Research Article, J Mar Biol Oceanogr Vol: 6 Issue: 2
Main Ecological Features of Benthic Macrofauna in Mediterranean and Atlantic Intertidal Eelgrass Beds: A Comparative Study
Nawfel Mosbahi1, Hugues Blanchet2,3, Nicolas Lavesque2,3, Xavier de Montaudouin2,3, Jean-Claude Dauvin4* and Lassad Neifar1
1Laboratoire de Biodiversité et Ecosystèmes Aquatiques, Faculté des Sciences de Sfax, Université de Sfax, BP 1171, 3038, Sfax, Tunisie
2Univ. Bordeaux, EPOC, UMR 5805, Station Marine d’Arcachon, 2 Rue du Professeur Jolyet, 33120 Arcachon, France
3CNRS, EPOC, UMR 5805, Station Marine d’Arcachon, 2 Rue du Professeur Jolyet, 33120 Arcachon, France
4Normandie Univ, UNICAEN, UNIROUEN, Laboratoire Morphodynamique Continentale et Côtière, CNRS, UMR 6143 M2C, 24 Rue des Tilleuls, 14000 Caen, France
*Corresponding Author : Jean-Claude Dauvin
Normandie Univ, UNICAEN, UNIROUEN, Laboratoire Morphodynamique Continentale et Côtière, CNRS, UMR 6143 M2C, 24 Rue des Tilleuls, 14000 Caen, France
Tel: +33(0)231565722
E-mail: jean-claude.dauvin@unicaen.fr
Received: June 16, 2017 Accepted: July 19, 2017 Published: July 25, 2017
Citation: Mosbahi N, Blanchet H, Lavesque N, Montaudouin X, Dauvin JC, et al. (2017) Main Ecological Features of Benthic Macrofauna in Mediterranean and Atlantic Intertidal Eelgrass Beds: A Comparative Study. J Mar Biol Oceanogr 6:2. doi: 10.4172/2324-8661.1000174
Abstract
Main Ecological Features of Benthic Macrofauna in Mediterranean and Atlantic Intertidal Eelgrass Beds: A Comparative Study
The present study compares the intertidal eelgrass macrofauna in two geographically and ecologically disparate localities (central Mediterranean and eastern Atlantic). Both coastal ecosystems are developed on extensive large mudflats with eelgrass beds, hosting a great diversity of water birds and providing important socio-economic assets. These two distinct and distant geographical ecosystems are affected by numerous anthropogenic pressures. By reflecting the response of the structure and functioning of benthic communities to climate change, the two eelgrass ecosystems provide a natural laboratory to investigate global warming. The macrobenthic fauna community of Zostera (Zosterella) noltei eelgrass beds was studied by sampling 34 stations in the Kneiss Islands and 48 stations in Arcachon Bay. A total of 148 species are identified in the Kneiss islands and 117 species in Arcachon Bay, but only 23 species are common to both ecosystems. Diversity, abundance and community structure are significantly different between the two study areas, which could be explained by differences between Mediterranean and Atlantic climatic conditions and by anthropic factors (e.g. fishing pressure, pollution, nutrient inputs) affecting each ecosystem. Multidimensional scaling (n-MDS) analysis identifies two distinct geographical station groups on the basis of species and familylevel abundance. On the contrary, three assemblages are identified on the basis of trophic groups distributed between the separate ecosystems. In terms of ecological quality status, the Kneiss site appears to have a good ecological condition and hosts a variety of sensitive species. On the other hand, biotic indices indicate that the Arcachon site is moderately perturbed and that the benthic communities are unbalanced. It is expected that the present-day functioning of the Kneiss Islands ecosystem will become typical of the situation in Arcachon Bay in several decades time, with the development of warmer and drier conditions.
Keywords: Zostera noltei meadows; Benthic communities; Anthropogenic pressures; Climate warming; Kneiss Islands; Arcachon Bay
Introduction
Sheltered and semi-sheltered coastal ecosystems rank among the most productive and important aquatic ecosystems on Earth [1,2]. These complex environments fulfil several vital functions which play a key role in controlling biodiversity, such as nursery and feeding areas for fish and birds [3,4]. Coastal ecosystems are often formed by a mosaic of interlinked habitats that should not be considered in isolation. However, each habitat has its own characteristics and hosts particular benthic assemblages [5-7].
Eelgrass meadows are distributed in intertidal and subtidal areas from tropical to temperate zones [8-10], representing important ecological and economic components of coastal zones worldwide [11,12]. Eelgrass meadows provide high-value ecosystem services with among the highest levels of primary production for submerged aquatic communities [13]. As engineer species, they attract and support rich faunistic assemblages [14,15] providing food and refuge for many commercial species and enhancing nutrient cycling, water quality and sediment stabilization [16-18].
The Kneiss Islands (Central Mediterranean) and Arcachon Bay (North-Eastern Atlantic) are two coastal ecosystems sharing many similar features (Table 1). They both comprise extensive mudflats covered with Zostera noltei meadows, alternating with a network of shallow tidal channels [6,19,20]. They host a great diversity of water birds species with different ecological requirements, and have consequently been recognized as Important Bird Areas [21]. Indeed, more than 45,000 water birds belonging to 50 species have been counted yearly on the wetlands of the Kneiss Islands [22] and more than 115,000 individuals belonging to 66 species in Arcachon Bay [23].
| Parameters/ Ecosystem | Kneiss Islands, Tunisia | Arcachon Bay, France | |
|---|---|---|---|
| Geomorphology | Latitude-Longitude | 34°20’N,10°9’E | 44°40’N,1°10’W |
| Surface (km²) | 220 | 180 | |
| Freshwater input (m3 yr-1) | Low | 1.25×109 | |
| Sea communication | Mediterranean Sea | Atlantic ocean | |
| Presence of islands | Four | One | |
| Intertidal flats (km²) | 147 | 117 | |
| Tidal channels (km²) | 73 | 63 | |
| Mean and maximal depth (m) | 6 and 10 | 8 and 15 | |
| Climate Water body |
Annual rain flow (mm) | 180-200 | 800-900 |
| Tidal range (m) | 0.3-2.3 | 0.9-4.9 | |
| Temperature: mean (range) (°C) | 22 (11-32) | 15 (5-25) | |
| Salinity | 37-41 | 25-35 | |
| pH | 7.80-8.15 | 8.2 | |
| Dissolved oxygen (mg.L-1) | 7-8 | 6-10 | |
| Transparency: mean (range) (FNU) | 3 (2-8) | 2 (1-6) | |
| Sediment | Organic matter (%) | 3-7 | 0-10 |
| Sediment type | Mud & muddy sand | Sand & mud | |
| Schorre (km²) | 6 | 8 | |
| Intertidal Zostera noltei (km²) | 68 | 70 | |
| Subtidal angiosperms (km²) | 5 | 3 | |
| Status, vertebrate occurrence | National importance | Nature reserve | Marine National Park |
| International importance | SPAMI, IBA, RAMSAR | IBA, Site Natura 2000 | |
| Mammal and turtles | Dolphin, turtle | Seal | |
| Water birds (number of species) | 50 | 66 | |
| Anthropic factors | Alien species (number) | 139 | 50 |
| Shellfishing | Clam, razor clam, mussels, crustaceans | Clam, cockle, mussel, shrimp | |
| Finfishing | Present | Present | |
| Cephalopod fishing | Cuttle fish, octopus | Cuttle fish | |
| Bait digging | Present | Present | |
| Aquaculture | Fish | Oyster | |
| Bottom trawling | Present | Absent | |
| Oil pollution | Not significant | Not significant | |
| Nutrients input | Absent | Present | |
| Macroalgae blooms | Not significant | Locally, occasionally | |
| Phytoplankton blooms | Present | Present | |
| Toxic blooms | Present | Present | |
| Dredging & sediment deposition | Absent | Present | |
| Phosphate industry | Present | Absent | |
| Coastal urbanisation | Not significant | Significant | |
| Coastal tourism | Absent | Significant | |
| Large coastal towns | Absent | Significant | |
| Agricultural activities | Not significant | Significant | |
| Tourism activities | Absent | Significant | |
| Marinas | Absent | Present | |
| Commercial port | Skhira (hydrocarbons and phosphates | Absent | |
| Fish port | One | One (small) |
Table 1: General characteristics of Kneiss Islands, and Arcachon Bay.
Likewise, the intertidal areas are of both ecological and socioeconomic interest, especially for traditional activities such as crustacean fishing, bait digging and clam harvesting [24] (Table 1). The latter activity is mainly artisanal in Tunisia and both artisanal and professional in France, plays an important economic role in both countries. For example, clam harvesting provides up to 45% of the total production in Tunisia [25], while ca. 40% of the aquaculture production of the Manila (Asari) clam Ruditapes philippinarum in France comes from Arcachon Bay [26].
Arcachon Bay has also developed intense activities involving oyster culture and tourism (including power boating), while the Kneiss Islands is subject to stronger influence from industrial fishing as well as artisanal bottom trawling which is banned in Arcachon Bay.
However, we consider that climate is the most significant factor which could impact the functioning of communities in these two broadly similar seagrass ecosystems, with the Kneiss Islands on average undergoing drier and warmer conditions than Arcachon Bay (Table 1). According to climate change predictions [27], warmer and drier conditions could prevail in Arcachon Bay in the future, leading to a situation comparable to the present-day Kneiss Islands. It is well accepted that climatic variation along latitudinal gradients provides an excellent natural laboratory to investigate the role of temperature and the potential impacts of climate warming at different sites [28-30]. Moreover, the climatic gradient is expected to have an even greater impact in intertidal/ shallow systems than in deeper subtidal systems [31-33].
The main aims of this study are: 1) to compare the structural assemblages and diversity of the benthic macrofauna community associated with Zostera noltei intertidal eelgrass beds in the Kneiss Islands and Arcachon Bay ecosystems based on taxonomic and ecological approaches as well as the study of trophic groups. While taxonomic differences are expected between contrasted biogeographic areas, this study focuses on similarities and proposes some possible explanations (exotic species present in one area or in both, species with particularly extensive range worldwide, eventually related to efficient larval dispersal); 2) to provide a reliable assessment of the general ecological status in each ecosystem, testing different benthic indicators of ecological quality. The expected discrepancies between index values (between ecosystems but also within ecosystems) can help reveal the strengths and weaknesses of these indices in these particular habitats (seagrasses); 3) to identify the role of the main environmental factors that determine the structure and functioning of the benthic community, using the latitudinal gradient to predict the response of benthic communities in these geographically distinct ecosystems; and 4) to compare one aspect of functional trait diversity (i.e. trophic diversity) in seagrass habitats of the Kneiss Islands and Arcachon Bay. This comparison aims to hierarchize the factors structuring benthic communities in seagrass environments, leading us to consider two main alternative hypotheses: either i) both areas display a similar trophic diversity, and we can assume that Zostera noltei is a highly effective engineer species structuring benthic functional diversity (in this case trophic functioning), independently of other factors (climate, anthropic activity, sediment grain-size, etc.); or ii) trophic guilds are contrasted between the two studied areas, suggesting that the presence of seagrass does not on its own ensure the stability of functioning of this habitat and that functioning of the benthic communities can be modified by global change independently of the physical presence of eelgrasses.
Materials and Methods
Study area
Kneiss islands: The Kneiss Islands are located in the Gulf of Gabès (South Eastern Tunisia), characterized by an extensive continental shelf (34°10’-34°30’ N and 10-10°30’E) (Figure 1 and Table 1). Shoals of shallower depth are developed around four islets. The total surface-area of the studied area (220 km²) can be divided into two main sectors: the subtidal channels (maximum depth of 10 m) and the intertidal zone [20,34]. The tide is semi-diurnal, with amplitude varying from 0.3 to 2.3 m [35]. The seawater temperatures vary seasonally between 11.3°C to 32.0°C. The salinity ranges between 37 and 41 ppt, according to annual precipitation, which does not exceed 200 mm, as well as inputs of freshwater from Ouadrane wadi during flood periods. At low tide, the Kneiss Islands are surrounded by vast mud and sand flats [36], with an abundant and diversified benthic macrofauna [20], making this site the most important area for wintering of migratory waders in the Mediterranean region [37]. The intertidal mudflats of the Kneiss Islands are colonized by Zostera noltei eelgrass beds (68 km2). Due to their marine biodiversity, the Kneiss Islands were established as a “Specially Protected Area of Mediterranean Importance” (SPAMI) in 2001, an “Important Bird Area” (IBA) in 2003 and designated as a “RAMSAR site” since 2007.
Arcachon Bay
Arcachon Bay is a triangular-shaped macro-tidal area situated on the South Western coast of France (44°40’ N, 1°10’ W) (Figure 1 and Table 1). It communicates with the Atlantic Ocean through a 2km wide channel. The tide is semi-diurnal, with amplitude varying from 0.8 to 4.6 m. The average sea water temperatures vary seasonally between 6°C and 22.5°C, and fluctuations in freshwater inputs from rivers and rainwater influence the water salinity, which ranges between 22 and 35. The total surface-area of the bay (180 km²) can be divided into two domains: the subtidal channels (63 km²), and the intertidal zone (117 km²). The main channels have a maximum depth of 25 m and are fed by a secondary network of shallower channels. The intertidal zone comprises sandy to sandy-mud flats, with most of their area (60%, i.e. 70 km²) being covered by Zostera noltei eelgrass beds (70 km²) [6,38]. The lagoon receives freshwater inputs mostly from the Leyre River, situated in the South-Eastern part of the Bay, which contributes 73% of the total annual freshwater inflow (813 million m3y-1). Arcachon Bay represents a site of international interest in terms of ornithological diversity (Important Bird Area) [23] and Natura 2000 site.
Sampling procedure
The sampling method was similar for the two study areas. At low tide, the top 20-30 cm of the sediment was collected with a 0.0225 m² corer, covering a total sampling area of 0.09 m² (i.e. four replicates per station). Using an unbalanced design, a total of 34 and 48 stations were sampled during the spring campaigns of 2013 and 2014 (Figure 1) in the Kneiss Islands (Figure 1) and 2002 in Arcachon Bay, respectively, when the eelgrass beds fully extended over the tidal flats. Sediment was sieved through a 1-mm mesh; the retained fraction was fixed in 4% buffered formalin and stained with Rose Bengal. In the laboratory, the macrofauna was sorted, identified to the lowest possible taxonomic level (usually species level) and counted.
Sediment analysis and organic matter
The topmost 3 cm sediment layer was also sampled for grainsize analysis. Sediment from each sample was homogenized and wet-sieved through a 63 μm mesh to separate mud (including silt and clay) and sandy fractions (retained in the sieve). After being oven dried to constant weight at 60°C, sandy fractions were separated using a mechanical shaker (column of six sieves of mesh sizes 2000, 1000, 500, 250, 125 and 63 μm) during 10 min. All fractions (including<63 μm) were then weighed and their percentages determined. For the organic matter content analyses, sediment samples were dried at 60°C to constant weight and ground to a fine powder. Organic matter content was determined on the powder samples by ‘loss on ignition’ at 450°C for 4 h.
Data analysis
Univariate analysis: Faunal parameters were calculated at each station to compare the macrozoobenthic biodiversity and ecological status of the studied areas: abundance A (number of individuals per m²), species number S (number of species per 0.09 m²), Shannon- Wiener index H’ [39] and evenness J’ [40]. The abundance of trophic groups was compared to highlight differences in the functioning of the benthic food web among both eelgrass systems. Species were classified into six trophic groups: non-selective deposit feeders (NSDF; burrowers which ingest the sediment from which they take their food), selective deposit feeders (SDF; taxa feeding on organic particles on the sediment surface), suspension feeders (SF; taxa feeding on suspended food in the water column), carnivores (C; predatory animals), herbivores (H; species mainly feeding from macrophytes, and micro-grazers (μG; feeding on benthic microalgae, bacteria and detritus). Identified species were classified into trophic groups according to Fauchald and Jumars [41] and notably modified by Grall and Glémarec [42], Hily and Bouteille [43], Afli and Glémarec [44], Pranovi et al. [45], Afli et al. [46] and Jumars et al. [47].
One-factor ANOVAs (Analyses of Variance) were carried out to test the differences in the values of abundance (total abundance), species richness, diversity index and evenness between the samples from Kneiss Islands and Arcachon Bay (Table 2). A post hoc Tukey test (p< 0.05) was used for a posteriori multiple comparisons. Analyses to test the normality (Kolmogorov-Smirnov) and verify the homogeneity of variances (Barlett) were carried out prior to each ANOVA. A chi-square test was used to determine the significance of differences in phylum and the trophic-group abundance between the ecosystems. These statistical procedures were performed using the software SYSTAT 20 (SPSS).
| Kneiss Islands | Arcachon Bay | ANOVA test | ||
|---|---|---|---|---|
| F | p | |||
| Total number of species / site | 148 | 117 | 24.85 | p<0.01* |
| Mean S (per 0.09 m²) | 39 | 27 | ||
| Mean A (ind/m-2) | 14,709 | 20,553 | 3.39 | p>0.05 ns |
| Mean H’ | 4.4 | 2.2 | 149.71 | p<0.01* |
| Mean J’ | 0.85 Good | 0.45 Moderate | 125.7 | p<0.01* |
| Mean AMBI | 0.74 Good | 0.43 Moderate | 200 | p<0.01* |
| Mean M-AMBI | 0.029 High | 0.113 Good | 213.71 | p<0.01* |
| Mean BO2A | 0.8 Good | 0.7 Good | 39.02 | p<0.01* |
| Mean d-MISS | 1.6 Good | 3.5 Moderate | 112.18 | p>0.05 ns |
Table 2: Main characteristics of structural indices used to qualify the ecological status (EcoQ) of the benthic macrofauna in both ecosystems: ns, not significant; (*), significant. EcoQ scores are given here for different biotic indices (AMBI, M-AMBI, BO2A, d-MISS).
Ecological indicators: Four currently available Benthic Indicators (BIs) were used, namely AMBI (AZTI Marine Biotic index) [48], M-AMBI [49], BO2A (Benthic Opportunistic Annelids Amphipods Ratio) [50,51] and d-MISS (Macrobenthic Index in Sheltered Systems) [19,52]. The first three biotic indices (BIs) are based on a classification of species into ecological groups according to their level of sensitivity/tolerance to stress. AMBI (AZTI Marine Biotic Index) is based on previous studies by Grall and Glémarec [42]. It considers five ecological groups [53] ranging from sensitive species (EGI) to first-order opportunistic species (EGV) [48]. M-AMBI (Multivariate AMBI) combines AMBI with the Shannon diversity and species richness, which are very strongly dependent on habitat type and many other factors. The application of M-AMBI requires the definition of reference conditions related to the typology under study [49]. The BO2A (Benthic Opportunistic Annelids Amphipods index) is based on the ratio of opportunistic polychaetes (i.e. polychaetes of ecological groups IV and V of the AMBI) and amphipods (except for the genus Jassa). The d-MISS (Macrobenthic Index in Sheltered Systems) index is a multimetric approach using 14 metrics describing the biological integrity of the macrobenthic fauna [19,54]
Multivariate analysis: Multivariate analysis was performed to compare the macrozoobenthic community structure of the two areas. Abundances were square-root transformed to minimize the influence of the most dominant taxa (for species and families). A non-metric multidimensional scaling method (n-MDS) based on the Bray-Curtis similarity allowed us to visually assess differences in macrofaunal assemblages among stations of the studied areas. SIMPER tests were used to determine which species contribute to within-group similarity. These analyses were performed using PRIMER®-v6 [55].
A similarity matrix was constructed from the fourth-root transformed abundance data using the Bray-Curtis similarity measure; non-metric multidimensional scaling (n-MDS) ordination was then applied to assess the differences in trophic groups between the two ecosystems.
Results
Sediment characteristics
Sediment grain-size analysis shows that sediments in the Kneiss Islands consist of muddy to muddy sands depending on the station, with median grain-size varying from 120 to 380 μm. In Arcachon Bay, sediments are mainly mud (mean grain-size=33-59 μm) with high silt and clay content (mean=57-75%). Organic matter content ranges from 3 to 7% in the Kneiss Islands and from 3 to 10% in Arcachon Bay. A comparison of sediment grain-size and organic matter contents between the two ecosystems reveals no significant difference (p>0.05).
Macrofaunal characteristics
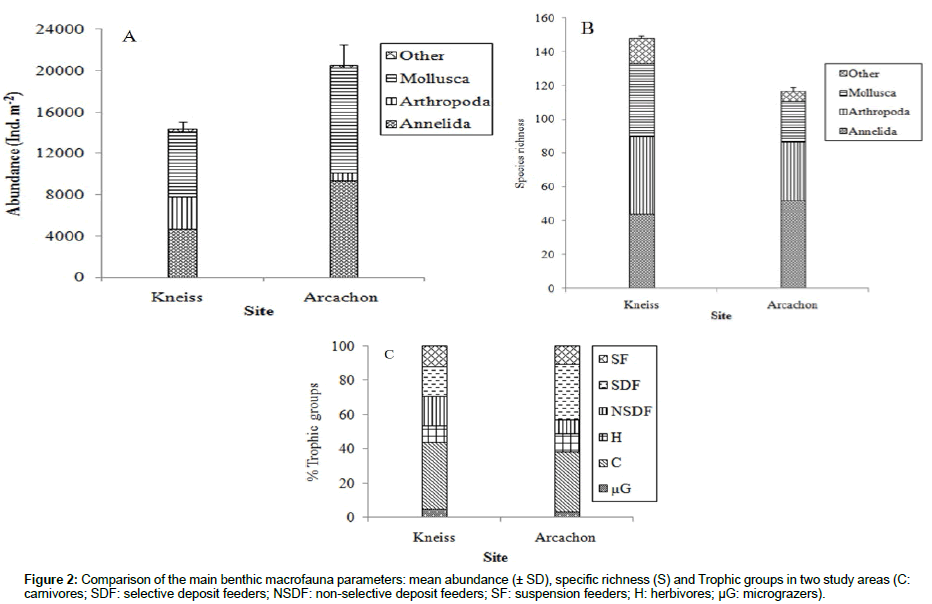
In the Kneiss Islands, a total of 148 species are identified from 34 stations, with an unequal distribution among sampling stations. The number of species in a sample (S) varies between 22 and 64 species per 0.09 m², with a mean of 39. Abundance (A) varies from 9,200 to 36,800 ind.m-2 (with a mean abundance of 14,709 ± SD=900 ind.m-2), evenness (J’) from 0.79 to 0.92 (mean= 0.85) and Shannon index (H’) from 3.5 to 5.2, with a mean of 4.4 bits ind-1 (Figure 2 and Table 2). In Arcachon Bay, 117 species are identified from 48 stations. The mean number of species observed in a sample (S) is 27.5, ranging from 8 to 49 species per 0.09 m². Abundance (A) ranges between 1,700 and 64,000 ind. m-2, with a mean abundance of 20,553 ± 2,100 ind. m-2. Finally, evenness (J’) varies from 0.19 to 0.87 (mean= 0.45) and Shannon index (H’) from 1.2 to 4.1, with a mean of 2.2 bits ind-1. The average values of S, J’ and H’ are higher in the Kneiss Islands than in Arcachon Bay (onefactor onefactor ANOVA; S: F=24.85; p<0.01; J’: F=125.7; p<0.01; H’: F=149.7; p<0.01). Abundance is similar in the Kneiss Islands and Arcachon Bay (ANOVA; F=3.39; p>0.05) (Figure 2A and Table 2). A total of 23 species are common to both ecosystems (Table 3), while 125 species are restricted to the Kneiss Islands, and 94 species to Arcachon Bay. The Kneiss Islands are characterized by a higher proportion of mollusc species (X2=5.388; p<0.05), while the proportions of arthropod and annelid species are similar in both systems (with X2=1.49; p>0.05 for arthropods and X2=0.66; p>0.05 for annelids) (Figure 2B).
| Phylum | Species |
|---|---|
| Annelida | Arenicola marina |
| Cirriformia tentaculata | |
| Euclymene oerstedii | |
| Eunice vittata | |
| Leiochone clypeata | |
| Marphysa sanguinea | |
| Melinna palmata | |
| Lysidice unicornis | |
| Nephtys hombergii | |
| Hediste diversicolor | |
| Platynereis dumerilii | |
| Crustacea | Ampelisca brevicornis |
| Melita palmata | |
| Microdeutopus gryllotalpa | |
| Corophium insidiosum | |
| Cyathura carinata | |
| Mollusca | Polititapes aureus |
| Ruditapes philippinarum | |
| Scrobicularia plana | |
| Bittium reticulatum | |
| Cyclope neritea | |
| Loripes orbiculatus | |
| Echinodermata | Amphipholis squamata |
Table 3: List of species, occurring both in the Kneiss Islands and Arcachon Bay.
For both ecosystems, annelids and molluscs are the dominant groups in terms of abundance, followed by arthropods (Table 4). In the Kneiss Islands, Scrobicularia plana, Cerithium scabridum and Pirenella conica are the three most abundant mollusc species (18% of total abundance), whereas Peringia ulvae, Abra segmentum and Bittium reticulatum are dominant in Arcachon Bay (47% of total abundance). In the Kneiss Islands, Cirratulus cirratus, Perinereis cultrifera and Euclymene lumbricoides are the most abundant annelid species. In Arcachon Bay, oligochaetes represent 33% of the total abundance.
| Site | Species | Phylum | Mean density per m² | % of presence |
|---|---|---|---|---|
| Kneiss Islands | Scrobicularia plana | Mollusca | 1010 | 71 |
| Cerithium scabridum | Mollusca | 866 | 91 | |
| Pirenella conica | Mollusca | 812 | 73 | |
| Cirratulus cirratus | Annelida | 780 | 79 | |
| Loripes orbiculatus | Mollusca | 529 | 85 | |
| Perinereis cultifera | Annelida | 528 | 38 | |
| Euclymene lombricoides | Annelida | 430 | 73 | |
| Gammarus insensibilis | Crustacea | 414 | 62 | |
| Bittium reticulatum | Mollusca | 392 | 50 | |
| Euclymene oerstedii | Annelida | 335 | 53 | |
| Arcachon Bay | Peringia ulvae | Mollusca | 9002 | 96 |
| Oligochaeta | Annelida | 6795 | 40 | |
| Heteromastus filiformis | Annelida | 967 | 94 | |
| Abra segmentum | Mollusca | 592 | 94 | |
| Melinna palmata | Annelida | 332 | 37 | |
| Pygospio elegans | Annelida | 274 | 48 | |
| Idotea chelipes | Crustacea | 189 | 90 | |
| Bittium reticulatum | Mollusca | 149 | 69 | |
| Chironomidae | Crustacea | 144 | 52 | |
| Nemertina | Nemertina | 137 | 92 |
Table 4: Top dominant taxa in the Kneiss Islands and Arcachon Bay.
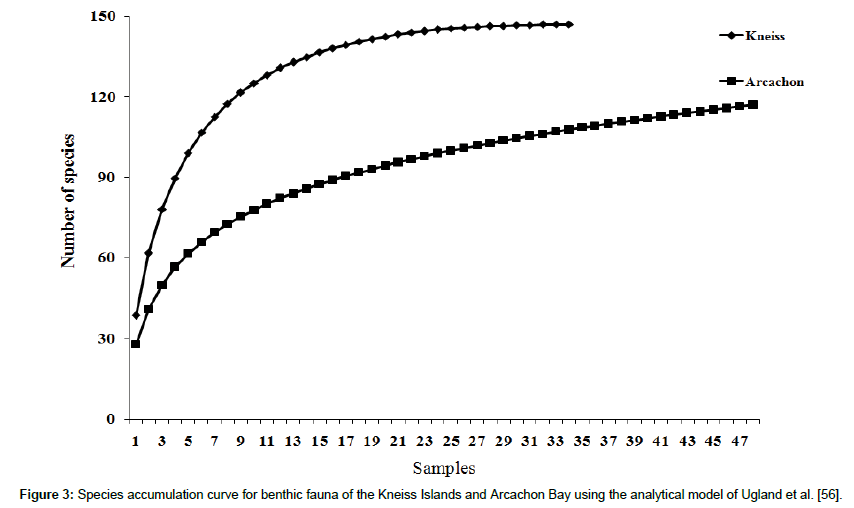
Based on the analytical model of Ugland et al. [56], the number of species recorded in the Kneiss Islands is approaching an asymptote, while the number of species continues to rise with sampling effort in the case of Arcachon Bay. This suggests that, in the case of Arcachon Bay, the sampling effort has probably been insufficient to record all the species present in this habitat (Figure 3). For a similar effort (34 stations), the expected number of species is 148 in the Kneiss Islands, as against 117 for Arcachon Bay (Figure 3).
Figure 3: Species accumulation curve for benthic fauna of the Kneiss Islands and Arcachon Bay using the analytical model of Ugland et al. [56].
The trophic structure analysis shows that, in the Kneiss Islands, carnivores (40%) and NSDF (18%) are the dominant trophic groups in terms of abundance. In Arcachon Bay, carnivores (36%), and SDF (33%) are the two most abundant trophic groups. However, SDF is the only trophic group displaying a different proportion (higher in Arcachon Bay) (micrograzers: X2=0.02; p>0.05; carnivores: X2=0.22; p>0.05; NSDF: X2=2.79; p>0.05; suspension feeders: X2=0.48; p>0.05; SDF: X2=4.90; p<0.05) (Figure 2C).
Ecological status
Out of the four biotic indices compared between the two studied areas, three of them provide different results in terms of ecological quality status. AMBI and M-AMBI indicate that the Kneiss Islands have a good ecological status compared to Arcachon Bay, which shows a moderate status. BO2A yields a high status for the Kneiss Islands and a good status for Arcachon Bay (p<0.01). The values of d-MISS are similar in both ecosystems, and correspond to good ecological status (ANOVA, F=112.18; p>0.05) (Table 2).
Identification of macrofaunal assemblages
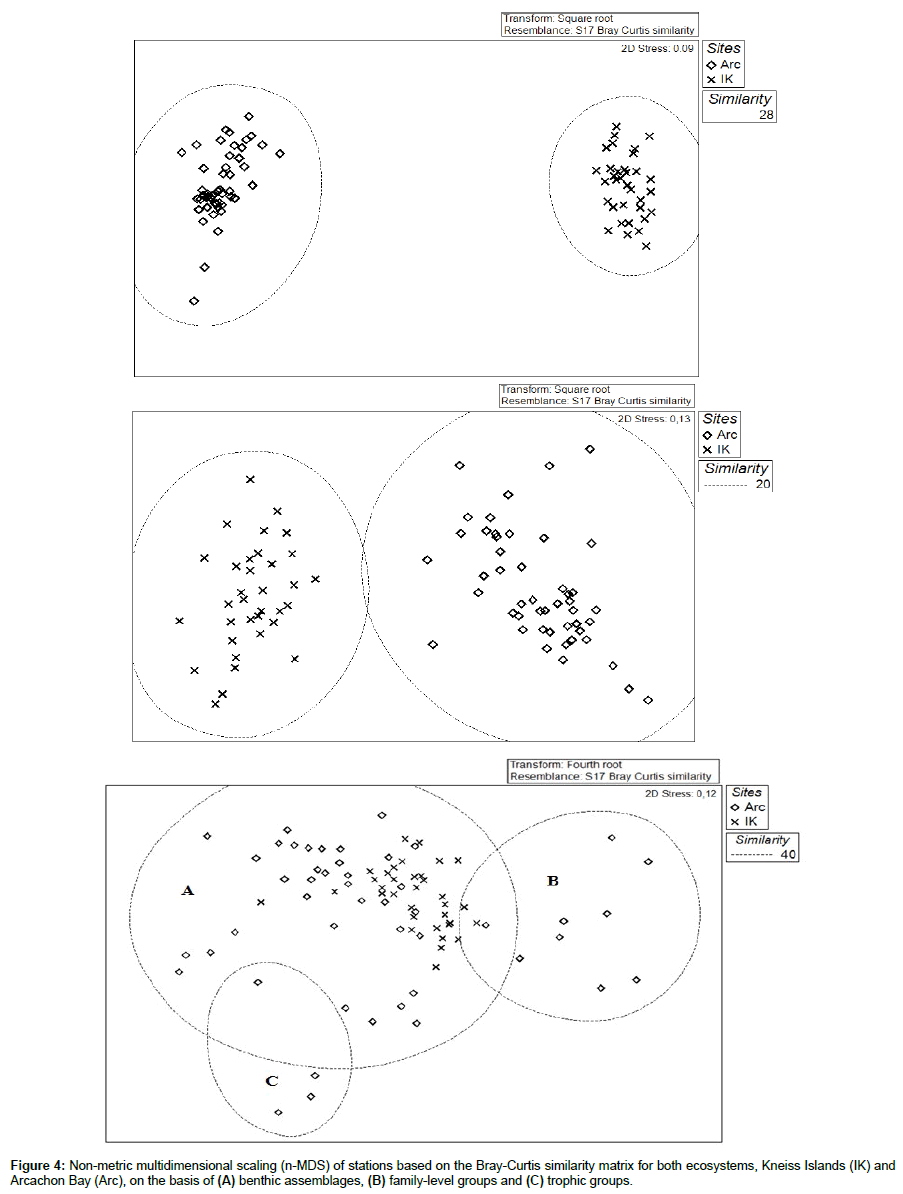
Using the MDS ordination plot based on species, we can discriminate two different groups, one corresponding to the Kneiss Islands and the other to Arcachon Bay (Figure 4A). Both ecosystems are also clearly separated on the basis of clades (i.e. molluscs, arthropods, annelids, etc.) (Figure 4B). Conversely, we find certain trends of similarity on the basis of trophic groups (Figure 4C). Three groups of stations are distinguished. Based on trophic groups (Figure 4C), the largest group (A) includes all the stations in the Kneiss Islands and the most of the stations in Arcachon Bay (67%). The two other groups (B and C) are restricted to stations in Arcachon Bay, located in a more exposed oceanic position: the station group B (12 stations) is dominated by NSDF, and is mostly represented by Heteromastus filiformis and oligochaetes. The station group C (4 stations) mainly includes SDF and carnivore species, such as Melinna palmata, Hexaplex trunculus and Anthozoa.
According to SIMPER analysis, Arcachon Bay is mainly characterized by the mud snail Peringia ulvae, as well as Oligochaetes, Heteromastus filiformis, Abra segmentum, Idotea chelipes, Nemertina, Ruditapes philippinarum and Bittium reticulatum. The macrofaunal community in the Kneiss Islands is represented by Cerithium scabridum, Cirratulus cirratus, Scrobicularia plana, Pirenella conica, Loripes orbiculatus, Ruditapes decussatus, Cerastoderma glaucum, Euclymene lombricoides and Cerithium vulgatum.
Discussion
Many studies on macrobenthic eelgrass bed communities have compared parameters such as abundance, biomass or species richness between adjacent sediments with or without vegetation [57,58], or between areas with different kind of vegetation [59,60]. On the other hand, there are few studies comparing macrobenthic communities in eelgrass beds at different spatial scales [61]. The originality of the present study is to compare the structure of benthic communities associated with intertidal Zostera noltei beds in two geographically distinct ecological communities, i.e. a Mediterranean ecosystem (Kneiss Islands) and a French Atlantic coastal ecosystem (Arcachon Bay). The aim of this approach is to identify differences and similarities between two similar habitats in terms of spatial heterogeneity (i.e. eelgrass physical presence) and to understand the response of benthic communities to other environmental variables, particularly climate, independently of the biogeographic area. In other words, we investigate whether the structure and functioning of macrofaunal communities in Z. noltei meadows can be compared at a large scale
A total of 232 taxa are recorded, associated with eelgrass beds, unequally distributed among the sampling stations. Annelids and molluscs are the dominant groups at both sites. High species diversity and abundance are frequently reported for macrofauna from seagrass habitats [61-66] compared with unvegetated sediments. Eelgrass meadows increase habitat complexity and provide living space and shelter for a diverse animal community [67,68]. These differences are related to the above-ground component of eelgrass, favouring the successful recruitment and colonization of animals, and the belowground structural complexity of the interlacing rhizome layer and roots increasing sediment stability [69-71].
A total of 148 species are identified In the Kneiss Islands and 117 species in Arcachon Bay, these values describing the γ-diversity of each site. The total number of species in the Kneiss Islands is higher on average than in Arcachon Bay, even when taking into account the difference in sampling effort. The species-accumulation curve for the Kneiss Islands stabilizes around an asymptotic value suggesting a correct assessment of γ-diversity. However, the lack of an asymptotic value for Arcachon Bay implies that the sampling effort is insufficient to provide an exhaustive assessment of diversity. This curve also indicates that diversity is not homogeneously distributed within Arcachon Bay, where an increased number of stations would have yielded ‘more species’. This may be related to a greater diversity of habitats within the eelgrass beds in Arcachon Bay compared to the Kneiss Islands, i.e. a higher β-diversity [72].
A comparison of faunal composition between Z. noltei meadows at the two sites shows the existence of 23 ‘shared’ species (i.e. common to both sites). Loripes orbiculatus Poli, 1791 (lucinid bivalve) is the most common bivalve species. This species has also been observed in other climatic zones and is present in 97% of tropical eelgrass sites, 90% of subtropical meadows, and 56% of temperate eelgrass beds. The presence of L. orbiculatus in eelgrass is also related to the special adaptation of lucinid bivalves to sulphide-rich sediments, due to symbiosis with gill bacteria [73]. Thus, seagrass meadows may offer an optimal habitat for these bivalves and their symbionts by indirectly stimulating sulphide production through high organic matter input, while also providing oxygen through radial oxygen release from the roots. In turn, lucinids remove sulphide from the sediment, which could relieve stress on eelgrass growth caused by sulphide accumulation due to the degradation of organic matter [73-75].
The abundance of macrobenthic fauna communities in both ecosystems is similar, but with different patterns. The Kneiss Islands represent a balanced species abundance pattern, as shown by high values of H’ and J’. By contrast, the macrobenthic community associated with Z. noltei beds in Arcachon Bay is dominated by small grazing molluscs such as Peringia ulvae (Pennant, 1777) (44% of total abundance), and oligochaete deposit-feeders (33% of total abundance). The abundance of these dominant taxa is similar to that observed in other Atlantic Z. noltei beds, including brackish habitats in the North Sea and Atlantic areas [69,76-78].
The differences in diversity between Arcachon Bay and the Kneiss Islands is probably a response to human activities (e.g. fishing pressure, port and aquaculture activities, as well as nutrient inputs), environmental factors (sediments characteristics, salinity) and different climatic conditions. In fact, the Gulf of Gabès is a semi-arid Mediterranean coastal zone [79], characterized by an arid climate (average annual precipitation: ≤ 200 mm year-1) with higher temperature and salinity. The invertebrate macrofauna in this inshore region is directly under the influence of salty and warm Mediterranean waters well known for their high intrinsic diversity [80,81]. General oceanographic conditions in the Mediterranean basin have been described in detail elsewhere [82-84]. Areas bordering the Mediterranean Sea have a dry-summer subtropical type climate. The summer is hot and dry, and the winter is cool and rainy. These general features, along with some other regional characteristics such as fluctuations in fluvial inputs, temperature and salinity, give rise to the particularities of Mediterranean communities [85,86]. Afli et al. [46,87] proposed that environmental conditions, particularly temperature and salinity, play a major role in the structuring of communities and the exclusion of certain species or groups of species in Mediterranean ecosystems. This explains why the Kneiss Islands have a larger number of species not common to both sites and why a taxonomy-based MDS allows a clear discrimination between the sea grass habitats (Kneiss Islands and Arcachon Bay).
In both studied areas, the abundance of species within trophic groups appears similar. This suggests the consistency of a similar functioning of the benthic food web in these two geographically distant Zostera noltei habitats. In both ecosystems, the trophic structure is dominated by carnivores. Normally, the presence of carnivores in a balanced ecosystem should not exceed a certain proportion. Their role is to control the community and prevent the monopolization of resources (food and space) as well as competition from prey populations [44,88]. The results of the MDS analysis clearly show that three functional trophic groups can be identified in both eelgrass ecosystems: the major group is represented by all the stations of the Kneiss Islands and the majority of Arcachon stations, strongly dominated by carnivores, NSDF and SDF. The two other groups are restricted to the Arcachon Bay stations, mainly dominated by NSDF such as oligochaetes, and SDF such as Melinna palmata and Cirratulus cirratus. The similarity in trophic functioning detected between the two different eelgrass beds shows that, in response to the climate difference between the Mediterranean and the Atlantic Ocean, many species in the Kneiss Islands play the same ecological role in Arcachon Bay.
Beyond comparing the specific and trophic functioning of the Kneiss Islands and Arcachon Bay, we also analyse the biotic indices (BIs) (sensu Water Framework Directive). Most of these BIs are based on sensitivity to environmental stress. Therefore, independently of species composition, BIs represent a relevant way to compare the seagrass communities in terms of fitness, or at least the distribution of associated species according to their resistance to stress (mainly related to organic matter in the sediment). The overall pattern of ecological quality status is different according to the selected BI. All BIs, except d-MISS, reveal a difference between the ecosystems (p<0.01). AMBI, M-AMBI and BO2A all classify the Kneiss Islands seagrass as having good or high ecological status (unpolluted status), highly dominated by sensitive species (GI). The fact that the Shannon index tends to increase slightly in finer sediments is due to the important influence of species richness and abundance and the lack of ecological terms in the formulae. However, these three BIs classify Arcachon Bay as having a poor to moderate ecological status. These lower values are more closely related to the sediment type than pollution conditions and physical disturbances [89]. Indeed, eelgrass in Arcachon Bay is associated with fine sediments and high organic matter content. Consequently, since the proportion of opportunistic species is high, this also influences the BIs [89]. Generally, most of these BIs (e.g., AMBI and BO2A) yield poor scores for semi-enclosed ecosystems where the natural benthic habitat consists of muddy, organic matter enriched sediments [52,89]. As a result, biotic indices based on these functional groups (e.g., BENTIX and AMBI) are not well adapted to study different types of pollution, such as physical pollution or metal contamination [46,87,90]. d-MISS is based on 14 metrics describing the ecological community, trophic composition and pollution indicator species, and is considered to be more efficient than other BIs in detecting perturbations in this kind of ecosystem [19,52]. Unlike the other calculated indices, the d-MISS index places both the Kneiss Islands and Arcachon Bay ecosystems in the good ES category.
Climatic conditions in Arcachon Bay will change over the next decades [27]. Indeed, the temperature is expected to increase by 10°C up to 2100, and may induce lower fluvial input (-5% to - 35%) around the years 2046-2065. These conditions will be similar to those occurring currently in the Gulf of Gabès. Change in climatic conditions in the eastern Atlantic means that western stenothermal cold water species would be negatively affected by any future warming, ultimately leading to an increase of species diversity and a reduction in the abundance, biomass and benthic primary production of invertebrates resulting in modifications of the structure and functioning of the ecosystem [31,91]. Kröncke et al. [33] showed that most shifts in the community structure are directly or indirectly correlated with the variability of the North Atlantic Oscillation Index (NAOI) in winter, especially the increase in NAOI since the late 1980s. This has resulted in the increase in warm-temperate species, a decrease in cold-temperate species and/ or the invasion of non-indigenous species (NIS).
In conclusion, this study shows that biogeography (and the associated climate conditions) is of prime importance in structuring benthic communities at the scale of the planet. Indeed, two comparable seagrass ecosystems under different climatic conditions host different species. Conversely, the habitat itself shapes the functioning of an ecosystem: seagrass habitats in the Kneiss Islands (arid climate) and Arcachon Bay (temperate oceanic climate) display similarities in terms of distribution of trophic guilds and overall characteristics (d-MISS). Besides, we also propose an interesting way to predict the response of benthic communities to climate warming by comparing two contrasted ecosystems in coastal areas of the Mediterranean Sea and Atlantic Ocean. The direct effects of climate change impact the performance of individuals at various stages in their life history cycle through changes in physiology, morphology and behaviour. Climate also has an impact at the population level via changes in transport processes that influence dispersal and recruitment [92,93]. Community-level effects are mediated by interacting species (e.g. predators, competitors, etc.), and include climate-driven changes in both the abundance and per capita interaction capability of these species. The combination of these proximal impacts leads to emergent ecological responses, which involve modifications in species distributions, biodiversity, productivity and micro-evolutionary processes. Crucially, the long-term research programme over the coming decade should provide much of the additional information that is required to assess and mitigate the potential impacts of climate change in the seagrass ecosystems of the Kneiss Islands and Arcachon Bay.
Acknowledgements
This work was supported by the Tunisian Ministry of Higher Education and Scientific Research. Authors wish to thank MSN Carpenter post-edited the English style and grammar for the English revision.
References
- Taylor DI, Nixon SW, Granger SL, Buckley BA (1999) Responses of coastal lagoon plant communities to levels of nutrient enrichment: a mesocosm study. Estuaries 22: 1041-1056.
- Cardoso PG, Raffaelli D, Pardal MA (2008) The impact of extreme weather events on the seagrass Zostera noltii and related Hydrobia ulvae population. Mar Poll Bull 56: 483-492.
- Odum EP (1983) Basic Ecology. (2nd edtn), Saunders College Publishing, Philadelphia, USA.
- Mc Lusky DS (1989) The estuarine ecosystem. (2nd edtn), Blackie & Son, London, UK.
- Morrisey DJ, Skilleter GA, Ellis JI, Burns BR, Kemp CE, et al. (2003) Differences in benthic fauna and sediment among mangrove (Avicennia marina var. australasica) stands of different ages in New Zealand. Estuar. Coast Shelf Sci 56: 581-592.
- Blanchet H, de Montaudouin X, Lucas A, Chardy P (2004) Heterogeneity of macrozoobenthic assemblages within a Zostera noltii seagrass bed: diversity, abundance, biomass and structuring factors. Estuar Coast Shelf Sci 61: 111-123.
- Boström C, O'Brien K, Roos C, Ekebom J (2006) Environmental variables explaining structural and functional diversity of seagrass macrofauna in an archipelago landscape. J Exp Mar Biol Ecol 335: 52-73.
- Den Hartog C (1970) The seagrasses of the world. (1st edtn), North Holland Publishers, Amsterdam, The Netherlands.
- Phillips RC, Meñez EG (1988) Smithsonian contributions to the Marine Sciences: Seagrasses. Smithsonian Institution Press, Washington DC. USA.
- Green EP, Short FT (2003) World Atlas of Seagrasses. University of California Press, California. USA.
- Waycott M, Duarte CM, Carruthers TJB, Orth RJ, Dennison WC, et al. (2009) Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proceedings of the National Academy of Sciences of the United States of America. 106: 12377 -12381.
- Larkum AWD, Orth RJ, Duarte CM (2006) Seagrasses: biology, ecology and conservation. (1st edtn), Springer. The Netherlands.
- Hillman K, Mc Comb, AJ, Walker DI (1995) The distribution, biomass and primary production of the seagrass Halophila ovalis in the Swan Canning estuary, Western Australia. Aqua Bot 51: 1- 54.
- Hemminga MA, Duarte CM (2000) Seagrass Ecology. (1st edtn), Cambridge University Press, Cambridge. UK.
- Williams SL, Heck Jr K.L. (2001) Seagrass community ecology: Marine Community Ecology. (1st edtn), Sinauer Associates, Massachusetts, USA.
- Duarte C.M (2002) The future of seagrass meadows. Envir Conser 29: 192- 206.
- Airoldi L, Beck MW (2007) Loss, status and trends for coastal marine habitats of Europe. Oceanog Mar Biol Ann Rev 45: 345-405.
- Ganthy F, Sottolichio A, Verney R (2013) Seasonal modification of tidal flat sediment dynamics by seagrass meadows of Zostera noltii (Bassin d'Arcachon, France). J Mar Sys 109-110.
- Do VT, de Montaudouin X, Blanchet H, Lavesque N (2012) Seagrass burial by dredged sediments: Benthic community alteration, secondary production loss, biotic index reaction and recovery possibility. Mar Poll Bull 64: 2340-2350.
- Mosbahi N, Boudaya L, Dauvin JC, Neifar N (2015) Spatial distribution and abundance of intertidal benthic macrofauna in the Kneiss Islands (Gulf of Gabès, Tunisia). Cah Biol Mar 56: 319-328.
- http://www.birdlife.org/
- Hamza F, Hammouda A, Chokri MA, Selmi S (2014) Wintering Waterbirds in the central area of the Gulf of Gabès in south-eastern Tunisia. J Afr Ornithol 87: 217-223.
- LPO (2011) Counting wetlands international assessment January 2010 site: Bassin d'Arcachon-3304. Editor: Amandine Theillout-LPO Aquitaine Reference: WI20113304.
- Mosbahi N, Pezy JP, Dauvin JC, Neifar L (2016) Short-term impact of bait digging on intertidal macrofauna of tidal mudflats around the Kneiss Islands (Gulf of Gabès, Tunisia). Aqua Liv Res 28: 111-118.
- Food and Agriculture Organization (2011) Guide to fishing gear used in Ghannouch and Akarit (Gulf of Gabes, Tunisia). Malaga, Spain.
- de Montaudouin X, Arzul I, Caill-Milly N, Khayati A, Labrousse J-M, et al. (2016a) Asari clam (Ruditapes philippinarum) in France: history of an exotic species 1972-2015. Bull Fish Res Agen Jap 2: 35-42.
- Le Treut H (2013) The impacts of climate change in Aquitaine. Revue Dynamiques environnementales, University Press of Bordeaux, France.
- Gam M, de Montaudouin X, Bazaïri H (2010) Population dynamics and secondary production of the cockle Cerastoderma edule: comparison between Merja Zerga (Moroccan Atlantic Coast) and Arcachon (French Atlantic coast). J Sea Res 63: 191- 201.
- De Frenne P, Graae BJ, Rodríguez-Sanchez F, Kolb A, Chabrerie O, et al. (2013) Latitudinal gradients as natural laboratories to infer species’ responses to temperature. J Ecol 101: 784-795.
- de Montaudouin X, Blanchet H, Bazairi H, Nazik A, Desclaux-Marchand C, et al. (2016b) Cockle infection by Himasthla quissetensis - II. The theoretical effect of climate change. J Sea Res 113: 108- 114.
- Hawkins SJ, Sugden HE, Mieszkowska N, Moore PJ, Poloczanska E, et al. (2009) Consequences of climate-driven biodiversity changes for ecosystem functioning of North European rocky shores. Mar Ecol Progr Ser. 396: 245-259.
- Hinz H, Capasso E, Lilley M, Frost M, Jenkins SR (2011) Temporal differences across a bio-geographical boundary reveal slow response of sub-littoral benthos to climate change. Mar Ecol Prog Ser 423: 69- 82.
- Kröncke I, Reiss H, Eggleton JD, Aldridge J, et al. (2011) Changes in North Sea macrofauna communities and species distribution between 1986 and 2000. Estuar Coast Shelf Sci 94: 1-15.
- Bali M, Gueddari M (2011) Les chenaux de marée autour des îles de Kneiss, Tunisie: sédimentologie et évolution. Hydr Sci J 56: 498-506.
- Sammari C, Koutitonsky VG, Moussa M (2006) Sea level variability and tidal resonance in the Gulf of Gabès, Tunisia. Conti Shelf Res 26: 338-350.
- Abdennadher A, Ramirez F, Romdhane MS, Ruiz LJ, Sanpera C (2010) Biomonitoring of coastal areas in Tunisia: Stable isotope and trace element analysis in the Yellow-legged Gull. Mar Poll Bull 60: 440-447.
- Hamza F, Hammouda A, Selmi S (2015) Species richness patterns of waterbirds wintering in the gulf of Gabès in relation to habitat and anthropogenic features. Estuar Coast Shelf Sci 165: 254- 260.
- Plus M, Sebastien D, Gilles T, Isabelle A, de Montaudouin X, et al. (2010) Long-term evolution (1988-2008) of Zostera spp. meadows in Arcachon Bay (Bay of Biscay). Estuar Coast Shelf Sci 87: 357- 366.
- Shannon CE, Weaver W (1963) The Mathematical theory of communication. (13th edtn), University Illinois Press, Urbana, USA.
- Pielou EC (1966) Shannon’s formula as a measure of specific diversity: its use and measure. Amer Natur 100: 463-465.
- Fauchald K., Jumars PA (1979) The diet of worms: a study of Polychaete feeding guilds. Ocean. Ann Rev Mar Biol 17: 173-284.
- Grall J, Glémarec M (1997) Biodiversity of maërl funds in Brittany: functional approach and anthropogenic impacts. Life Mil 47: 339-349.
- Hily C, Bouteille M (1999) Modifications of the specific diversity and feeding guilds in intertidal sediment colonized by an eelgrass meadow (Zostera marina) (Brittany, France). Comp Rend Acad Sci 322: 1121- 1131.
- Afli A, Glémarec M (2000) Long-term fluctuation of macrobenthic stands in the eastern Gulf of Moriban (Brittany, France). Cah Biol Mar 41: 67-89.
- Pranovi F, Curiel D, Rismondo A, Marzocchi M, Scattolin M (2000) Determination of food sources for benthic invertebrates in a salt marsh (Aiguillon Bay, France) by carbon and nitrogen stable isotopes: importance of locally produced sources. Open J Mar Sci 64: 303-388.
- Afli A, Ayari R, Brahim M (2008) Trophic organization of the macro-zoobenthic assemblages within coastal areas subjected to anthropogenic activities. J Mar Biol Ass UK 88: 663-674.
- Jumars PA, Dorgan KM, Lindsay SM (2014) Diet of Worms Emended: An Update of Polychaete Feeding Guilds. Ann Rev Mar Sci 7: 497-520.
- Borja A, Franco J, Perez V (2000) A marine Biotic Index to establish the ecological quality of soft-bottom benthos within European estuarine and coastal environments. Mar Poll Bull 40: 1100- 1114.
- Muxika I, Borja A, Bald J (2007) Using historical data, expert judgement and multivariate analysis in assessing reference conditions and benthic ecological status, according to the European Water Framework Directive. Mar Poll Bull 55: 16- 29.
- Dauvin JC, Ruellet T (2007) Polychaete/amphipod ratio revisited. Mar Poll Bull 55: 215- 224.
- Dauvin JC, Ruellet T (2009) The estuarine quality paradox: Is it possible to define an ecological quality status for specific modified and naturally stressed estuarine ecosystems? Mar Poll Bull 59: 38- 47.
- Lavesque N, Blanchet H, de Montaudouin X (2009) Development of a multimetric approach to assess perturbation of benthic macrofauna in Zostera noltii beds. J Exp Mar Biol Ecol 368: 101-112.
- http://www.azti.es
- Do VT, Blanchet H, de Montaudouin X, Lavesque N (2013) Limited Consequences of Seagrass Decline on Benthic Macrofauna and Associated Biotic Indicators. Estuar Coast 36: 795-807.
- Clarke KR, Gorley RN (2006) PRIMER v6: User Manual/Tutorial. PRIMER-E Ltd, Plymouth.
- Ugland KI, Gray JS (2003) The species accumulation curve and estimation of species richness. J Anim Ecol 72: 888-897.
- Boström C, Bonsdorff E (1997) Community structure and spatial variation of benthic invertebrates associated with Zostera marina (L.) beds in the northern Baltic Sea. J Sea Res 37: 153-166.
- Connolly RM (1997) Differences in composition of small, motile invertebrate assemblages from seagrass and unvegetated habitats in a southern Australian estuary. Hydrobiologia 346: 137-148.
- Paula J, Fidalgo Ecosta P, Gove D (2001) Patterns of abundance of seagrasses and associated infaunal communities at Inhaca Island, Mozambique. Estuar Coast Shelf Sci 53: 307-318.
- Sfriso A, Birkemeyer T, Ghetti PF (2001) Benthic macrofauna changes in areas of Venice lagoon populated by seagrasses or seaweeds. Mar Environ Res 52: 323-349.
- Fredriksen S, De Backer A, Böstrom C, Christie H (2010) Infauna from Zostera marina L. meadows in Norway. Differences in vegetated and unvegetated areas. Mar Biol Res 6: 189-200.
- Bowden DA, Rowden AA, Martin JA (2001) Effect of patch size and in-patch location on the infaunal macroinvertebrate assemblages of Zostera marina seagrass beds. J Exp Mar Biol Ecol 259: 133-154.
- Barnes RSK, Barnes MKS (2012) Shore height and differentials between macrobenthic assemblages in vegetated and unvegetated areas of an intertidal sandflat. Estuar Coast Shelf Sci 106: 112-120.
- Teixeira H, Salas F, Pardal MA, Marques JC (2007) Applicability of ecological evaluation tools in estuarine ecosystems: the case of the lower Mondego estuary (Portugal). Hydrobiologia 587: 101-112.
- Diwara M, Zouari-Tlig S, Rabaoui L, Ben Hassine OK (2008) Impact of management on the diversity of macrobenthic communities in Tunis north lagoon: systematic. Cah Biol Mar 49: 1-16.
- Do VT, de Montaudouin X, Lavesque N, Blanchet H, Guyard H (2011) Seagrass colonization: knock-on effects on zoobenthic community, populations and individual health. Estuar Coast Shelf Sci 95: 458-469.
- Mazzella L, Buia MC, Gambi MC, Lorenti M, Russo GF, et al. (1992) Plant animal trophic relationships in the Posidonia oceanica ecosystem of the Mediterranean Sea: a review: Plant-animals interactions in the marine benthos. (46th edtn), Clarendon Press, Oxford, USA.
- Duffy JE (2006) Biodiversity and the functioning of seagrass ecosystems. Mar Ecol Prog Ser 311: 233-250.
- Bachelet G, de Montaudouin X, Auby I, Labourg PJ (2000) Seasonal changes in macrophyte and macrozoobenthos assemblages in three coastal lagoons under varying degrees of eutrophication. ICES. J Mar Sci 57: 1495-1506.
- Yamada K, Hori M, Tanaka Y, Hasegawa N, Nakaoka M (2007) Temporal and spatial macrofaunal community changes along a salinity gradient in seagrass meadows of Akkeshi-ko estuary and Akkeshi Bay, northern Japan. Hydrobiologia. 592: 345-358.
- Leopardas V, Uy W, Nakaoka M (2014) Benthic macrofaunal assemblages in multispecific seagrass meadows of the southern Philippines: Variation among vegetation dominated by different seagrass species. J Exp Mar Biol Ecol 457: 71-80.
- Blanchet H (2004) Structure and functioning of benthic stands of the Bassin d'Arcachon. PhD thesis, University of Bordeaux, France.
- van der Heide T, Govers LL, de Fouw J, Olff H, van der Geest M, et al. (2012) A Three-Stage Symbiosis Forms the Foundation of Seagrass Ecosystems. Science 363: 1432-1432.
- Cavanaugh CM (1983) Symbiotic chemoautotrophic bacteria in marine invertebrates from sulphide-rich habitats. Nature 302: 58-61.
- Anderson AE (1995) Metabolic responses to sulfur in lucinid Bivalve. Anim Zoolo 35: 121-131.
- Jacobs RPWM, Huisman WHT (1982) Macrobenthos of some Zostera beds in the vicinity of Roscoff (France) with special reference to relations with community structure and environmental factors. Proceedings Royal Dutch Academy of Weddings C85: 335-356.
- Castel J, Labourg PJ, Escaravage V, Auby I, Garcia ME (1989) Influence of seagrass beds and Oyster Park on the abundance and biomass patterns of meio- and macrobenthos in tidal flats. Estuar Coast Shelf Sci 28: 71-85.
- Reise K, Herre E, Sturm M (1994) Biomass and abundance of macrofauna in intertidal sediments of Känigshafen in the northern Wadden Sea. Helgol Meeres 48: 201-215.
- Nakamura Y, Turner JT (1997) Predation and respiration by the small cyclopoid copepod Oithona similis: how important is feeding on ciliates and heterotrophic flagellates? J Plank Res 19: 1275-1288.
- Bel Hassen M, Drira Z, Hamza A, Ayadi H, Akrout F, et al. (2008) Summer phytoplankton pigments and community composition related to water mass properties in the Gulf of Gabès. Estuar Coast Shelf Sci 77: 645-656.
- Coll M, Piroddi C, Steenbeek J, Kaschner K, Ben Rais Lasram F, et al. (2010) The biodiversity of the Mediterranean Sea: estimates, patterns, and threats. PLoS One 8: 11842.
- Bethoux JP (1979) Budgets of the Mediterranean Sea. Their dependence on the local climate and on the characteristics of the Atlantic waters. Oceanologica Acta 2: 157-163.
- Pinardi N, Zavatarelli M, Arneri E, Crise A, Ravaioli M (2006) The physical, sedimentary and ecological structure and variability of shelf areas in the Mediterranean Sea: The Sea: The Global Coastal Ocean.(14th b edtn), Harvard University Press, Cambridge, USA.
- Bas C (2009) The Mediterranean: A synoptic overview. Contrib Sci 5: 25-39.
- Albertelli G, Covazzi-Harriague A, Danovaro R, Fabiano M, Fraschetti S, et al. (1999) Differential responses of bacteria, meiofauna and macrofauna in a shelf area (Ligurian Sea, NW Mediterranean): role of food availability. J Sea Res 42: 11-26.
- Salen-Picard C, Arlhac D (2002) Long-term changes in a Mediterranean benthic community: relationships between the polychaete assemblages and hydrological variations of the Rhône River. Estuar Coast 25: 1121-1130.
- Afli A, Boufahja F, Sadraoui S, Ben Mustapha K, Aissa P, et al. (2009) Functional organization of the benthic macrofauna in the Bizerte lagoon (SW Mediterranean Sea), semi-enclosed area subject to strong environmental/anthropogenic variations. Cah Biol Mar 50: 105-117.
- Paine RT (1966) "Food Web Complexity and Species Diversity". Am Nat 100: 65-75.
- Blanchet H, Lavesque N, Ruellet T, Dauvin JC, Sauriau PG, et al. (2008) Use of biotic indices in semi-enclosed coastal ecosystems and transitional waters habitats-Implications for the implementation of the European Water Framework Directive. Ecol Indic 8: 360-372.
- Reiss H, Kroncke I (2005) Seasonal Variability of Benthic Indices: An Approach to Test the Applicability of Different Indices for Ecosystem Quality Assessment. Mar Poll Bull 50: 1490-1499.
- Hawkins SJ, Moore PJ, Burrows MT, Poloczanska E, Mieszkowska N, et al. (2008) Complex interactions in a rapidly changing world: responses of rocky shore communities to recent climate change. Clim Res 37: 123 -133.
- Harley CDG, Hughes AR, Hultgren KM, Miner BG, Sorte CJB, et al. (2006) The impacts of climate change in coastal marine systems. Ecol Letters 9: 228-241.
- Helmuth B, Mieszkowska N, Moore P, Hawkins SJ (2006) Living on the edge of two changing worlds: forecasting responses of rocky intertidal ecosystems to climate change. Ann Rev Ecol Evol Syst 37: 373- 404.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi