Commentary, J Phys Res Appl Vol: 5 Issue: 6
Materials of Solid State Physics and Metallurgy
William Kane*
*Corresponding author: William Kane, Department of Engineering, University of Maryland, Baltimore, USA, Tel: +1 487598562; E-mail: kanewllm@med.edu
Received date: November 01, 2021; Accepted date: November 15, 2021; Published date: November 22, 2021
Abstract
It grew out of an amalgam of solid-state physics, metallurgy, and chemistry, since the rich variety of materials properties cannot be understood within the context of any single classical discipline [1]. With a basic understanding of the origins of properties, materials can be selected or designed for an enormous variety of applications, ranging from structural steels to computer microchips. Materials science is therefore important to engineering activities such as electronics, aerospace, telecommunications, information processing, nuclear power, and energy conversion
Description
Materials science, the study of the properties of solid materials and structure. It grew out of an amalgam of solid-state physics, metallurgy, and chemistry, since the rich variety of materials properties cannot be understood within the context of any single classical discipline [1]. With a basic understanding of the origins of properties, materials can be selected or designed for an enormous variety of applications, ranging from structural steels to computer microchips. Materials science is therefore important to engineering activities such as electronics, aerospace, telecommunications, information processing, nuclear power, and energy conversion [2]. The process of corrosion of steels in hydrogen-sulfide media of different concentrations with regard for the specific features of the formation of sulfides of different chemical compositions and their influence on the course of redox reactions. We present different points of view on their role in corrosion processes. On the basis of generalization of the influence of iron sulfides on electrode reactions, release and absorption of hydrogen by Armco iron, U8 steel, and 45 steel of different structures, it is established that, despite the fact that iron sulfides mainly reduce the overvoltage of cathodic reactions and increase the amount of hydrogen released under cathodic polarization, they do not always promote the hydrogenation of these materials. Iron sulfides predominantly affect the reaction of hydrogen release, whereas its absorption depends on the structures of steels. By an example of 17G1S-U steel, we show that the corrosion rate is affected by the nature of sulfides formed on the surface. Indeed, for their concentrations varying within the range 25–100 mg/dm3, the corrosion rate decreases by almost an order of magnitude. Moreover, for hydrogen-sulfide concentration CH2S ≥ 500 mg/dm3, we observe the formation of porous sulfides, corrosion defects are localized, and the intensity of absorption of hydrogen by steel becomes almost twice higher than at lower concentrations, which promotes the development of cracking and corrosion cracking [3]. It is shown that the procedure of preliminary cyclic hydrogenation-dehydrogenation of ferritepearlite pipe steel strongly affects its ability to absorb electrochemical hydrogen whose amount increases with the number of these cycles. The concentration of hydrogen adsorbed by the subsurface layers of the metal, the concentration of hydrogen absorbed by the volume of the metal, and its total concentration in the metal are found depending on the number of hydrogenation-dehydrogenation cycles. It is established that, for the analyzed cases, the procedure of preliminary hydrogenation-dehydrogenation increases the ability of steel to absorb hydrogen by a factor of 1.2–2.
Gas and Electrochemical Hydrogenation
A brief survey of the experimental works on gas and electrochemical hydrogenation of the alloys with high magnesium contents including, in particular, the alloys based on R2Mg17 and RMg12. Some methods for the improvement of the hydrogen sorption properties of magnesium-based composites and the influence of various additions are studied. It is shown that the indicated properties of the composites depend on the duration of grinding and the amount of added catalysts. In almost all cases, this influence is positive and the main parameters can increase by a significant fraction. Young's modulus is a measure for stiffness of a material and a higher E means a higher stiffness. The respective polymorphism of the feather corneous beta-protein gene causes the replacement of glycine by cysteine. We looked for possible effects of the three FCBP genotypes on E in the 10th primaries of racing pigeons. However, we did not find a statistically significant difference of E between the genotypes, even within the sexes and/or within different locations under our test conditions. Our findings do not preclude the possibility that under other conditions (temperature, moisture) an influence of the glycine/cysteine polymorphism on E may exist. Compared to the more proximal locations of the rachis (base and middle) we observed lower values for E in the distal region (tip). The 10th primary constitutes the leading edge of the pigeon wing and this special function may require higher stiffness in the proximal parts of the shaft. We observed significantly higher values of E in females than in males, which result only from statistically significantly higher values in the middle region [4]. The higher stiffness of female primaries may also contribute to the better results of hens compared to cocks in pigeon races. This article approaches the subject of materials science through five major fields of application: energy, ground transportation, aerospace, computers and communications, and medicine. The discussions focus on the fundamental requirements of each field of application and on the abilities of various materials to meet those requirements.
Materials for Energy
Classifying energy materials as passive or active or in relation to conventional, advanced, or future energy systems is useful because it provides a picture of the nature and degree of urgency of the associated materials requirements. But the most illuminating framework for understanding the relation of energy to materials is in the materials properties that are essential for various energy applications. Because of its breadth and variety, such a framework is best shown by examples. In oil refining, for example, reaction vessels must have certain mechanical and thermal properties, but catalysis is the critical process [5]. The many materials studied and applied in materials science are usually divided into four categories: metals, polymers, semiconductors, and ceramics. The sources, processing, and fabrication of these materials are explained at length in several articles: metallurgy; elastomer (natural and synthetic rubber); plastic; man-made fibre; and industrial glass and ceramics. Atomic and molecular structures are discussed in chemical elements and matter. The applications covered in this article are given broad coverage in energy conversion, transportation, electronics, and medicine.
References
- Sabzi HE (2016) The effects of bimodal grain size distributions on the work hardening behavior of a TRansformation-TWinning induced plasticity steel. Mat Sci Eng 678: 23-32.
- Cheng QL, Qing WC, Dong ET, Xiao L (2016) Influence of ferrite matrix and precipitation status on the mechanical properties of low carbon low alloy steel during high temperature tension. Mat Sci Eng 678: 1-9.
- Khani S (2016) Microstructural development during equal channel angular pressing of as-cast AZ91 alloy. Mat Sci Eng 678: 44-56.
- Wang Y, Dong J, Zhang M, Yao Z (2016) Stress relaxation behavior and mechanism of AEREX350 and Waspaloy superalloys. Mat Sci & Eng 678: 10-22.
- Zhang C (2016) The kinetics and cellular automaton modeling of dynamic recrystallization behavior of a medium carbon Cr-NiMo alloyed steel in hot working process. Mat Sci Eng 678: 33-43.
Track Your Manuscript
Explore SciTechnol
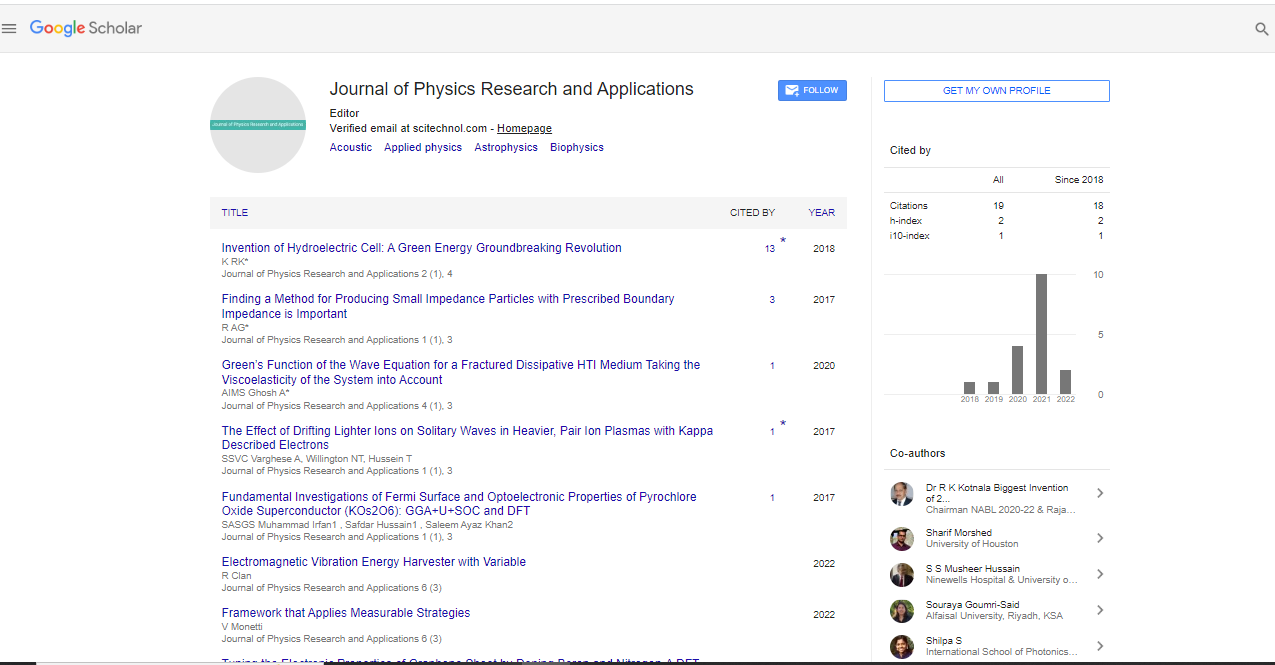
Google Scholar citation report
Citations : 21
Journal of Physics Research and Applications received 21 citations as per Google Scholar report
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi