.79-10.63 | |||
| Inferior S (%) | |||||
| Mean ±SD | 18.38 ± 4.50 | 13.25 ± 4.78 | 0.005 | ||
| Range | 11.52-22.61 | 8.49-22.43 | |||
Table 5: Comparison between the two stages of CKD group regarding systolic strain of left atrial walls (S%).
Discussion
Many studies demonstrated increased cardiovascular mortality in ESRD [17,18] patients with stage 4 and 5 CKD (e GFR≤ 30 ml/min/ 1.73 m) had increased fluid overload and LV hypertrophy that independently alter LA size and function and leads to abnormalities in traditional echocardiographic parameters. Our study was conducted to detect early cardiac involvement using newer echocardiographic parameters in patients with early stages of CKD. We include patients with stage 2 and 3 CKD with mild hypertension compared to hypertensive patients with same age and normal kidney function and to healthy control subjects, we specifically used a group with HT alone to evaluate the effect of concomitant CKD on LA function, as hypertension independently causes LA changes [18,19].
LVMI was higher significantly in CKD compared to controls this result is agree with the result of Nakanishi et al. [20] our study reported that LVMI was higher in HT group compared with CKD and control groups which is the same results of Kadappu et al. [21].
This study found that LV diastolic function was impaired in early stages of CKD which was the same finding of Takenori et al. [22] who studied left ventricular diastolic dysfunction in the early stages of CKD, compared to 40 non CKD subjects and concluded that impaired diastolic function of LV was observed even in patients with early stages of CKD. LA enlargement in ESRD independent related to LVMI, LVEF and diastolic dysfunction was reported by Tripepi et al. [8,23] they found that “LAVI was an independent predictor of adverse cardiovascular out comes in patients with ESRD”.
Our study found that (LAVI) was larger significantly in early CKD and hypertensive groups compared to controls, with no significant difference between CKD and HT group; (LAVI) could not differentiate between the effects of hypertension alone and hypertension with CKD. Our results were in the same line with the study of Kadappu et al. [21].
However LA systolic strain was reduced significantly in CKD group compared with hypertensive and control groups despite similar (LAVI) in both hypertensive and CKD groups, in the study of Boyd et al. [24] who examine age –related changes at atrial strain measurement in 188 healthy individuals, reported that “atrial strain was altered before volume parameters with aging, strain analysis more sensitive in detecting subclinical atrial dysfunction”.
This study found that traditional parameter of left atrial function (peak A velocity) was significantly higher in CKD group compared with other groups, and this represent compensatory increase in late diastolic filling due to the reduction in early diastolic filling and impaired LA compliance with a reduction in reservoir function, these finding was in the same line with the study of Nakanishi et al. [20] who studied the association of CKD with impaired left atrial reservoir function in 69 patients with CKD compared with 289 non CKD subjects, reported that “LA reservoir function was impaired significantly in CKD patients without overt cardiac disease, while LA maximum volume index did not differ between the studied groups”.
These results suggest that CKD may alter LA reservoir function before LA enlargement which occurs subsequently as renal dysfunction progress and LA enlargement represent a late marker of LA remodeling.
In our study there was a significant reduction in E` velocity and increase in E/e` ratio in patients of CKD and HT groups compared to controls with no significant difference between HT group and CKD group, hence TDI could not differentiate cardiovascular involvement in CKD patients with HT from those with only HT. Evaluation of global and segmental LA function using systolic strain parameter considered to be a sensitive measure [25,26].
Our study found that LA global strain reduced significantly in CKD group compared to HT and control groups this may be secondary to increased activation of RASS in CKD group which lead to atrial fibrosis and in turn alters LA function and volume, our results demonstrated that LA strain is affected earlier than LA volume in early CKD as left atrial fibrosis would alter LA function (GS) earlier than LA dilatation, which is amore chronic process, these results was the same results of Kadappu et al. [21]. This study demonstrated that alterations in LA function (systolic strain) precede changes in LV function (EF) in patients with CKD and suggest that alterations in LA function may be a predictor of adverse cardiovascular out comes as reported in previous studies [27,28].
In the study of Kadappu et al. [29] to evaluate LA function and volume in patients with stage 3 CKD compared with patients matched for risk factors and age with normal kidney function and healthy controls, found that LA systolic strain were altered in CKD group, concluded that “LA strain and LAVI may be useful to detect early myocardial involvement in stage 3 CKD” which was the same results of ours.
In the study of Kadappu et al. [29] to evaluate LA function and volume in patients with stage 3 CKD compared with patients matched for risk factors and age with normal kidney function and healthy controls, found that LA systolic strain were altered in CKD group, concluded that “LA strain and LAVI may be useful to detect early myocardial involvement in stage 3 CKD” which was the same results of ours.
Conclusion
Left atrial dysfunction and enlargement are evident in patients with early CKD, LA systolic strain was reduced in CKD earlier than LA enlargement which occur later, hence LA systolic strain and LAVI may be useful to detect myocardial involvement in early CKD and LA strain is more sensitive parameter.
Ethical standards
This study was approved by the Ethics Committee in the Faculty of Medicine, Tanta University and with ethical standards of Helsinki Declaration of 1964 and later revision. Written consent was obtained from all patients included in the study.
Disclosure of any funding to the study
This study did not receive any specific grant from funding agencies.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Levey AS, Coresh J, Bolton K, Culleton B, Harvey KS, et al. (2002) K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1-266.
- Kottgen A, Russell SD, Loehr LR, Crainiceanu CM, Rosamond WD, et al. (2007) Reduced kidney function as a risk factor for incident heart failure: The atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol 18:1307–1315.
- Alonso A, Lopez FL, Matsushita K, Loehr LR, Agarwal SK, et al. (2011) Chronic kidney disease is associated with the incidence of atrial ï¬Âbrillation: The Atherosclerosis Risk in Communities (ARIC) study. Circulation 123: 2946-2953.
- Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, et al. (2007) The Framingham predictive instrument in chronic kidney disease. J Am Coll Cardiol 50: 217-224.
- Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, et al. (2005) Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 67: 2089-2100.
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C (2004) Chronic kidney dis-ease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296-1305.
- Roberts WC, Waller BF (1983) Cardiac amyloidosis causing cardiac dysfunction: analysos pf 54 necropsy patients. Am J Cardiol 52: 137-146.
- Tripepi G, Benedetto FA, Mallamaci F, Tripepi R, Malatino L, et al. (2006) Left atrial volume in end-stage renal disease: A prospective cohort study. J Hypertens 24:1173–1180.
- Eshoo S, Ross DL, Thomas L (2009) Impact of mild hypertension on left atrial size and function. Circ Cardiovasc Imaging 2: 93-99.
- Kadappu KK, Boyd A, Eshoo S, Haluska BA, Yeo AET, et al. (2012) Changes in left atrial volume in diabetes mellitus: More than diastolic dysfunction? Eur Heart J Cardiovasc Imaging 13: 1016-1023.
- Ehrlich JR, Hohnloser SH, Nattel S (2006) Role of angiotensin system and effects of its inhibition in atrial ï¬Âbrillation: Clinical and experimental evidence. Eur Heart J 27: 512-518.
- Siragy HM, Carey RM (2010) Role of the intrarenal renin-angiotensin-aldosterone system in chronic kidney disease. Am J Nephrol 31: 541-550
- Vianna-Pinton R, Moreno CA, Baxter CM (2009) Two-dimensional speckle-tracking echocardiography of the left atrium: feasibility and regional contraction and relaxation differences in normal subjects. J Am Soc Echocardiogr 22: 299-305.
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, et al. (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echo Cardiogr 28:1-39.
- Nagueh SF, Appleton CP, Gillebert TC, Byrd BF, Dokainish H, et al. (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 22:107-133.
- Foppa M, Duncan BB, Rohde LE (2005) Echocardiography-based left ventricular mass estimation. How should we deï¬Âne hypertrophy? Cardiovasc Ultrasound 3:17.
- Liu YW, Su CT, Huang YY, Yang CS, Huang JW, et al. (2011) Left ventricular systolic strain in chronic kidney disease and hemodialysis patients. Am J Nephrol 33: 84-90.
- Levin A, Singer J, Thompson CR, Ross H, Lewis M (1996) Prevalent left ventricular hypertrophy in the predialysis population: identifying opportunities for intervention. Am J Kidney Dis 27: 347-354.
- McIntyre CW, John SG, Jefferies HJ (2008) Advances in the cardiovascular assessment of patients with chronic kidney disease. NDT Plus 6: 383-391.
- Nakanishi K, Jin Z, Russo C, Homma S, Elkind MS, et al. (2017) Association of chronic kidney disease with impaired left atrial reservoir function: A community-based cohort study. European Journal of Preventive Cardiology 4: 392-398.
- Kadappu KK, Kuncoro AS, Hee L, Aravindan A, Spicer ST, et al. (2014) Chronic Kidney Disease is Independently Associated with Alterations in Left Atrial Function. Echocardiography 31: 956-964.
- Takenori O, Makoto S, Hisao Y, Sugi K (2009) Left ventricular diastolic dys-fucntion in the early stage of chronic kidney disease. J Cardiol 54: 199-204.
- Tripepi G, Benedetto FA, Mallamaci F, Tripepi R, Malatino L, et al. (2007) Left atrial volume monitoring and cardiovascular risk in patients with end-stage renal disease: A prospective cohort study. J Am Soc Nephrol 18: 1316-1322
- Boyd AC, Richards DA, Marwick T, Thomas L (2011) Atrial strain rate is a sensitive measure of alterations in atrial phasic function in healthy ageing. Heart 97:1513-1519.
- Abd El Rahman MY, Hui W, Timme J, Ewert P, Berger F, et al. (2005) Analysis of atrial and ventricular performance by tissue Doppler imaging in patients with atrial septal defects before and after surgical and catheter closure. Echocardiography 22: 579-585.
- Inaba Y, Yuda S, Kobayashi N, Hashimoto A, Uno K, et al. (2005) Strain rate imaging for noninvasive functional quantiï¬Âcation of the left atrium: Comparative studies in controls and patients with atrial ï¬Âbrillation. J Am Soc Echocardiogr 18: 729-736.
- Cameli M, Lisi M, Focardi M, Reccia R, Natali BM, et al. (2012) Left atrial deformation analysis by speckle tracking echocardiography for pre-diction of cardiovascular outcomes. Am J Cardiol 110: 264-269.
- Shaikh AY, Maan A, Khan UA, Aurigemma GP, Hill JC, et al. (2012) Speckle echocardiographic left atrial strain and stiffness index as predictors of maintenance of sinus rhythm after cardioversion for atrial fibrillation: a prospective study. Cardiovasc Ultrasound 10: 48.
- Kadappu KK, Abhayaratna K, Boyd A, French JK, Xuan W, et al. (2016) Independent echocardiographic markers of cardiovascular involvement in chronic kidney disease: the value of left atrial function and volume. J Am Soc Echocardiogr 4: 359-367.
- Dimitroula H, Damvopoulou E, Giannakoulas G, Dalamanga E, Dimitroulas T, et al. (2010) Effects of renin-angiotensin system inhibition on left atrial function of hypertensive patients: an echocardio-graphic tissue deformation imaging study. Am J Hypertens 23: 556-561.
- Kokubu N, Yuda S, Tsuchihashi K, Hashimoto A, Nakata T, et al. (2007) Noninvasive assessment of left atrial function by strain rate imaging in patients with hypertension: a possible beneficial effect of renin-angiotensin system inhibition on left atrial function. Hypertens Res 30: 13-21.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi