Research Article, Int J Cardiovasc Res Vol: 7 Issue: 1
Multisite Pacing in Intact Myocardium and after Acute Myocardial Infarction: Experimental Study
Matthaios I*, Kaladaridou A, Skaltsiotes E, Agrios J, Antoniou A, Georgiopoulos G, Papadopoulou E and Toumanidis S
Department of Clinical Therapeutics, Medical School, National and Kapodistrian University of Athens, “Alexandra” Hospital, 80 Vas. Sophias Ave Lourou, Athens, 11528, Greece
*Corresponding Author : Ioannis Matthaios MD,PhD
Department of Clinical Therapeutics, “Alexandra” Hospital, 80 Vas. Sophias Ave – Lourou, Athens, 11528, Greece
Tel: 0030 210 3381497
Fax: 0030 210 3381497
E-mail: giannismat@gmail.com
Received: November 24, 2017 Accepted: January 12, 2018 Published: January 16, 2018
Citation: Matthaios I, Kaladaridou A, Skaltsiotes E, Agrios J, Antoniou A, et al. (2018) Multisite Pacing in Intact Myocardium and after Acute Myocardial Infarction: Experimental Study. Int J Cardiovasc Res 7:1. doi: 10.4172/2324-8602.1000337
Abstract
Background: Left ventricular (LV) pacing is unsuccessful in a significant number of patients, mainly due to sub-optimal LV pacing location. Nevertheless, data about the impact of different pacing sites on LV function in intact and ischemic myocardium are scarce.
Objectives: To investigate the effect of combinations of alternative LV pacing sites on LV mechanics, in the intact myocardium and after experimental acute anterior myocardial infarction (AMI), in order to define the optimal configuration.
Methods: Atrioventricular epicardial pacing at alternative pacing sites was performed in 16 healthy pigs simultaneously, before and after AMI. Hemodynamic parameters together with classic and novel echocardiographic indices were used to evaluate the effect of each pacing combination. Speckle tracking technique using Echo PAC software was used.
Results: In intact myocardium, most LV performance variables measured, including deformation parameters were adversely affected during pacing (in all combinations, all variables p<0.05). After AMI the pacing combination of LV apex lateral wall and LV basal posterior wall had the most favourable effect on LV function, leading to similar hemodynamic and torsional effects with sinus rhythm (all variables p>0.05).
Conclusions: In intact myocardium, LV function is depressed in comparison to sinus rhythm, in every combination of pacing sites studied. However, during AMI the combination of pacing LV apex lateral wall and LV basal posterior wall managed to maintain the LV function at a level comparable to the sinus rhythm.
Keywords: Pacing; Torsion; Acute myocardial infarction; Cardiac mechanics
Introduction
More than a half a century has passed since Furman and Schwedel published their work on endocardial lead placement for cardiac pacing in humans [1]. Since then, the apex of the right ventricle (RV) remains the standard of choice regarding the site of lead placement due to the accessibility, feasibility, and the safety it provides.
However, in the last decade there is growing evidence which demonstrates that pacing the apex of the RV has unfavourable effects, influencing morbidity and mortality [2-5]. These findings are consistent with Wigger’s observations, who, in 1925, reported about the adverse left ventricular (LV) hemodynamic effects that occurred, secondary to RV apical pacing [6]. These harmful effects are produced, mostly due to the alteration of the normal conduction pathway. The electrical stimulation bypasses the atrioventricular node and the highly specialised His-Purkinje system, and is spread slowly through the common myocardium, causing abnormal ventricular contraction and reducing pump function.
Obviously, it is time to seek alternative pacing sites to minimise the adverse clinical outcomes of RV apex stimulation. Investigation in this area is also extensive due to the introduction of cardiac resynchronisation therapy (CRT) and the fact that almost one-thirds of patients receiving this therapy do not respond to treatment [7,8].
A number of studies have been published about alternative pacing sites, mostly in patients with heart failure [9-11]. However, data about the impact of different pacing sites on LV function in intact myocardium and myocardium under ischemia is scarce. Moreover, the evaluations of cardiac mechanics in this condition, with novel techniques such as two-dimensional speckle tracking echocardiography (STE), are limited. The STE allows detailed evaluation of LV mechanics, including LV mechanical dyssynchrony, LV strain, and LV torsion, and provides important additional information for the selection of the optimal pacing site [12,13]. Data based on STE- comparing the effects of different LV pacing and sites on the LV mechanics, LV strain, and LV torsion-are still limited [14,15].
The purpose of this study was to assess the acute hemodynamic and echocardiographic response to different pacing configurations in intact myocardium after experimental acute myocardial infarction (AMI). The ultimate goal was to determine the optimal combination of pacing sites that preserve LV function under ischemic conditions.
Methods
The protocol complied with the “Principles for the Care of Experimental Animals” and the “Guidelines for the Care and Use of Experimental Animals” issued by the US National Academy of Sciences and National Institute of Health (version 85-23, revision 1996), and was approved by the Scientific Committee of our Hospital.
Surgical preparation
16 healthy pigs, weighing 40 ± 5 kg, were pre-medicated with intramuscular administration of ketamine potassium 5 mg/kg, and midazolam 5 mg/kg. Anaesthesia was induced with thiopental sodium 5 mg/kg intravenously (IV), and the animals were then intubated and ventilated by mechanical ventilation (Sulla 808V, Drager Medizintechnik GmbH Germany). Throughout the experiment anaesthesia was maintained with IV propofol 0.1-0.2 mg/kg, and analgesia was maintained with the administration of opioid-fentanyl. Lead II of the standard electrocardiogram (ECG), haemoglobin oxygen saturation, and the animal’s temperature with a rectal thermometer were monitored constantly by a multichannel device (Dynamap Plus Vital Signs Monitor, Criticon, Tampa, FL, USA).
A 6F sheath was inserted into the right internal jugular vein for the delivery of drugs and fluids. A suprapubic urinary catheter was inserted to measure urine output, and fluid loss was compensated by continuous infusion of saline into the right jugular vein. Moreover, left external carotid artery was cannulated and a 6F pigtail catheter was placed into the LV cavity and used for the measurement of LV pressure and peak rate of LV pressure increase (dP/dtmax). To avoid endovascular thrombus formation, a 5000 IU heparin bolus was administered. Loading conditions were kept constant during the different manoeuvres.
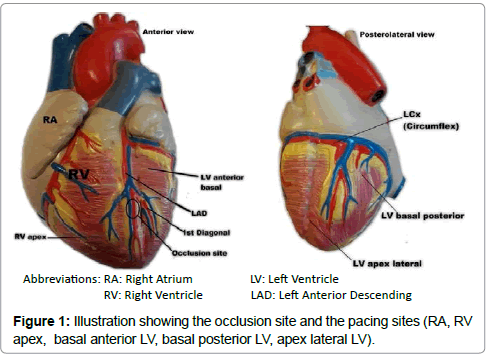
We used the same surgical procedure that we have developed in our previous experiments [15,16]. Briefly, a regular median sternotomy was performed after thyme resection, and a longitudinal pericardiotomy was performed. The left anterior descending (LAD) coronary artery was surgically exposed, and two 3-0 Prolene sutures (Ethicon, Johnson & Johnson Co., European Logistics Centre, Sint- Stevens- Woluwe, Belgium) were placed after the origin of the first diagonal branch of the LAD, to be used for future ligation. Before ligation, left coronary artery entrapment was confirmed by upward traction. The apex of the LV was observed for evidence of myocardial blanching indicating interruption to coronary flow, confirming epicardial ischemia (Figure 1).
Pacing
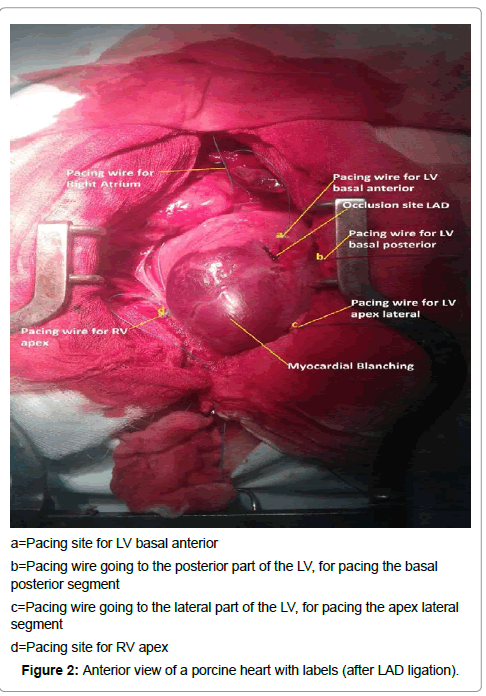
Temporary myocardial pacing leads (Medtronic, type 6500, Minneapolis, MN, USA) were attached to the surface of the right atrium, to the epicardium of the RV apex, and in different positions of the epicardium of the LV. These different positions of the LV were the basal posterior wall, the basal anterior wall, and the apex lateral wall (Figure 2). All pacing positions were kept outside the infarcted zone.
a=Pacing site for LV basal anterior
b=Pacing wire going to the posterior part of the LV, for pacing the basal
posterior segment
c=Pacing wire going to the lateral part of the LV, for pacing the apex lateral
segment
d=Pacing site for RV apex
Figure 2: Anterior view of a porcine heart with labels (after LAD ligation).
The pacing leads were connected to an external pacemaker (Medtronic Model 5388 dual chamber temporary pacemaker, Minneapolis, MN, USA). Pacing was unipolar with an indifferent electrode positioned in between the intercostal muscles. The AV delays were short enough to produce an activation wave originating from the ventricular pacing lead, and were not modified between the different pacing configurations. Pacing was performed at about twice the stimulation threshold and the pacing rate was set at 10 beats/min above the intrinsic heart rate in each case.
Standard echocardiography
The echocardiographic study was performed using a Vivid i digital ultrasound system (GE Medical Systems Ultrasound Israel Ltd., Tirat Hacarmel, Israel) and a 3.5 MHz phased array transducer. Echocardiographic measurements were performed intrathoracically, by placement of the probe directly on the epicardium. A soft silicone pad, placed between the probe and the epicardium, acted as a cushion for the moving heart and as an offset to prevent near-field artifacts. Two-dimensional grey-scale echocardiographic images were obtained, using second-harmonic imaging. Instrument settings were held constant for each experiment. Three consecutive cardiac cycles were stored in cineloop format for offline analysis. Averaged values were calculated for each parameter.
Two-dimensional speckle tracking echocardiography
An assessment of the LV rotation and twist was obtained by the acquisition of specific short-axis planes with internal landmarks: the basal plane was acquired at the level of the mitral valve leaflets while excluding the mitral annulus and the apical plane was acquired distal from the papillary muscles. The frame rate range was 65-80/s. In each phase, three consecutive cardiac cycles’ cineloop images were stored for offline analysis with a dedicated platform Echo Pac PC (version 7.0, GE Medical Systems).
Experimental protocol
After completion of the surgical preparations, a steady-state period of 15 minutes was allowed. Epicardial atrioventricular pacing in multiple sites was performed simultaneously. The combinations of pacing sites we studied are demonstrated in Table 1. These pacing combinations were performed in a random order. Baseline measurements were obtained in sinus rhythm, and then repeated during every pacing combination. Measurements were taken after 5 minutes of pacing at each combination, separated by 5-minute intervals in sinus rhythm. Data were obtained 30 minutes after ligation of the LAD. The ligation was placed in the same position in all the animals.
| Pacing Configuration 1 | RV apex + LV basal posterior wall (similar to CRT) |
| Pacing Configuration 2 | RV apex + LV apex lateral wall |
| Pacing Configuration 3 | RV apex + LV basal anterior wall |
| Pacing Configuration 4 | RV apex + LV basal anterior wall + LV basal posterior wall (triple) |
| Pacing Configuration 5 | LV basal anterior wall + LV apex lateral wall. |
| Pacing Configuration 6 | LV basal posterior wall + LV apex lateral wall |
| Pacing Configuration 7 | LV basal posterior wall + LV basal anterior wall |
Table 1: Combinations of the different pacing sites we studied.
Statistical analysis
Continuous variables are displayed as medians, and 25th/75th percentiles. Statistical analysis was performed on absolute values, and each experiment served as its own control. Differences in variables of interest among various pacing sites were established with the Friedman non-parametric test for k-related samples (where k=number of alternative pacing sites). Pair-wise comparisons were subsequently performed, using the Wilcoxon Signed Rank Analysis, for two related samples. Bonferroni correction was implemented to account for multiple comparisons.
Variation of main hemodynamic, echocardiographic, and torsional parameters among different pacing sites was further assessed by linear mixed models with unstructured variance-covariance matrix. All mixed models incorporated at least one random effect (i.e., random intercept) to address heterogeneity among subjects. Diagnostics for linear mixed models were based on graphical analysis of the corresponding residuals (Q-Q plots). The level of significance was set at p<0.05. The statistical software package SPSS for Windows, version 17, was used for data analysis (SPSS Inc., Chicago, IL, USA).
Results
A total of 16 experiments were performed and completed by protocol. Three animals were excluded from the analysis due to unsuccessful recovery from lethal ventricular fibrillation, immediately after LAD ligation.
Intact myocardium
Data analysis revealed that in intact LV myocardium sinus rhythm is superior of all the different combinations of epicardial pacing sites we studied in most of hemodynamic and conventional echocardiographic parameters (Table 2).
| Intact Myocardium |
Control (sinus rhythm) |
( 1 ) RV apex + LV basal posterior (CRT) |
( 2 ) RV apex + LV apex lateral |
(3 ) RV apex + LV basal anterior |
( 4 ) RV apex + LV basal posterior + LV basal anterior |
( 5 ) LV basal anterior + LV apex lateral |
( 6 ) LV basal posterior + LV apex lateral |
( 7 ) LV basal posterior + LV basal anterior |
|---|---|---|---|---|---|---|---|---|
| EDV (ml) | 57.3 (54.0-71.0) |
59.5 (53.1-62.6) p=0.3 |
56.4 (51.6-63.4) p=0.3 |
57.2 (51.5-62.3) p=0.2 |
55.5 (50.2-64.9) p=0.1 |
55.0 (50.6-60.3) p=0.08 |
57.1 (47.6-63.1) p=0.1 |
58.0 (55.2-60.4) p=0.4 |
| ESV (ml) | 32.0 (26.0-35.7) |
35.3 (32.2-40.5) p=0.08 |
32.3 (29.9-36.6) p=0.1 |
34.7 (28.1-40.1) p=0.4 |
33.5 (28.7-38.9) p=0.1 |
32.0 (27.1-36.1) p=0.5 |
31.2 (26.9-38.4) p=0.6 |
34.0 (32.1-38.8) *p=0.006 |
| SV (ml) | 29.8 (25.1-33.9) |
22.0 (18.4-24.2) *p=0.002 |
24.2 (20.7-28.1) *p=0.004 |
22.7 (21.1-24.1) *p=0.003 |
23.0 (20.2-26.1) *p=0.003 |
22.5 (21.1-26.7) *p=0.006 |
23.6 (21.5-27.4) *p=0.01 |
22.7 (20.3-25.9) *p=0.006 |
| EF (%) | 49.5 (45.2-55.7) |
38.5 (32.7-42.1) *p<0.001 |
42.5 (38.0-46.0) *p<0.001 |
41.5 (37.1-44.7) *p<0.001 |
40.5 (37.2-44.1) *p=0.001 |
42.0 (39.5-46.5) *p=0.001 |
44.0 (38.7-45.7) *p=0.002 |
38.5 (34.2-41.1) *p=0.001 |
| CO (L/min) | 3.05 (2.37-3.36) |
2.42 (2.08-2.93) *p=0.04 |
2.81 (2.41-2.99) p=0.6 |
2.60 (2.26-3.19) p=0.12 |
2.56 (2.13-2.84) p=0.12 |
2.82 (2.47-3.15) p=0.6 |
2.81 (2.46-3.34) p=0.6 |
2.72 (2.31-3.05) p=0.23 |
| HR (beats/min) | 95.0 (86.5-113.8) |
110.0 (101.2-127.0) *p=0.001 |
110.0 (100.8-123.9) *p=0.005 |
110.5 (105.0-123.8) *p=0.001 |
110.0 (100.0-123.9) *p=0.004 |
120.0 (105.2-132.7) *p<0.001 |
117.5 (106.2-129.0) *p<0.001 |
113.0 (105.0-125.5) *p<0.001 |
| QRS (ms) | 65.0 (61.0-73.0) |
86.0 (77.5-97.3) *p=0.001 |
90.5 (83.9-94.9) *p<0.001 |
87.7 (83.5-92.9) *p<0.001 |
84.7 (76.3-92.9) *p<0.001 |
89.4 (80.8-95.4) *p<0.001 |
84.2 (78.1-90.5) *p<0.001 |
93.0 (82.5-98.2) *p<0.001 |
| e/e′ ratio | 6.71 (4.83-8.93) |
8.79 (6.94-11.03) *p=0.008 |
8.01 (6.03-10.9) p=0.2 |
7.20 (5.91-8.01) p=0.8 |
8.02 (5.69-10.3) p=0.2 |
8.02 (7.04-12.08) p=0.02 |
8.87 (6.52-10.93) p=0.1 |
7.81 (6.5-10.21) *p=0.04 |
| dP/dtmax (mmHg/s x103 ) | 1.40 (0.90-1.67) |
1.20 (1.00-1.60) p=0.3 |
1.25 (0.90-1.60) p=0.3 |
1.20 (0.92-1.60) p=0.5 |
1.25 (1.00-1.60) p=0.9 |
1.20 (0.80-1.60) p=0.1 |
1.10 (0.90-1.50) p=0.07 |
1.45 (0.97-1.70) p=0.9 |
| SAP (mmHg) | 97.1 (83.9-105.3) |
94.2 (77.2-103.6) p=0.1 |
91.9 (84.1-101.0) *p=0.03 |
93.6 (79.9-101.7) *p=0.01 |
92.7 (80.6-103.7) p=0.07 |
93.3 (80.1-99.9) *p=0.02 |
91.7 (78.5-100.8) *p=0.01 |
94.9 (88.9-103.4) p=0.1 |
| Mean AP (mmHg) | 51.6 (45.8-63.1) |
54.4 (43.1-62.7) p=0.6 |
56.6 (49.6-60.6) p=0.8 |
55.5 (46.2-62.3) p=0.9 |
55.2 (43.9-61.4) p=0.5 |
54.7 (44.5-60.2) p=0.7 |
55.9 (46.4-59.6) p=0.3 |
57.3 (51.9-60.1) p=0.8 |
| -dP/dtmax (mmHg/s x103 ) | -1.90 -(1.65-2.45) |
-1.80 -(1.40-2.11) p=0.06 |
-1.85 -(1.60-2.17) p=0.09 |
-1.95 -(1.50-2.07) *p=0.04 |
-1.65 -(1.50-2.15) *p =0.007 |
-1.75 -(1.62-2.2) p =0.09 |
-1.60 -(1.40-2.07) *p =0.004 |
-1.85 -(1.45-2.15) *p =0.03 |
Values are displayed as medians and 25th/75th percentiles.
Asterisk (*) indicates significant difference from reference (sinus rhythm) as derived from Wilcoxon signed rank test for two related samples,
𑃠indicates the overall significance of the effect of pacing site on each variable by non-parametric test analysis (Friedman test)
Abbreviations: CO: cardiac output, EDV: LV end-diastolic volume, EF: LV ejection fraction, ESV: LV end-systolic volume, e: mitral early filling velocity, ð‘’’:early diastolic mitral annulus velocity by tissue Doppler imaging, HR: heart rate; Ld: LV end-diastolic long-axis dimension, Ls: LV end-systolic long-axis dimension, SAP: LV systolic arterial pressure, Sd: LV end-diastolic short-axis dimension, SR: sinus rhythm, Ss: LV end-systolic short-axis dimension, SV: LV-stroke volume.
Other abbreviations as in Table 1.
Table 2: Comparison of hemodynamic and conventional echocardiographic variables during sinus rhythm and during pacing with the different configurations in intact myocardium.
In the intact myocardium, pacing with every configuration we tested significantly decreased most of the systolic and diastolic parameters. The stroke volume and the ejection fraction were reduced (in all sites p<0.05) even though the cardiac output seemed to be preserved in all sites, except from site 1 (p=0.04), mostly because the heart rate was higher during pacing (all sites p<0.05). The changes in dP/dtmax and mean arterial pressure were not significantly affected by pacing (all sites p>0.05). Moreover, the e/e΄ ratio, which is an index of the diastolic performance of the LV, was increased by all pacing configurations although it reached a statistically significant level in three of them (configuration 1 p=0.008, configuration 5 p=0.02 and configuration 7 p=0.04) [17]. For every pacing mode and pacing site, the heart rate was significantly higher and the QRS duration significantly longer in comparison to sinus rhythm (all sites p<0.05).
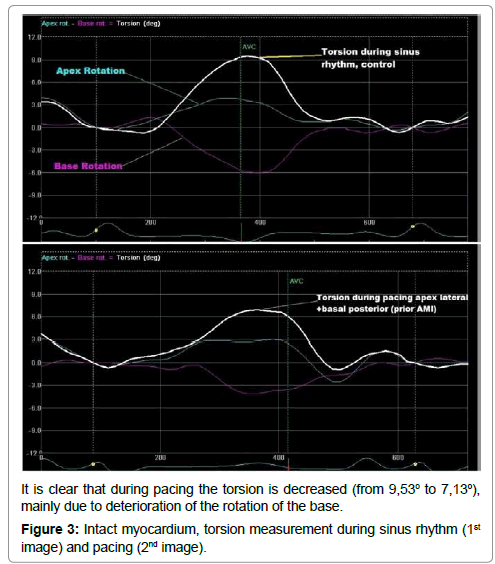
In addition, data analysis revealed that most of the LV torsional and strain parameters also deteriorated significantly during pacing in the intact myocardium (Table 3 and Figure 3). Torsion and twist were reduced significantly (all sites p<0.05). Pacing with every configuration led to reduction of rotation, circumferential and radial strain of the apex and the base of the LV. However, this reduction was not uniform and in some pacing sites it reached a significance level while in others it remained statistically insignificant. The untwisting rate, which is a novel indicator of the relaxation of the LV, was reduced significantly only in site 4 (p=0.03) and site 6 (p=0.03) [18]. On the contrary, the torsion rate, which provides additional information about the systolic performance of the LV, remained statistically unaffected by pacing (all sites p>0.05) [19].
| Intact myocardium |
Control (sinus rhythm) |
( 1 ) RV apex + LV basal posterior (CRT) |
( 2 ) RV apex + LV apex lateral |
( 3 ) RV apex + LV basal anterior |
( 4 ) RV apex + LV basal posterior + LV basal anterior |
( 5) LV basal anterior + LV apex lateral |
( 6 ) LV basal posterior + LV apex lateral |
( 7) LV basal posterior + LV basal anterior |
|---|---|---|---|---|---|---|---|---|
| Rotation Base (°) | -4.90 -(4.47-5.45) |
-4.30 -(3.16-5.23) *p=0.03 |
-4.06 -(2.61-5.30) *p=0.02 |
-4.92 -(3.12-4.90) p=0.18 |
-4.46 -(3.04-5.40) p=0.11 |
-4.78 -(3.26-6.90) p=0.69 |
-3.90 -(3.14-5.90) p=0.07 |
-4.06 -(2.57-5.30) *p=0.03 |
| Rotation Apex (°) | 4.58 (2.86-6.44) |
3.15 (2.41-4.41) *p=0.03 |
3.55 (2.08-5.61) p=0.47 |
3.09 (1.55-4.22) *p=0.07 |
2.86 (1.51-3.99) *p=0.03 |
2.32 (1.55-3.42) *p=0.02 |
3.41 (1.85-4.93) p=0.2 |
4.18 (2.54-5.64) p=0.4 |
| Torsion (°) | 9.49 (7.53-11.2) |
6.71 (5.04-8.57) *p=0.004 |
6.02 (5.54-9.81) *p=0.05 |
6.99 (3.49-8.41) *p=0.006 |
5.89 (3.49-8.16) *p=0.006 |
5.88 (4.62-8.08) *p=0.005 |
6.48 (4.03-9.15) *p=0.03 |
6.60 (4.98-9.22) *p=0.05 |
| Twist (°/mm) | 0.142 (0.123-0.178) |
0.112 (0.090-0.126) *p=0.02 |
0.097 (0.084-0.148) *p=0.05 |
0.103 (0.054-0.122) *p=0.004 |
0.084 (0.052-0.118) *p=0.01 |
0.102 (0.068-0.115) *p=0.004 |
0.100 (0.057-0.140) *p=0.02 |
0.109 (0.082-0.137) *p=0.01 |
| Torsion rate (°/s) | 72.9 (59.0-84.7) |
70.2 (64.9-90.9) p=0.38 |
75.6 (59.2-92.2) p=0.64 |
65.9 (62.2-78.7) p=0.33 |
71.2 (50.5-89.1) p=0.78 |
62.0 (57.1-81.8) p=0.78 |
77.6 (59.3-92.9) p=0.47 |
66.0 (43.1-93.6) p=0.64 |
| Untwisting rate (°/s) | -102.9 -(67.3-119.4) |
-68.1 -(55.2-93.8) p=0.03 |
-63.5 -(58.6-108.3) p=0.1 |
-81.5 -(62.6-110.6) p=0.18 |
-50.3 -(41.6-85.7) *p=0.03 |
-82.7 -(56.6-140.6) p=0.95 |
-64.4 -(53.2-93.6) *p=0.03 |
-83.3 -(42.8-102.1) p=0.17 |
| Circumferential base (%) | -13.3 -(11.7-15.3) |
-10.8 -(9.4-11.7) *p=0.006 |
-11.6 -(10.2-13.2) *p=0.06 |
-10.5 -(9.1-13.7) *p=0.004 |
-10.8 -(9.2-12.1) *p=0.02 |
-10.6 -(9.1-12.8) *p=0.006 |
-11.1 -(9.6-12.6) *p=0.008 |
-10.5 -(8.7-12.5) *p=0.005 |
| Circumferential apex (%) | -13.3 -(9.5-17.4) |
-10.1 -(6.6-13.1) *p=0.004 |
-10.4 -(7.9-13.4) *p=0.08 |
-12.0 -(7.4-15.2) p=0.18 |
-10.5 -(8.8-12.6) *p=0.02 |
-10.8 -(7.1-13.9) *p=0.02 |
-12.7 -(11.1-14.9) p=0.88 |
-13.6 -(9.7-15.4) p=0.35 |
| Radial base (%) | 28.2 (21.5-41.2) |
25.4 (21.1-36.7) p=0.4 |
27.4 (17.5-36.1) p=0.5 |
24.1 (21.1-36.6) p=0.6 |
20.2 (16.1-28.2) *p=0.03 |
25.0 (18.1-30.9) *p=0.02 |
25.4 (20.8-32.9) p=0.4 |
19.9 (15.2-24.3) *p=0.006 |
| Radial apex (%) | 43.5 (33.6-52.5) |
32.7 (26.6-39.7) *p=0.02 |
24.1 (20.3-37.9) *p=0.02 |
18.4 (8.71-28.2) *p=0.002 |
28.6 (22.1-44.8) *p=0.01 |
30.7 (20.7-34.5) *p=0.01 |
29.5 (20.7-50.5) *p=0.06 |
30.7 (23.9-42.3) *p=0.001 |
Values are displayed as medians and 25th/75th percentiles.
Asterisk (*) indicates significant difference from reference (sinus rhythm) as derived from Wilcoxon signed rank test for two related samples.
𑃠indicates the overall significance of the effect of pacing site on each variable by non-parametric test analysis (Friedman test).
Abbreviations as in Table 1.
Table 3: Comparison of left ventricular torsional and strain parameters during sinus rhythm and during pacing with the different configurations in intact myocardium.
In conclusion, by linear mixed model analysis, sinus rhythm was superior than pacing sites for major hemodynamic parameters (ejection fraction and stroke volume) as well as for twist index.
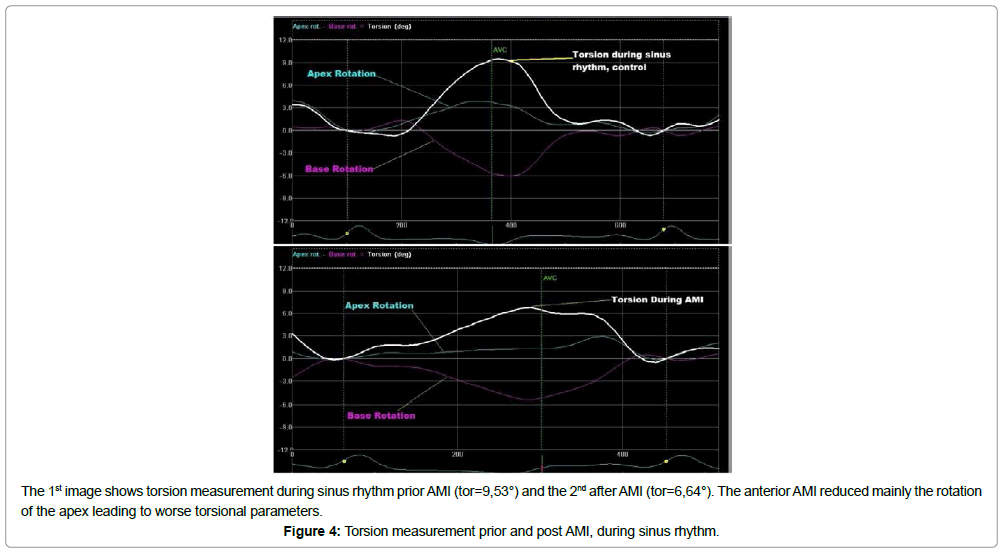
Post AMI
Measurements after AMI revealed a significant deterioration in LV performance compared to the intact myocardium (Figure 4 and Table 4). Moreover after AMI and during pacing, the hemodynamic and conventional echocardiographic variables were altered to a different degree in comparison to sinus rhythm (Table 4). Stroke volume and ejection fraction were reduced significantly in all sites, except site 2 and site 6 (p>0.05). Additionally, the QRS duration was prolonged (all p>0.05). Cardiac output did not present a statistically significant change, mainly due to an increase in heart rate (all p<0.05) during pacing. Furthermore, the e/e΄ratio was increased in absolute values in all pacing sites, but a statistically significant increment was found only in site 1 (p=0.03) and site 7 (p=0.04). Hemodynamic parameters, such dP/dtmax, LV systolic pressure, and mean arterial pressure, did not decrease to statistically significant levels (p>0.05).
| Post AMI |
Control (sinus rhythm) |
( 1 ) RV apex + LV basal posterior (CRT) |
( 2 ) RV apex + LV apex lateral |
(3) RV apex + LV basal anterior |
( 4 ) RV apex + LV basal posterior + LV basal anterior |
( 5 ) LV basal anterior + LV apex lateral |
( 6 ) LV basal posterior + LV apex lateral |
( 7 ) LV basal posterior + LV basal anterior |
|---|---|---|---|---|---|---|---|---|
| EDV (ml) | 64.0 (61.7-70.7) | 59.0 (49.6-66.2) *p=0.008 |
63.0 (60.1-65.7) p=0.4 |
61.5 (55.9-64.7) p=0.07 |
60.0 (53.2-64.3) *p=0.005 |
58.7 (55.5-62.6) p=0.3 |
58.7 (55.1-62.5) *p=0.02 |
60.5 (55.2-63.5) *p=0.02 |
| ESV (ml) | 41.0 (38.2-44.3) | 40.2 (34.4-43.2) p=0.3 |
41.0 (36.1-43.6) p=0.7 |
43.0 (36.8-45.2) p=0.8 |
40.0 (35.6-42.1) p=0.09 |
38.8 (36.6-43.1) p=0.4 |
37.2 (33.8-40.7) *p=0.05 |
41.5 (38.2-44.7) p=0.6 |
| SV (ml) | 24.5 (22.5-27.5) | 19.2 (15.2-22.5) *p=0.004 |
23.0 (19.6-25.1) p=0.1 |
20.0 (17.7-20.6) *p=0.01 |
19.5 (17.3-20.5) *p=0.004 |
19.7 (16.2-22.1) *p=0.01 |
24.5 (21.5-28.8) p=0.08 |
22.0 (20.2-22.9) *p=0.005 |
| EF (%) | 37.0 (35.5-39.5) | 32.0 (31.0-35.7) *p=0.01 |
34.0 (33.0-40.2) p=0.3 |
31.0 (29.0-37.0) *p=0.008 |
34.0 (31.0-35.0) *p=0.007 |
33.5 (29.2-35.0) *p=0.01 |
36.5 (34.0-39.5) p=0.6 |
32.0 (31.0-34.0) *p=0.006 |
| CO (L/min) | 3.07 (2.37-3.33) | 2.47 (2.34-3.06) p=0.3 |
3.12 (2.69-3.55) p=0,07 |
2.79 (2.43-2.99) p=0.2 |
2.77 (2.42-2.95) p=0.3 |
2.72 (2.28-3.13) p=0.7 |
2.83 (2.66-3.62) p=0.8 |
2.68 (2.54-2.91) p=0.2 |
| HR (beats/min) | 125.0 (103.0-136.0) | 140.0 (126.2-156.7) *p=0.002 |
143.5 (135.0-152.5) *p=0.002 |
135.5 (120.0-153.2) p=0.005 |
145.0 (132.0-157.5) *p=0.002 |
140.0 (131.0-160.5) *p=0.002 |
133.7 (126.2-151.0) *p=0.02 |
145.0 (132.5-149.0) *p=0.002 |
| QRS (msec) | 65 (61.9-70.5) | 84 (76.0-98.7) *p=0.003 |
97.3 (87.7-102.0) *p=0.002 |
89.7 (87.0-99.1) *p=0.001 |
88 (83.0-98.5) *p=0.001 |
90.7 (78.0-96.5) *p=0.002 |
85.7 (80.0-95.7) *p=0.002 |
96.5 (91.0-100.5) *p=0.001 |
| LD/SD ratio | 2.75 (2.60-3.14) | 2.86 (2.43-2.94) p=0.7 |
2.77 (2.38-2.89) p=0.5 |
2.78 (2.54-2.92) p=0.6 |
2.74 (2.41-2.96) p=0.2 |
2.73 (2.44-2.85) p=0.3 |
2.73 (2.49-2.91) p=0.7 |
2.84 (2.47-3.08) p=0.8 |
| LS/SS ratio | 3.76 (3.29-4.36) | 3.73 (3.19-3.97) p=0.2 |
3.42 (3.22-4.35) p=0.5 |
3.87 (3.21-4.36) p=0.4 |
3.54 (3.35-4.42) p=0.5 |
3.8 (3.22-4.18) p=0.3 |
3.49 (3.08-4.40) p=0.4 |
3.57 (3.33-4.17) p=0.06 |
| e/e′ ratio | 8.20 (5.34-10.04) | 11.20 (6.10-14.25) *p=0.03 |
7.66 (6.10-12.93) p=0.1 |
8.21 (5.90-10.16) p=0.5 |
9.92 (7.10-12.90) p=0.1 |
7.86 (5.9-10.95) p=0.3 |
8.49 (6.14-11.20) p=0.5 |
9.88 (7.50-11.83) *p=0.04 |
| dp/dtmax (mmHg/s x103 ) | 1.10 (0.95-1.80) | 1.10 (0.81-1.42) p=0.5 |
1.00 (0.90-1.55) p=0.7 |
1.00 (0.90-1.45) p=0.6 |
1.10 (0.96-1.27) p=0.6 |
1.10 (0.95-1.50) p=0.7 |
1.10 (0.90-1.65) p=0.8 |
1.2 (1.02-1,60) p=0,5 |
| SAP (mmHg) | 84.4 (67.2-98.2) | 73.3 (62.1-85.8) p=0.2 |
76.5 (65.3-89.3) p=0.5 |
83.4 (74.8-84.1) p=0.2 |
80.3 (61.5-86.8) p=0.8 |
81.8 (68.3-91.3) p=0.3 |
80.9 (66.4-90.6) p=0.06 |
83.4 (63.2-84.8) p=0.1 |
| meanAP(mmHg) | 48 (41.1-62.2) | 45.6 (39.4-53.7) p=0.9 |
44.6 (35.8-47.5) p=0.6 |
49.1 (43.0-50.0) p=0.4 |
45.2 (41.8-52.7) p=0.6 |
48.8 (39.2-53.8) p=0.5 |
45.8 (38.1-53.3) p=0.1 |
48.6 (37.8-52.7) p=0.1 |
| -dp/dtmax (mmHg/s x103 ) |
-1.65 -(1.4-2.07) |
-1.35 -(1.10 -1.77) *p=0.01 |
-1.40 -(1.12-1.60) *p=0.007 |
-1.45 -(1.3-1.67) *p=0.004 |
-1.40 -(1.22-1.87) *p=0.05 |
-1.50 -(1.17-1.8) *p=0.01 |
-1.40 -(1.15-1.80) *p=0.006 |
-1.40 -(1.12-1.77) *p=0.007 |
Values are displayed as medians and 25th/75th percentiles
* Indicates significant difference from reference (sinus rhythm) as derived from Wilcoxon signed rank test for two related samples
𑃠indicates the overall significance of the effect of pacing site on each variable by non-parametric test analysis (Friedman test)
Abbreviations are CO: cardiac output, EDV: LV end-diastolic volume, EF: LV ejection fraction, ESV: LV end-systolic volume, e: mitral early filling velocity, ð‘’’:early diastolic mitral annulus velocity by tissue Doppler imaging;, Ld: LV end-diastolic long-axis dimension, Ls: LV end-systolic long-axis dimension, SAP: LV systolic arterial pressure, Sd: LV end-diastolic short-axis dimension, SR: sinus rhythm, Ss: LV end-systolic short-axis dimension, SV: LV-stroke volume.
Table 4: Comparison of the hemodynamic and conventional echocardiographic variables during sinus rhythm and during pacing with the different configurations after AMI.
Torsional and strain parameters of the LV were also affected by the different pacing configurations in comparison with sinus rhythm (Table 5 and Figure 5). Pacing mostly affected the base of the heart, leading to a decrease in the rotation and the circumferential and radial strain of the base. The rotation and the circumferential strain of the apex did not change remarkably in comparison with the sinus rhythm— although the radial strain was reduced significantly-except in site 4, where it increased significantly (22.1% vs. 19.9%, p<0.01). Furthermore, the torsion and the twist were reduced during pacing but this reduction was only significant during pacing with the combination 7 (p=0.01, and p=0.02, respectively). The torsion rate and the untwisting rate also did not change significantly (all p>0.05).
| Post AMI | Control (Sinus Rhythm) |
( 1 ) RV apex + LV basal posterior (CRT) |
( 2 ) RV apex + LV apex lateral |
( 3 ) RV apex + LV basal anterior |
( 4) RV apex + LV basal posterior + LV basal anterior |
( 5) LV basal anterior + LV apex lateral |
( 6 ) LV basal posterior + LV apex lateral |
( 7) LV basal posterior + LV basal anterior |
|---|---|---|---|---|---|---|---|---|
| Rotation Base (°) | -5.16 -(4.06-7.05) | -3.51 -(2.20-5.30) *p=0.01 |
-3.24 -(2.92-5.10) *p=0.03 |
-4.82 -(3.55-6.30) p=0.1 |
-4.34 -(2.90-5.54) *p=0.04 |
-4.77 -(3.17-5.05) *p=0.04 |
-4.35 -(2.90-5.96) p=0.2 |
-4.33 -(3.04-5.20) *p=0.01 |
| Rotation Apex (°) | 1.55 (1.02-3.23) | 2.0 (1.71-3.14) p=0.2 |
2.29 (0.97-2.74) p=0.8 |
1.89 (1.25-2.68) p=0.4 |
1.71 (1.25-2.47) p=0.9 |
2.28 (1.65-3.13) p=0.2 |
1.88 (0.89-3.30) p=0.5 |
1.52 (1.00-2.72) p=0.65 |
| Torsion (°) | 6.58 (4.64-7.56) | 4.53 (2.96-7.07) p=0.1 |
4.7 (3.07-6.45) p=0.06 |
6.08 (3.91-7.29) p=0.3 |
3.62 (2.89-7.26) p=0.07 |
4.87 (3.76-7.16) p=0.2 |
5.81 (3.34-7.63) p=0.8 |
3.65 (2.70-5.52) *p=0.01 |
| Twist (°/mm) | 0.098 (0.072-0.105) | 0.070 (0.045-0.102) p=0.5 |
0.071 (0.048-0.095) p=0.06 |
0.084 (0.058-0.111) p=0.5 |
0.057 (0.043-0.950) p=0.1 |
0.072 (0.064-0.103) p=0.3 |
0.092 (0.049-0.110) p=0.9 |
0.059 (0.036-0.083) *p=0.02 |
| Torsion rate (°/s) | 63.9 (42.1-93.6) | 58.4 (47.1-64.5) p-0.9 |
58.6 (46.7-78.7) p=0.7 |
63.7 (51.6-81.1) p=0.8 |
64.5 (38.6-77.4) p=0.6 |
57.9 (45.8-84.5) p=0.8 |
45.2 (37.5-63.2) p=0.5 |
74.6 (51.7-91.4) p=0.7 |
| Untwisting rate (°/s) | -73.3 -(48.8-100.4) | -79.3 -(50.5-98.9) p=0.7 |
-66.8 -(50.5-77.4) p=0.2 |
-76.7 -(57.9-102.5) p=0.5 |
-61.5 -(49.6-83.7) p=1.0 |
-76.9 -(58.3-102.7) p=0.1 |
-71.5 -(46.4-100.2) p=0.5 |
-58.5 -(44.6-87.0) p=0.9 |
| Circumferential base (%) | -13.52 -(12.31-14.49) | -11.87 -(9.32-13.08) *p=0.004 |
-11.33 -(11.05-14.32) p=0.2 |
-11.49 -(10.51-14.22) *p=0.04 |
-10.55 -(9.30-12.03) *p=0.03 |
-12.25 -(9.71-14.09) p=0.4 |
-11.45 -(9.47-13.67) *p=0.04 |
-11.88 -(8.59-13.40) *p=0.006 |
| Circumferential apex (%) | -8.52 -(5.31-10.16) | -7.93 -(4.28-9.43) p=0.6 |
-6.10 -(2.75-7.77) p=0.06 |
-6.88 -(5.86-10.47) p=0.4 |
-8.01 -(6.14-9.40) p=0.6 |
-7.38 -(4.91-10.37) p=0.6 |
-7.15 -(4.03-9.05) p=0.4 |
-8.20 -(5.40-9.65) p=0.6 |
| Radial base (%) | 43.5 (33.6-52.5) | 30.9 (26.6-39.2) *p=0.03 |
24.1 (20.1-37.9) *p=0.02 |
33.8 (24.1-37.4) *p=0.05 |
28.6 (19.7-44.8) *p=0.01 |
30.7 (20.7-36.7) *p=0.03 |
29.5 (20.5-48.1) p=0.08 |
30.7 (19.7-42.3) *p=0.003 |
| Radial apex (%) | 19.9 (11-29.1) | 14.0 (8.1-18.3) *p=0.01 |
18.4 (8.7-28.2) *p=0.01 |
19.5 (12.8-29.3) *p=0.002 |
22.1 (14.2-25.4) *p=0.01 |
17.8 (8.6-25.4) *p=0.01 |
19.3 (12.3-34.6) p=0.06 |
16.5 (11.4-26.7) *p=0.001 |
Values are displayed as medians and 25th/75th percentiles.
* Indicates significant difference from reference (sinus rhythm) as derived from Wilcoxon signed rank test for two related samples
𑃠indicates the overall significance of the effect of pacing site on each variable by non-parametric test analysis (Friedman test)
Table 5: Comparison of left ventricular torsional and strain parameters during sinus rhythm and during pacing with the different configurations after AMI.
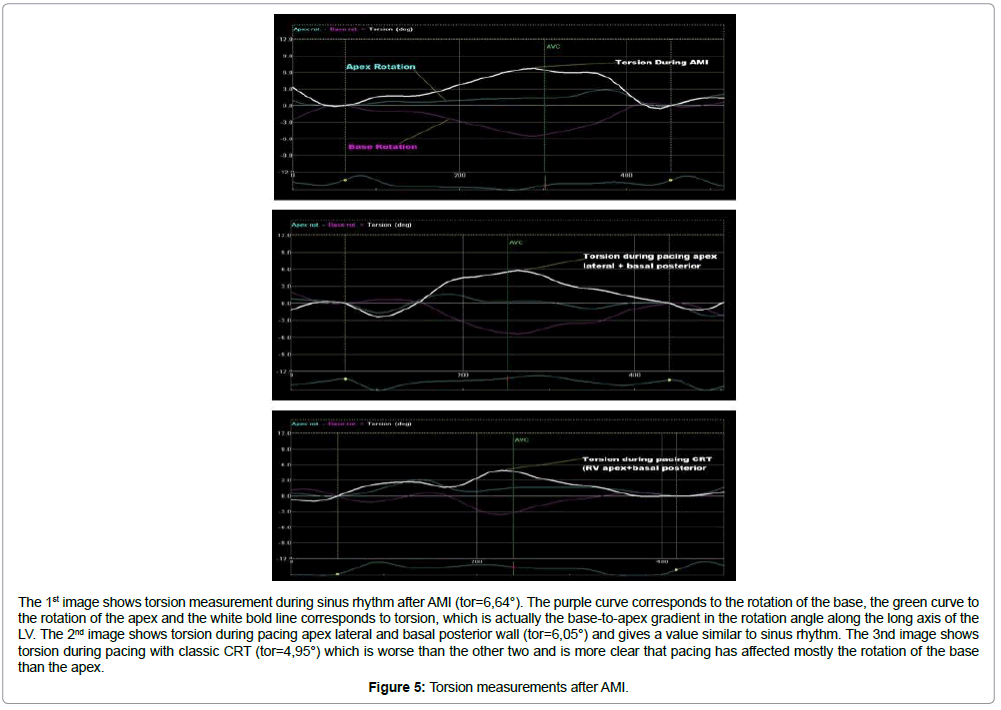
The 1st image shows torsion measurement during sinus rhythm after AMI (tor=6,64°). The purple curve corresponds to the rotation of the base, the green curve to the rotation of the apex and the white bold line corresponds to torsion, which is actually the base-to-apex gradient in the rotation angle along the long axis of the LV. The 2nd image shows torsion during pacing apex lateral and basal posterior wall (tor=6,05°) and gives a value similar to sinus rhythm. The 3nd image shows torsion during pacing with classic CRT (tor=4,95°) which is worse than the other two and is more clear that pacing has affected mostly the rotation of the base than the apex.
Figure 5: Torsion measurements after AMI.
Data analysis also revealed, that the combination of pacing LV apex lateral wall and LV basal posterior wall (site 6), manages to preserve almost all hemodynamic, echocardiographic, and rotational parameters at levels similar to sinus rhythm.
Discussion
This study is one of the few experimental works in the area of pacing, that combines hemodynamic data, conventional echocardiographic variables, and torsional and deformation parameters, to investigate the effects of different pacing sites in the intact and infarcted myocardium. The usage of only echocardiographic parameters was avoided, as the results of the PROSPECT study revealed that echocardiographic mechanical indices alone were unreliable measures of optimal LV response during biventricular pacing, although the STAR study (Speckle Tracking and Resynchronisation study) showed that speckle tracking echocardiography is a moderately helpful tool to predict CRT response [20,21].
Previous studies have demonstrated that single site LV pacing is superior to RV pacing [22-24]. In addition, these studies have revealed that LV apex pacing has a favourable hemodynamic effect, probably because pacing from the apex creates a relatively physiological propagation of electrical conduction [15,25-27]. Prior studies have demonstrated that the earliest part that is activated is the middle inferior septum. From there, the wave of depolarisation sweeps rapidly to the entire septal and apical myocardium, leaving the basal and posterior aspects of the heart to be the latest areas excited [28- 30]. However, it is the system of the Purkinje fibres that will pass the depolarisation through the myocardium. These fibres extend from the apex, where their concentration is high toward the atrioventricular septum and the base of the heart. Hence, pacing the apex could justify a favourable effect, since this is the site where the impulses exit the Purkinje system [31-33].
Right atrial-single site LV pacing, even though superior to right atrial-RV pacing, was not studied here, as it is not considered sufficiently reliable, especially in pacemaker-dependent patients with transvenously implanted LV leads, because of their higher dislodgement rate [34,35].
In this study, the pacing leads were kept outside the infarct zone. This was considered essential, since in our previous research works we managed to demonstrate that after LAD ligation, pacing inside the infarcted area deteriorated the LV function as compared to pacing in sites away from this area [16,36]. The same conclusion in clinical settings has been announced by Bleeker et al., who found that an LV lead, positioned in an area with a transmural scar, reduces the beneficial effects of CRT on the clinical outcome and cardiac performance [37].
The use of simultaneous biventricular pacing was considered necessary, since this is the usual modality of CRT to date. Moreover, as stated in the current European Society of Cardiology guidelines for CRT, this practice is largely empirically derived from pathophysiological reasoning, and from the evidence provided by earlier clinical trials [38]. Additionally, the RHYTHM II ICD trial has demonstrated that VV delay (interventricular delay) optimisation with sequential biventricular pacing did not improve hemodynamic response during CRT [39].
AV delays, as mentioned at the methodology, were not modified between the different pacing configurations. We decided to not include AV delays as a variable in an already too complex protocol, since there are a number of studies, which investigated pacing sites with different AV delays and the majority of them have concluded that the pacing site is more important than AV delay optimisation in enhancing a favourable pacing result [40,41]. This reflects the current European guidelines for CRT, where it is stated that current evidence does not strongly support the performance of AV and VV optimisation routinely, in all patients receiving CRT [38].
The present study shows that, in the intact myocardium, sinus rhythm is superior of all the different combinations of epicardial pacing sites that were studied. However after AMI, the pacing combination of LV apex lateral and LV basal posterior wall (site 6) preserves LV function, at a level similar to sinus rhythm. This finding probably has to do with the fact that this configuration combines two regions of the LV with discrete characteristics. Firstly, the basal posterior wall is a region of late activation. Studies have shown that pacing at the site of latest mechanical activation resulted in superior echocardiographic response after six months of CRT, and better prognosis during long-term follow-up [42,43]. This is the reason why most electro physiologists are targeting a posterolateral vein to place the LV lead at CRT. On the contrary, the lateral apex provides the nearest region to the apex of the LV outside the infarct zone, where the impulses exit the Purkinje system, leading to faster engagement of the pacing impulse.
Our findings indicated that the best LV performance after AMI did not occur at the shortest QRS length; however, this is further supported by the results of other researchers [44,45]. In addition, this is also one of the reasons that QRS duration after lead implantation is not the best indicator of CRT response.
Although in the present study, only the acute effects of different pacing sites were investigated, there are a number of studies in clinical settings, showing that the immediate response of LV twist after CRT predicts LV reverse remodelling [46,47]. In concordance with this, in the current study, LV twist was mostly preserved during pacing configuration 6, as compared to all other configurations, suggesting this to be an important site to be considered in future investigations.
Clinical implications
The quest for finding the best pacing site and the best pacing combination in the impaired myocardium is in progress. This quest is imperative, considering the numerous patients with an impaired myocardium that have to undergo cardiac surgery. Pacing wires are frequently inserted at the end of cardiac surgical procedures. Their main use is to improve haemodynamic function in the presence of arrhythmias, as well as to suppress atrial and ventricular tachyarrhythmias [48,49]. Moreover, pacing leads are used perioperative as a precaution of dangerous bradyarrhythmias. Our study provides additional information and options for pacing these patients.
Cardiac resynchronisation therapy is a major breakthrough in treatment for advanced congestive heart failure patients. However, there is substantial rate of non-response to this therapy, and this problem might become increasingly important, because it is anticipated that larger groups of heart failure patients are indicated to undergo the therapy [7,8]. Hence, there is extensive research in exploring various ways to increase the response to the technique. Our findings could guide the way to patients with anterior myocardial infarction that requires CRT. For these patients, we provide evidence that pacing the LV apex lateral and LV basal posterior wall is superior to the classic CRT. It is worth mention, that CRT is provided using epicardial pacing electrodes, because conventional access for lead positioning (via coronary sinus or by thoracotomy) results in an epicardial location of the LV pacing electrode. With the currently available leads, the LV apex lateral could be reached using the trans coronary venous approach, if the lead can be advanced far enough. Alternatively, a minimally invasive thoracotomy can be used to place the lead in this position, a technique that is already in use for patients who, anatomically, are not suitable for a transvenous approach [50].
Limitations
This is an experimental study in animals and the results should be used with caution in clinical situations. In addition, the present study assessed only acute effects of the different pacing sites on LV mechanics, even though in swine models, acute coronary occlusion, due to the lack of collaterals, depresses the LV function and prompts neurohormonal activation, thus satisfying several criteria characteristic of the heart failure phenotype [51]. The impact of the pacing site in patients with chronic ischemia, is more clinically relevant, and needs to be further investigated. In addition, to avoid intrinsic ventricular conduction, the pacing rate was set at 10 beats/ min above the intrinsic heart rate in each case, resulting in a high heart rate. These non-physiological conditions may preclude us from drawing conclusions about the long-term effects of chronic pacing with different combinations on LV performance. Moreover, this was an animal study focused on anterior myocardial infarction, and the potential future clinical application should be specifically aimed at patients with this type of infarction. Extrapolation of the data from the present study in other types of failing myocardium should be done with care.
Conclusions
In the present study, hemodynamic data, combined with speckle tracking analysis applied to conventional 2D echocardiography was used, to investigate the acute effects of different combination pacing sites on LV mechanics. In pig hearts with an intact myocardium sinus rhythm is superior from every pacing combination we studied, however after anterior AMI, the pacing combination of LV apex lateral wall and LV posterior wall managed to preserve LV function similar to sinus rhythm.
References
- Furman S, Schwedel JB (1959) An intracardiac pacemaker for Stokes-Adams seizures. N Engl J Med 261: 943-948.
- Toff WD, Camm AJ, Skehan JD (2005) Single-chamber versus dual-chamber pacing for high-grade atrioventricular block. N Engl J Med 353:145-155.
- Sharma AD, Rizo-Patron C, Hallstrom AP, O'Neill GP, Rothbart S, et al. (2005) Percent right ventricular pacing predicts outcomes in the DAVID trial.. Heart Rhythm. 2005; 2:830-834.
- Sweeney MO, Prinzen FW (2006) A new paradigm for physiologic ventricular pacing. J Am Coll Cardiol 47: 282-288.
- Tops LF, Schalij MJ, Holman ER, van Erven L, van der Wall EE, et al. (2006) Right ventricular pacing can induce ventricular dyssynchrony in patients with atrial fibrillation after atrioventricular node ablation. J Am Coll Cardiol 46: 1642-1648.
- Wiggers C (1925) The muscular reactions of the mammalian ventricles to artificial stimuli. Am J Physiol 73: 346-378.
- Cazeau S, Leclercq C, Lavergene T, Walker S, Varma C, Linde C, et al. (2001) Effects of multisite biventricular pacing in patients with heart failure and interventricular conduction delay. N Engl J Med 344:873-880.
- Cleland JGF, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al (2005) The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 352:1539-1549.
- Giudici MC, Karpawich PP (1999) Alternative pacing sites: it's time to define terms. Pacing Clin Cardiol 22:551-553.
- Deshmukh P, Casavant DA, Romanyshyn M, Anderson K (2000) Permanent, direct His-bundle pacing: a novel approach to cardiac pacing in patients with normal His-Purkinje activation. Circulation 101: 869-877.
- de Cock CC, Giudici MC, Twisk JW(2003)Comparison of the haemo-dynamic effects of right ventricular outflow-tract pacing with right ventricular apex pacing. Europace 5:275-280.
- Reisner SA, Lysyansky P, Agmon Y, Mutlak D, Lessick J, et al. (2004) Global longitudinal strain: a novel index of left ventricular systolic function. J Am Soc Echocardiogr 17:630-633.
- Leitman M, Lysyansky P, Sidenko S, Shir V, Peleg E, et al (2004) Two-dimensional strain-A novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr 17:1021-1029.
- Tops L, Delgado V, Bax JJ (2009) The role of speckle tracking strain imaging in cardiac pacing. Echocardiography 26: 315-323.
- Toumanidis S, Kaladaridou A, Bramos D, Skaltsiotes E, Agrios J, et al. (2014) Atrioventricular left ventricular apical pacing improves haemodynamic, rotational, and deformation variables in comparison to pacing at the lateral wall in intact myocardium: experimental study. Cardiol Res Pract 2014: 316290.
- Toumanidis ST, Kaladaridou A, Bramos D, Skaltsiotes E, Agrios JN, et al.(2013) Apical rotation as an early indicator of left ventricular systolic dysfunction in acute anterior myocardial infarction: experimental study. Hellenic J Cardiol 54:264-272.
- Arques S, Roux E, Luccioni R(2007) Current clinical applications of spectral tissue Doppler echocardiography (E/E' ratio) as a noninvasive surrogate for left ventricular diastolic pressures in the diagnosis of heart failure with preserved left ventricular systolic function. Cardiovasc Ultrasound 5:16.
- Takeuchi M, Borden WB, Nakai H, Nishikage T, Kokumai M, et al. (2007) Reduced and delayed untwisting of the left ventricle in patients with hypertension and left ventricular hypertrophy: a study using two-dimensional speckle tracking imaging. Eur Heart J 28:2756-2762.
- Arts T, Prinzen F, Delhaas T (2009) Potentials and limitations of ventricular torsion as indicator of cardiac function. IEEE Eng Med Biol Soc: 181-184.
- Bax JJ, Gorcsan J 3rd (2009) Echocardiography and noninvasive imaging in cardiac resynchronization therapy: results of the PROSPECT (Predictors of Response to Cardiac Resynchronization Therapy) study in perspective. J Am Coll Cardiol 53:1933–1943.
- Tanaka H, Nesser HJ, Buck T, Oyenuga O, Jánosi RA, et al. (2010) Dyssynchrony by speckle-tracking echocardiography and response to cardiac resynchronization therapy: results of the Speckle Tracking and Resynchronization (STAR) study. Eur Heart J14:1690-1700.
- Blanc JJ, Etienne Y, Gilard M, Mansourati J, Munier S, et al. (1997) Evaluation of different ventricular pacing sites in patients with severe heart failure: results of an acute hemodynamic study. Circulation 96: 3273-7327.
- Kass DA, Chen CH, Curry C, Talbot M, Berger R, et al. (1999) Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation 99:1567-1573.
- Auricchio A, Stellbrink C, Block M, Sack S, Vogt J, et al. (1999) Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. The Pacing Therapies for Congestive Heart Failure Study Group. The Guidant Congestive Heart Failure Research Group. Circulation. 99:2993–3001.
- LISTER JW, KLOTZ DH, JOMAIN SL, STUCKEY JH, HOFFMAN BF (1964) EFFECT OF PACEMAKER SITE ON CARDIAC OUTPUT AND VENTRICULAR ACTIVATION IN DOGS WITH COMPLETE HEART BLOCK. Am J Cardiol 14: 494-503.
- Tyers GFO (1970) Comparison of the effect on cardiac function of single-site and simultaneous multiple site ventricular stimulation after A-V block. J Thorac Cardiovasc Surg 59:211-217.
- Fei L, Wrobleski D, Groh W, Vetter A, Duffin EG, et al. ( 1999) Effects of multisite ventricular pacing on cardiac function in normal dogs and dogs with heart failure. J Cardiovasc Electrophysiol 10:935–946.
- Cassidy DM, Vassallo JA, Marchlinski FE, Buxton AE, Untereker WJ, et al. (1984) Endocardial mapping in humans in sinus rhythm with normal left ventricles: activation patterns and characteristics of electrograms. Circulation 70: 37-42.
- Durrer D, Van Dam RTh, Frend GE, Janse MJ, Meijler FL, et al.(1970)Total excitation of the isolated human heart. Circulation, 41: 899.
- Scher AM, Young AC, Malmgren AL, Erickson RV (1955) Activation of the interventricular septum. Circ Res 3: 56-64.
- Hondeghem M, Stroobandt R (1973) Purkinje fibers of sheep papillary muscle: occurrence of discontinuous fibers. Am J Anat 141:251-262.
- Spach MS, Huang SN, Ayers CR (1963) Electrical and anatomic study of the Purkinje system of the canine heart. Am Heart J 65: 664-673.
- Purkyne JE (1845) Mikroskopisch-neurologische Beobachtungen. Arch. f. Anat. Physiol. wiss. Med. 12: 281-295.
- Bildirici U, Vural A, Agacdiken A, Sahin T, Celikyurt U, et al. (2008) Comparison of the effects of left vs. right ventricular pacing on left ventricular remodelling. Europace 10: 1387-1391.
- Cojoc A, Reeves JG, Schmarkey L, Strieper MJ, Joyner RW, (2006) Effects of single-site versus biventricular epicardial pacing on myocardial performance in an immature animal model of atrioventricular block. J Cardiovasc Electrophysiol 17: 884-889.
- Toumanidis ST, Takos DJ, Tsirikos N, Bramos D, Kottis G (2011) Pacing within the ischemic area significantly decreases the left ventricular ejection fraction during experimental acute myocardial infarction. Pacing Clin Electrophysiol 34: 63-71.
- Bleeker GB, Schalij MJ, van der Wall EE, Bax JJ (2006) Postero-lateral scar tissue resulting in non-response to cardiac resynchronization therapy. J Cardiovasc Electrophysiol 17: 899-901.
- Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, et al. (2013) 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Europace. 8: 1070-1118.
- Boriani G, Muller CP, Seidl KH, Grove R, Vogt J, et al. (2006) Randomized comparison of simultaneous biventricular stimulation versus optimized interventricular delay in cardiac resynchronization therapy. Am Heart J 151: 1050–1058.
- Peschar M, de Swart H, Michels KJ, Reneman RS, Prinzen FW (2003) Left ventricular septal and apex pacing for optimal pump function in canine hearts. J Am Coll Cardiol 41: 1218-1226.
- Ypenburg C, van Bommel R, Delgado V, Mollema S, Bleeker G, et al. (2008) Optimal left ventricular lead position predicts reverse remodeling and survival after cardiac resynchronization therapy. J Am Coll Cardiol. 52: 1402-1409.
- Murphy RT, Sigurdsson G, Mulamalla S, Agler D, Popovic ZB, et al. (2006) Tissue synchronization imaging and optimal left ventricular pacing site in cardiac resynchronization therapy. Am J Cardiol 97: 1615-1621.
- Park RC, Little WC, O'Rourke RA (1985) Effect of alteration of left ventricular activation sequence on the left ventricular end-systolic pressure-volume relation in closed-chest dogs. Circ Res 57: 706-717.
- Buckingham TA, Candinas R, Schlapfer J, et al. (1997) Acute hemodynamic effects of atrioventricular pacing at different sites in the right ventricle individually and simultaneously. Pacing Clin Electrophysiol 20: 909–915.
- Devecchi P, Bolzani V, Sarasso G, Piccinino C, Marti G, et al. (2009) Left ventricular torsion in paced patients. J Cardiovasc Med (Hagerstown) 10: 921-927.
- Bertini M, Marsan NA, Delgado V, van Bommel RJ, Nucifora G, et al. (2009) Effects of cardiac resynchronization therapy on left ventricular twist. J Am Coll Cardiol 54:1317-1325.
- Janousek J, Vojtovic P, Hucin B, Tláskal T, Gebauer RA, et al. (2001) Resynchronization pacing is a useful adjunct to the management of acute heart failure after surgery for congenital heart defects. Am J Cardiol. 88:145–149.
- Zimmerman FJ, Starr JP, Koenig PR, Smith P, Hijazi ZM, et al. (2003) Acute hemodynamic benefit of multisite ventricular pacing after congenital heart surgery. Ann Thorac Surg 75: 1775-1780.
- Pham PP, Balaji S, Shen I, Ungerleider R, Li X, et al. (2005) Impact of conventional versus biventricular pacing on hemodynamics and tissue Doppler imaging indexes of resynchronization postoperatively in children with congenital heart disease. J Am Coll Cardiol 46: 2284-2289.
- Jaroszewski DE, Altemose GT, Scott LR, Srivasthan K, Devaleria PA, et al. (2009) Nontraditional surgical approaches for implantation of pacemaker and cardioverter defibrillator systems in patients with limited venous access. Ann Thorac Surg 88: 112-116.
- White FC, Bloor CM (1981) Coronary collateral circulation in the pig: correlation of collateral flow with coronary bed size. Basic Res Cardiol 76: 189-196.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi