Research Article, J Plant Physiol Pathol Vol: 13 Issue: 1
Physiochemical Characterization of Citrus Fruits for Identification of Potential Cultivars in Pakistan
Muhammad Aqib Shah1, Hafiz Saif Ur Rehman Shah2, Ume Habiba1, Muhammad Amjad Farooq3, Saqib Hussain Bangash4, Irsa Ejaz5, Ume Hani6, Daniel Adjibolosoo5, Tahir Abbas Khan7, Iftikhar Ali Ahmad8, Raheela Yasin9, Ume Laila10 and Iza Fatima11*
1Department of Horticulture, MNS-University of Agriculture, Multan, Punjab, Pakista
2Department of Horticulture, Bahauddin Zakariya University, Multan, Punjab, Pakistan
3Department of Horticulture Sciences, University of Agriculture, Faisalabad-38040, Pakistan
4Department of Agriculture, Guangxi University, Nanning, Guangxi, China
5Department of Agronomy and Biotechnology, China Agricultural University, Beijing, China
6Department of Forestry and Wood Sciences, Czech University of Life Sciences, Prague, Czech Republic
7Department of Agronomy, Jiangxi Agricultural University, Nanchang, Jiangxi, China
8Department of Resources and Environment, Huazhong Agricultural University, Wuhan, 430070, Hubei, China
8Department of Life Sciences and Technology, Guangxi University, Nanning, Guangxi, China
9Department of Biological and Environmental Science, University of Gothenburg, Gothenburg, Sweden
10Department of Entomology and Plant Pathology, Oklahoma State University, Stillwater, OK 74078, USA
11Department of Entomology and Plant Pathology, Oklahoma State University, Stillwater, OK 74078, USA
*Corresponding Author:Iza Fatima
Department of Entomology and Plant Pathology, Oklahoma State University, Stillwater, OK 74078, USA
E-mail:ifatima@okstate.edu
Received date: 11 December, 2023, Manuscript No. JPPP-23-122537;
Editor assigned date: 13 December, 2023, PreQC No. JPPP-23-122537 (PQ);
Reviewed date: 27 December, 2023, QC No. JPPP-23-122537;
Revised date: 16 January, 2025, Manuscript No. JPPP-23-122537 (R);
Published date: 23 January, 2025, DOI: 10.4172/2329-955X.1000372
Citation: Shah MA, Shah HSUR, Habiba U, Farooq MA, Bangash SH (2025) Physiochemical Characterization of Citrus Fruits for Identification of Potential Cultivars in Pakistan. J Plant Physiol Pathol 13:1.
Abstract
The present study aimed to analyze the variability in fruit quality parameters from the citrus germplasm unit in Arifwala, Pakistan. Healthy, uniform, and disease-free fruits were sampled at commercial maturity from 10-year-old citrus plants grafted on rough lemon rootstock grown at the citrus germplasm unit during 2021-2022. Twenty-eight cultivars from five citrus groups (sweet orange, mandarin, grapefruit, lemon, and lime) were kept under investigation. Sampled cultivars were subjected to the following fruit quality parameters: Physical (fruit weight, fruit diameter, number of seeds per fruit, juice, peel, and rag content) and biochemical (juice pH, total soluble solids, titratable acidity, ripening index, vitamin C, total phenolic contents, antioxidant, anthocyanin, and sugars (sucrose and fructose)) and data was recorded. The results of twenty-eight cultivars demonstrated a significant difference among physiochemical parameters. Rhode red valencia cultivar from the sweet orange group; minniola and honey cultivars, from the mandarin group, had significant quality traits. Reed and frost marsh, from the grapefruit group; mozero lemon from the lemon group and Persian lime from the lime group were considered best cultivars with good quality traits. The cultivars identified from the current study were considered valuable from the view of physiochemical parameters.
Keywords: Citrus, Genetic variation, Germplasm, Fruit quality, Physiological; Biochemical
Introduction
Wild relatives, old accessions, and landraces held in germplasm collections offer an important genetic supply for existing and new breeding challenges. Genetic variability is estimated by morphological, agronomic, and biochemical methods. The selection of suitable cultivars from a cultivar’s panel requires basic information on the morphological and biochemical characteristics of variable genotypes for consideration in the breeding program. Moreover, the morphological and biochemical descriptions are certified and widely accepted for registration and conservation of germplasm. In Pakistan, citrus is cultivated on an area of 194 thousand hectares with a 2.4 million tons annual yield. Interestingly, the citrus industry is recognized for the large production of kinnow and mandarin. Currently, the kinnow industry is facing many challenges due to the low yield potential of trees, poor tree health, and many postharvest issues. This monoculture system creates great risk for the citrus industry if any hazard occurs, and it will destroy the citrus industry in Pakistan. Citrus growers are also disappointed by these challenges. The appraisal of promising grafted citrus cultivars with the best morphological and biochemical properties is the best strategy to strengthen the citrus industry and improve the quality traits of fruits.
Grafting is also a common practice in fruit crops to enhance the desirable traits. In a study by Ishfaq, et al. four grapefruit cultivars grafted onto rough lemon rootstock in the citrus groves of Barani Agricultural Research Institute, Chakwal, were examined for different fruit quality traits [1]. The results showed that shamber and red blush had maximum juice contents (51%), and fruit weight was 503 to 510 g/fruit, 31% peel, and 18% rag. A study was conducted by Bermejo and Cano to examine the changes occurring in organic acids, sugars, and vitamin C [2]. Results showed that sugars are produced during fruit growth and development. Besides the fruit maturity stage, the type of cultivar and rootstock were also found to be influencing the concentrations of organic and ascorbic acid. Six pigmented grapefruit cultivars (Fire, star ruby, red blush, pink ruby, shamber, and rio red) were analyzed by Usman, et al. for chemical and quantitative trials [3]. In quantitative characteristics, total dissolvable solids were higher in 'star ruby' (8.51°Brix) than in 'pink ruby' (8.87°Brix). The high values of TSS: TA (6-7.8) and ascorbic acid contents (61-73 mg/100 g) were found in 'red blush' and 'pink ruby'.
Citrus fruits are known for their unique taste and many health beneficial properties. The consumption of citrus fruits provides sugars, organic acids, minerals, dietary fiber, and several phytochemicals, such as vitamins A, B, C, and E, and antioxidant compounds. Citrus fruits have wide genetic diversity; commercially cultivated groups are lemon, lime, sweet orange, grapefruit, mandarin, and pomelo. Citrus fruit quality standards are particularly difficult to define due to genetic variability since external and internal quality, as well as nutritional and nutraceutical qualities can differ significantly. Furthermore, many factors (agronomic, environmental, harvesting time, and cultural practices) influence the phytochemicals and nutrient accumulation in fruits, which greatly depends upon the fruit’s maturation process. Usually, peel color, TSS, acidity, and juice content are important quality attributes; nevertheless, the relative relevance of such attributes varies depending on the variety of citrus.
Several studies have been performed to identify the best citrus cultivars of grapefruit and sweet orange based on morphological and biochemical traits; however, fewer studies have been performed on the postharvest changes of mandarin, lemon, and lime. Thus, the present study aims to screen potential citrus cultivars by investigating morphological and physiochemical traits in fresh fruits of citrus cultivars grafted on rough lemon rootstock that exhibit desirable fruit yield and quality traits. The findings of this study will provide valuable insights into the selection of appropriate citrus cultivars for commercial production, thereby contributing to the growth and development of the citrus industry.
Materials and Methods
Geographical location
The study was conducted at the citrus germplasm unit, Arifwala, which is situated in Punjab province, Pakistan. The geographical coordinates of the study site are 30°17'0"N and 73°4'0"E.
Experiment material
Healthy, uniform, and disease-free fruits were harvested at commercial maturity from 10-year-old citrus plants grown at the citrus germplasm unit, Arifwala district Pakpattan, Punjab, Pakistan, during 2021-2022. Scion cultivars were grafted onto the ‘Rough Lemon’ rootstock. Twenty-eight cultivars were selected from sweet orange (Rhode red valencia, casa granda, at wood early navel, navelina, marrs early, pine apple, amber sweet, musambi, succari, salusatina and cara cara navel), mandarin (Seedless kinnow, kinnow, danscy, minniola and honey), grapefruits (Red blush, star ruby, frost marsh, reed, flame and shamber), lemon (Mozero lemon, China lemon, frost lisbon and M-H R. 173) and lime (Persian lime and Kaghzi lime) (Table 1). In total, eighteen uniform-size and mature fruits were harvested from one plant for each cultivar. There were three replications and each replication had six fruits. For assessing the fruit quality, the physiochemical parameters were examined in the postharvest science and technology laboratory at the Muhammad Nawaz Shareef University of Agriculture, Multan, Pakistan.
Materials and Methods
Geographical location
The study was conducted at the citrus germplasm unit, Arifwala, which is situated in Punjab province, Pakistan. The geographical coordinates of the study site are 30°17'0"N and 73°4'0"E.
Experiment material
Healthy, uniform, and disease-free fruits were harvested at commercial maturity from 10-year-old citrus plants grown at the citrus germplasm unit, Arifwala district Pakpattan, Punjab, Pakistan, during 2021-2022. Scion cultivars were grafted onto the ‘Rough Lemon’ rootstock. Twenty-eight cultivars were selected from sweet orange (Rhode red valencia, casa granda, at wood early navel, navelina, marrs early, pine apple, amber sweet, musambi, succari, salusatina and cara cara navel), mandarin (Seedless kinnow, kinnow, danscy, minniola and honey), grapefruits (Red blush, star ruby, frost marsh, reed, flame and shamber), lemon (Mozero lemon, China lemon, frost lisbon and M-H R. 173) and lime (Persian lime and Kaghzi lime) (Table 1). In total, eighteen uniform-size and mature fruits were harvested from one plant for each cultivar. There were three replications and each replication had six fruits. For assessing the fruit quality, the physiochemical parameters were examined in the postharvest science and technology laboratory at the Muhammad Nawaz Shareef University of Agriculture, Multan, Pakistan.
| Sr. no. | Citrus groups | Varieties |
| 1 | Sweet orange | At wood early navel |
| 2 | Navelina | |
| 3 | Marrs early | |
| 4 | Pine apple | |
| 5 | Amber sweet | |
| 6 | Musambi | |
| 7 | Succari | |
| 8 | Salusatina | |
| 9 | Cara cara navel | |
| 10 | Rhode red valencia | |
| 11 | Casa granda | |
| 12 | Mandarin | Seedless kinnow |
| 13 | Kinnow | |
| 14 | Danscy | |
| 15 | Minniola | |
| 16 | Honey | |
| 17 | Grape fruit | Red blush |
| 18 | Star ruby | |
| 19 | Frost marsh | |
| 20 | Reed | |
| 21 | Flame | |
| 22 | Shamber | |
| 23 | Lemon | Mozero lemon |
| 24 | China lemon | |
| 25 | Frost lisbon | |
| 26 | M-H-R. 173 | |
| 27 | Lime | Persian lime |
| 28 | Kaghzi lime |
Table 1: List of citrus groups and varieties.
Physical parameters
Fruit weight, juice content, rag content, and peel content were determined by using a digital weight balance (NVT-2000 OHAUS Corporation, USA), and the average was calculated among three replications. The diameter of the Fruit (horizontally and vertically) was determined by using a Vernier caliper (Mitutoyo, 938882 Seiko Apan's Corporation). The number of seeds per fruit, healthy and unhealthy seeds was counted and the average was computed.
Bio-chemical parameters
Total soluble solids (°Brix): Total Soluble Solids (TSS) in citrus juice were calculated using a portable refractometer (PAL-1, Atago, Japan). On the refractometer prism, 1-2 drops of fruit juice were dropped and the results were obtained in °Brix. Before and after sample analysis, the calibrated instrument was washed with distilled water.
Titratable acidity (mg/100 g): Titratable Acidity (TA) in citrus juice samples was measured through a method used by Horwitz, in which 10 ml of fruit juice was poured into a 100 ml conical flask and diluted with distilled water to a volume of 50 ml [4]. Then titration was performed against 0.1 N NaOH by adding 2-3 drops of phenolphthalein indicator until the pinkish color endpoint was obtained. The total titratable acidity was determined using the formula below.
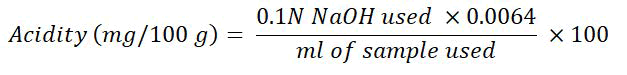
Ripening index (TSS/TA): The ripening index was found after sthe division of the TSS value by the respective TA value of each sample.
Juice pH: A pH meter (Starter 3100 OHAUS Corporation, USA) was used to determine the pH of citrus fruit juice. The juice was collected in a beaker. The bulb of the pH meter was dipped in fruit juice and a constant reading was noted.
Ascorbic acid (mg/100 ml): Ruck's method was applied to determine the ascorbic acid level of juice [5]. The final volume was produced by adding a 0.4 percent oxalic acid solution to a 10 ml juice sample and put into a 100 ml volumetric flask. A 5 ml filtrate was collected and its titration with 2,6-dichlorophenolindophenol dye was performed till a bright pink color endpoint (which lasted for a minimum of 15 sec). The ascorbic acid content was estimated by applying the formula below.
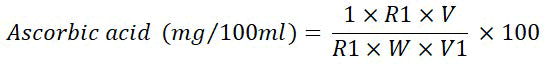
Where,
R=Dye used (ml) to titrate against 2.5 ml of reference solution (1 ml standard ascorbic acid+1.5 ml 0.4 percent oxalic acid) (standard reading)
R1=ml of dye used to titrate against aliquot V1 (sample reading)
V=Volume of oxalic acid with 0.4 percent oxalic acid
V1=ml of juice was used to titrate
W=ml of juice taken
(In a 200 ml volumetric flask, 42 mg NaHCO3 and 52 mg 2,6 dichlorophenol indophenol were added, and the final volume was made with distilled water).
Total phenolic contents (GAE mg/100): Total Phenolic Contents (TPC) in citrus fruit were measured by adopting the Folin-Ciocalteu procedure. In an Eppendorf, 100 µL supernatant and 200 µL F-C reagent (10%) were taken and mixed through a vortex. Afterward, 800 µL of Na2CO3 (700 mM) solution was added to it, followed by mixing and dark incubation for 30 min at room temperature. The absorbance of samples (200 µL) was recorded at 765 nm using the Epoch Eliza Reader.
Antioxidant capacity (% inhibition of DPPH): Antioxidant capacity was determined using the method described by Brand Williams, et al. in which 50 µL of supernatant was mixed with 5 ml of 0.004% DPPH (2,2-diphenyl-1-picrylhydrazyl) in a test tube, and incubated in the dark for 30 minutes at room temperature [6]. Then, 200 µL of the mixture was transferred to a microplate, which was analyzed with an Epoch Eliza Reader at 517 nm absorbance.
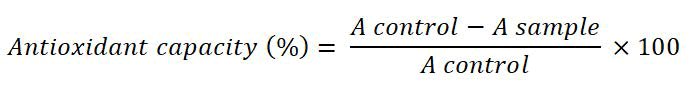
Where: A control=Absorbance of DPPH A sample=Absorbance of sample extract
Total anthocyanin content (mg/100 gFW): Total anthocyanin contents were analyzed by adopting the procedure of Giusti and Wrolstad [7]. A 1 ml frozen sample (juice) and 10 ml extraction mixture containing HCL+methanol (15:85) were added into the falcon tube. Incubation was done for 1 h at 25°C in dark conditions. Afterwards, the mixture was centrifuged at 4°C and 9000 rpm for 5 min. The supernatant was collected and subjected to the Epoch Elisa Reader to find the absorbance values at 530, 620, and 650 sssnm.
Anthocyanin (mg/100 gFW)=(A530–A620)–0.1 (A650–A620)
Where: A=Absorbance value at a specific wavelength
Sugar (sucrose and fructose) mg/100 ml: 1 ml of each standard was added to 5 ml of concentrated H2SO4 and 1 ml of phenol 5 percent aqueous solution. For color development, it was placed in a water bath at 100°C for 5 min and then vortexed for 30 sec. The reference solutions were made in the same way as the sample solutions, excluding the one in which 1 ml of the sample was substituted with distilled water. Then the standard's absorbance was recorded at 490 nm using an Epoch Eliza Reader. The data was collected, and a calibration curve was generated.
Sample preparation and analysis of total sugar content in fruit juice samples: A 104 times dilute solution was prepared by centrifuging the 10 ml of juice samples at 4000 rpm for 20 min. The total sugar content was determined using the above procedure. To determine the results, the following formulas were used for sucrose and fructose:
Sucrose=0.4493X–0.1402
Fructose=1.0715X–0.3019
Where:
X=Absorbance value at a specific wavelength
Experimental design and statistical analysis: The study was conducted under the Randomized Complete Block Design (RCBD). The experimental data was statistically analyzed through the application of ANOVA and the significant difference between or among treatments was determined by the mean comparison test at p ≤ 0.05 using “Agricolae” in RStudio.
Results
Physiological traits
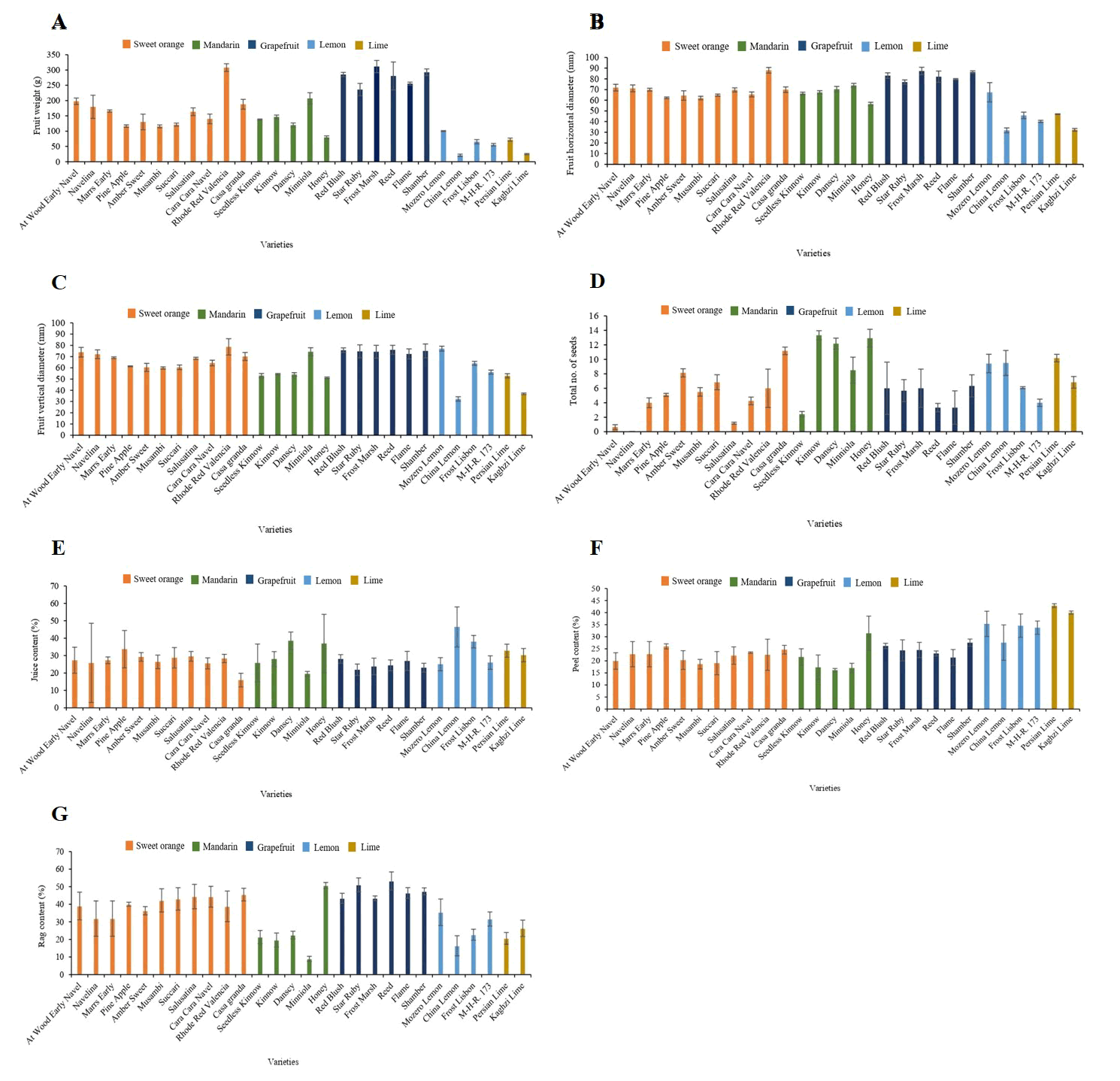
Fruit weight, size, and total number of seeds: A significant variation was found among sweet orange, mandarin, grapefruit, lemon, and lime cultivars in terms of average fruit weight. The maximum fruit weight of cultivars from the sweet orange, mandarin, grapefruit, lemon, and lime groups was observed in rhode red valencia (308.00 g), minniola (207.08 g), frost marsh (311.33 g), mozero lemon (100.33 g) and Persian lime (72.17 g), respectively. However, the minimum fruit weight of cultivars from the sweet orange, mandarin, grapefruit, lemon, and lime groups was observed in musambi (115.61 g), honey (79.83 g), star ruby (236.00 g), China lemon (21.17 g) and Kaghzi lime (25.00 g), respectively (Figure 1A).
Figure 1: Performance of twenty-eight citrus cultivars for physical parameters.
A statistically significant difference was found in the fruit’s horizontal and vertical diameters among the studied cultivars in each group. The maximum fruit horizontal and vertical diameter values from the sweet orange, mandarin, grapefruit, lemon, and lime groups were observed in Rhode red valencia (88 and 78.67 mm, respectively), minniola (73.83 and 74.33 mm, respectively), frost march (87.33 and 74.33 mm, respectively), mozero lemon (67.42 and 77.08 mm, respectively) and Persian lime (47.00 and 52.92 mm, respectively). The minimum fruit horizontal and vertical diameter values from grapefruit, mandarin, sweet orange, lime and lemon groups were observed in musambi (62.04 and 59.88 mm, respectively), honey (56.42 and 51.17 mm, respectively), star ruby (77.00 and 74.67 mm, respectively), China lemon (31.83 and 32.33 mm, respectively) and Kaghzi lime (32.33 and 36.83 mm, respectively) (Figure 1B and C).
A significant variation was found among sweet orange, mandarin, lemon, and lime cultivars in terms of the total number of seeds and healthy and unhealthy seed characteristics, except for grapefruit. The maximum number of seeds was recorded in casa granda 11.17 (sweet orange), kinnow 13.33 was statistically equal to danscy 12.17 and honey 12.92 (mandarin), China lemon 9.50 was statistically equal to mozero lemon 9.42 (lemon), and Persian lime 10.17 (lime group). The minimum number of seeds was observed in navelina and at wood early navel (0), followed by Salusatina (1.17) (sweet orange), seedless kinnow (2.42) (mandarin), reed and flame (3.33) (grapefruit), M-H-R. 173 (4.00) (lemon) and kaghzi lime (6.83) (lime), as shown in Figure 1D.
Fruit contents (juice, peel, and rag content): A significant variation was found among lemon cultivars in terms of juice percentage, and other groups of sweet orange, mandarin, grapefruit, and lime showed non-significant variation among the cultivars. The highest percentage of juice content in the lemon group was observed in China lemon (46.48%) and frost lisbon (38.01%). The minimum juice content of lemon was observed in M-H-R 173 (26.02%). Other citrus groups showed the maximum percentage of juice content; however, the casa granda cultivar (15.95%) of the sweet orange group showed the lowest percentage of juice content as shown in Figure 1E.
A significant variation was found among mandarin and lime cultivars in terms of the percentage of peel content, and other groups of sweet orange, grapefruit, and lemon showed non-significant variation among the cultivars. The highest percentage of peel content was recorded in the Persian lime (42.90%) of the lime group and the minimum was observed in kaghzi lime (39.98%), as shown in Figure 1F. As compared to other citrus group’s cultivars, danscy (16.14%) of the mandarin group showed the lowest peel content. The maximum percentage of peel content in the mandarin group was observed in the honey cultivar (31.45%).
A significant variation was found among sweet orange, mandarin, grapefruit, and lemon cultivars in terms of rag content, except for lime. The maximum percentage of rag content in the sweet orange group was observed in rhode red valencia (45.52%), in mandarin, honey (50.68%), and in grapefruit, reed (53.24%), which is statistically equal to star ruby and shamber (51.07 and 47.36%, respectively). In lemon, maximum rag content was recorded in mozero lemon and M-H-R 173 (35.46 and 31.64%, respectively). The minimum rag content of sweet orange was recorded in casa granda (14.04%) and in mandarin, while minniola (8.95%) has the minimum rag content. In grapefruit, red blush has a minimum rag content of 43.41%, which is statistically equal to frost marsh (43.43%), and in lemon, China lemon (16.38%) showed a minimum rag content. A statistically non-significant result was found among sweet orange, mandarin, grapefruit, lemon, and lime cultivars in terms of seed percentage character (Figure 1G).
Biochemical traits
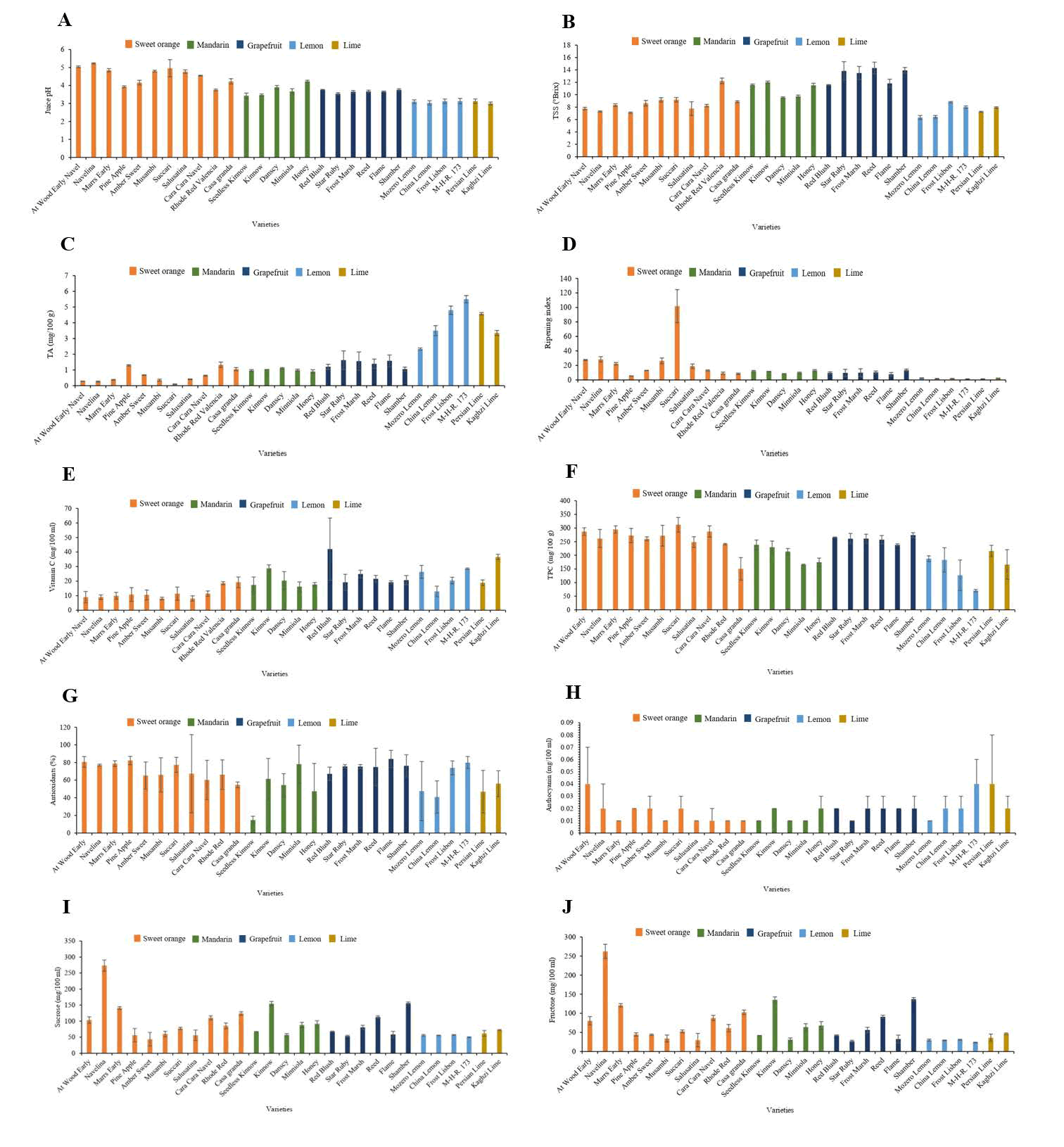
Juice pH, TSS, and TA: A significant variation was found among sweet orange, mandarin, and grapefruit cultivars in terms of pH character, and other groups, such as lemon and lime, showed non significant variation among the cultivars. The maximum juice pH of sweet orange was recorded in navelina (5.23) and at wood early navel (5.04), followed by Succari (4.96); in mandarin, it was maximum in Honey (4.23); and in grapefruit, red blush showed maximum juice pH (3.76), which was statistically equal to frost marsh, reed, flame and shamber (3.65, 3.67, 3.66 and 3.76, respectively), as shown in Figure 2A. The minimum juice pH of the sweet orange group was observed in rhode red valencia (3.75), which is statistically equal to pine apple (3.93). In mandarin, seedless kinnow showed a minimum juice pH of 3.43, followed by Kinnow (3.47), and in grapefruit, star ruby had a minimum juice pH of 3.54, as shown in Figure 2A.
Figure 2: Performance of twenty-eight citrus cultivars for biochemical parameters.
A significant variation was found among sweet orange, mandarin, grapefruit, lemon, and lime cultivars in terms of TSS content. The maximum TSS of sweet orange was observed in rhode red valencia (12.23°Brix), in mandarin, kinnow (12.00°Brix), grapefruit, reed (14.30°Brix), which was statistically equal to star ruby, frost marsh, and shamber (13.83°Brix, 13.50°Brix, and 13.93°Brix, respectively), in lemon, frost lisbon (8.80°Brix) and in lime, kaghzi lime (7.93°Brix) showed maximum TSS. However, the minimum TSS of the sweet orange group was recorded in pine apple (7.10°Brix), mandarin, danscy (9.53°Brix), followed by Minniola (9.73), lemon, mozero lemon (6.33°Brix) and in lime, Persian lime (7.23°Brix) (Figure 2B).
A significant variation was found among sweet orange, mandarin, lemon, and lime cultivars in terms of TA. Grapefruit showed nonsignificant results among the cultivars. The maximum TA of sweet orange was recorded in rhode red valencia and pine apple (1.33 and 1.29 mg/100 g, respectively). In mandarin, danscy (1.11 mg/100 g), which was statistically equal to kinnow (1.02 mg/100 g), in lemon, M H-R 173 (5.50 mg/100 g), and Persian lime (4.57 mg/100 g) showed maximum TA. The minimum TA of sweet orange was observed in succari (0.09 mg/100 g), in mandarin, honey (0.90 mg/100 g), in lemon, mozero lemon (2.33 mg/100 g) and in lime, kaghzi lime (3.35 mg/100 g) showed the minimum TA as shown in Figure 2C.
Ripening index and vitamin C: A significant variation was found among sweet orange, mandarin, lemon, and lime cultivars in terms of ripening index (TSS/TA). Grapefruit showed non-significant results among cultivars. The maximum ripening index of sweet orange was observed in succari (101.82), honey (12.98) from the mandarin group, which was statistically equal to seedless kinnow and kinnow (12.09 and 11.72, respectively), in lemon, mozero lemon (2.73) and in lime, Kaghzi lime (2.38), which showed the maximum ripening index. The minimum ripening index of sweet orange was recorded in pine apple (5.49), followed by casa granda (8.54). In mandarin, danscy (8.60), lemon, M-H-R. 173 (1.45) and in lime, Persian lime (1.58) showed a minimum ripening index (Figure 2D).
A significant variation was found among sweet orange, mandarin, grapefruit, lemon, and lime cultivars in terms of vitamin C contents. The maximum value of vitamin C in sweet orange was observed in casa granda and rhode red valencia (19.20 and 18.60 mg/100 ml, respectively), in mandarin kinnow (28.80 mg/100 ml), in grapefruit, red blush (42.00 mg/100 ml), in lemon M-H-R. 173 (28.50 mg/100 ml) and in lime, kaghzi lime (36.60 mg/100 ml). In the sweet orange group, minimum vitamin C content was recorded in musambi and salusatina (8.10 mg/100 ml), in mandarin, minniola (16.20 mg/100 ml), in grapefruit, star ruby, and flame (19.20 mg/100 ml), in lemon, China lemon (12.90 mg/100 ml) and in lime, Persian lime (18.90 mg/100 ml), which showed minimum vitamin C content, as shown in Figure 2E.
Content of total phenolic content, antioxidant, and anthocyanin: A significant variation was found among sweet orange, mandarin, and lemon cultivars in terms of Total Phenolic Contents (TPC); however, grapefruit and lime showed non-significant variation among the cultivars. The highest value of TPC of sweet orange was observed in Succari (312.10 GAE mg/100 g), which was statistically equal to at Wood Early Navel, marrs early, pine apple, musambi, and cara cara navel (287.01, 294, 272.64, 271.87 and 287.14 GAE mg/100 g, respectively). In mandarin, Seedless Kinnow (238.64 GAE mg/100 g), followed by kinnow and danscy (229.54 and 213.57 GAE mg/100 g, respectively), and in lemon, mozero lemon (187.60 GAE mg/100 g), which was statistically equal to China lemon and frost lisbon (183.03 GAE mg/100 g and 126.91 GAE mg/100 g, respectively). The minimum value of TPC in the sweet orange group was recorded in casa granda (150.40 GAE mg/100 g). In mandarin, minniola (165.31 GAE mg/100 g) and in lemon, M-H-R. 173 (70.40 GAE mg/100 g) showed the minimum TPC, as shown in Figure 2F. However, a non significant variation was found among sweet orange, mandarin grapefruit, lemon, and lime cultivars in terms of antioxidant and anthocyanin characters.
Among the five citrus groups, grapefruit showed the highest percentage of antioxidants. Flame cultivar showed the highest percentage of 84.06%, followed by shamber (76.24%) and star ruby (75.60%). The pine apple cultivar from the sweet orange group showed a maximum percentage of 82.18, followed by at wood early navel (80.58%) as shown in Figure 2G. The casa granda cultivar showed a percentage of 54.58, which is similar to the danscy cultivar (54.40%) from the mandarin group. The minimum antioxidants were observed in the seedless kinnow cultivar (14.72). As compared to others, kaghzi lime and Persian lime from the lime group showed fewer antioxidants (55.95 and 46.92%, respectively). Maximum antioxidants were observed in M-H-R. 173 cultivar (79.89%) of lemon group and minimum were observed in China lemon cultivar (40.86%).
The percentage of anthocyanin showed less variation among different cultivars, however, the wood early navel from the sweet orange group, M-H-R. 173 from the lemon group and Persian lime from the lime group showed a maximum anthocyanin percentage of 0.04. Marrs early, musambi, salusatina, cara cara navel, rhode red valencia, casa granda cultivar from the sweet orange group, seedless kinnow, danscy, minniola, from manadarin group, star ruby from grapefruit group, mozero lemon from the lemon group showed minimum anthocyanin of 0.01% (Figure 2H).
Sugar (sucrose and fructose): A significant variation was found among sweet orange, mandarin, grapefruit, and lemon cultivars in terms of sucrose and fructose content. A nonsignificant variation was found among lime cultivars. The maximum values of sucrose and fructose in sweet orange were observed in navelina (272.99 and 262.32 mg/100 g, respectively), in mandarin, kinnow (154.60 and 135.47 mg/100 g, respectively), in grapefruit, shamber (156.10 and 137.07 mg/100 g, respectively) and in lemon, frost lisbon (56.88 and 30.76 mg/100 g, respectively). The minimum values of sucrose and fructose in sweet orange were recorded in salusatina (55.91 and 29.71 mg/100 g, respectively), in mandarin, danscy (57.03 and 30.92 mg/100 g, respectively), in grapefruit, star ruby (53.36 and 26.99 mg/100 g, respectively) and in lemon, M-H-R. 173 (50.07 and 23.45 mg/100 g, respectively), which showed the lowest sugar content, as shown in Figure 2I and J.
Discussion
Physiological traits
Fruit weight, size, and total number of seeds: Variations in sweet orange, mandarin, grapefruit, lemon, and lime cultivars were detected based on the findings of the present research, indicating that temperature has a direct effect on the form and weight of fruit. Low fruit weight was observed in musambi, honey, star ruby, China lemon, and kaghzi lime cultivars, which is in harmony with the report of Ali, et al. where a decrease in strawberry fruit weight was observed as the mean temperature increased [8]. Furthermore, pre-harvest operations can affect citrus fruit weight and quality, as the use of different paper bags at the pre-harvest stage decreased fruit weight and size in pear fruit and increased it in mango. In the current study, fruit size differed among different citrus groups and cultivars. The highest fruit horizontal and vertical diameters were observed in rhode red valencia, minniola, frost march, mozero lemon, and Persian lime; however, less was observed in musambi, honey, star ruby, China lemon, and kaghzi lime, which suggests that fruit shapes and sizes are mostly quantitatively inherited. Based on the findings of this study, it is possible to hypothesize that the mutations that have resulted in increased fruit size could be due to increases in carpel and locule numbers.
In the present study, the maximum number of seeds was recorded in casa granda (sweet orange), kinnow (mandarin), China lemon (lemon), and Persian lime (lime group). This variation in the number of seeds is consistent with the previous study on the Nadorcott variety of mandarin group, which explains that the number of seeds in a cultivar varies on the method of pollination and application of different treatments. Navelina and at wood early navel (sweet orange), seedless kinnow (mandarin), reed and flame (grapefruit), M-H-R. 173 (lemon) and kaghzi lime (lime) showed less number of seeds, which could be due to the sulfur and other growth regulator treatment on trees at the flowering stage as sulfur treatment was reported to reduce pollen tube growth by 94-100%, saccharose treatment also led to growing most pollen tube from inside the flower stigma in citrus cultivars, which leads to poor seed growth or big size seeds in citrus cultivars.
Fruit contents (juice, peel, and rag content): Citrus juice differed by species as well as by variety. In this research, the highest percentage of juice content in the lemon group was observed in China lemon and frost lisbon, which agrees with the study of Tounsi, et al. [9]. The lemon group was reported with the highest juice aroma content, followed by blood orange and mandarin, however, blood orange achieved the highest juice percentage. Less juice percentage was observed in the casa granda cultivar of sweet orange, which explains that variation in citrus juice content is due to variations in species, cultivars, and regions. Maximum percentage of juice content was also observed in rhode red valencia of the sweet orange group, minniola in mandarin, and mozero lemon in the lemon group, which were also bigger in fruit size, leading to a hypothesis that the content of juice was high in large-diameter fruits and a similar observation was also reported in kiwifruit. The rag and peel content of citrus fruit also varies from species to species and variety to variety. Honey cultivar in the mandarin group and persian lime in the lime group showed maximum peel content and danscy and kaghzi lime showed minimum peel content, which could be due to high or low solute content resulting in thick and thin peel in citrus fruits.
Biochemical traits
Juice pH, TSS, and TA: Citrus cultivar genotype varies greatly for various physiochemical properties. According to the findings of this study, a huge and significant difference was observed among citrus cultivars from various citrus groups regarding juice pH. Navelina and at wood early navel of the sweet orange group, honey cultivar of mandarin, red blush of grapefruit group showed maximum juice pH, however, some cultivars of the same citrus groups such as rhode red valencia, seedless kinnow and star ruby have less juice pH. The CitPH1 and CitPH5 genes, which are in responsible for acidification in citrus fruit, could play a role in the pH differential between cultivars within citrus groups. In the lemon group, mozero lemon and M-H-R. 173 cultivar’s pH was lower, which is in harmony with the results of Irkin, et al. where the pH of the lemon group was less than mandarin [10].
The TSS and sugar content are highly influenced by the cultivar type, cultivation cycle, month of measurement, and storage time. By the results of the present study, the evaluated cultivars rhode red valencia, kinnow, reed, frost lisbon, and kaghzi lime produced higher TSS than pine apple, danscy, mozero lemon, and Persian lime, however, a study on postharvest evolution of cucumber revealed that the TSS and Dry Matter Content (DMC) were at their maximum levels at the collection time and gradually declined during the storage period (7, 14, 21, and 28 d). The TSS in cultivars of the grapefruit group was higher as compared to the cultivars of other groups, which is validated by the study of Baswal, et al. in which maximum TSS was observed in the ruby red cultivar of grapefruit [11]. The TSS findings from the current study indicated significant variation amongst citrus cultivars, leading to the hypothesis that the longer the period between the fruit’s harvesting time and the time of their consumption, the larger the TSS losses occur.
Fruit sensory quality is influenced by acidity, which directly influences the taste of fruit. The lowest TA of sweet orange was observed in succari and in mandarin it was observed in the honey cultivar, which is evident by Gloria, et al. that while malic acid concentration stayed steady, citric acid concentration declined as the growing season progressed on [12]. In the lemon group, the mozero lemon cultivar and in the lime group, the kaghzi lime cultivar also had less TA, therefore, it is possible to speculate that during fruit harvest season in which fruits are collected at various stages of maturation, there is a decrease in acid content with an increase in fruit’s maturity. The consumption of such component acids in fruit’s respiratory process may also contribute to the decrease in TA. The maximum TA was observed in rhode red valencia and pine apple varieties of sweet orange, Danscy variety of mandarin, and M-H-R 173 varieties of lemon. A previous study by Tounsi, et al. showed the opposite results the bitter orange group had the highest TA value, followed by the lemon group; however, the mandarin group, had the lowest TA value.
Ripening index and vitamin C: The ripening index ratio (TSS/TA) is another essential measure associated with citrus fruit quality features. The maximum TSS/TA in succari and honey varieties of sweet orange and mandarin groups with a ratio between 12-100, however, a contrary study in mandarins and oranges proposed that a TSS/TA ratio of 8 to 14 was required for optimum eating quality, depending on the variety and local production. The TSS/TA ratio was less than 3 in some cultivars of lemon and lime groups such as Mozero lemon and kaghzi lime but these cultivars showed high TA. Though the acidity of acid limes and lemons increase early in fruit development in these cultivars, mostly by the increase in citric acid levels, the TSS/TA ratio is not thought to be a reliable index for identifying the maturity of acid limes and lemons.
Citrus fruits are well-known for being a good source of vitamin C in the diet. In this study, vitamin C contents varied among sweet orange, mandarin, grapefruit, lemon, and lime cultivars, which might be due to genotypic variances that impact the amount of vitamin C in citrus fruits. Vitamin C content was much higher in the red blush variety of grapefruit using the inner parts of fruits such as pulp and seeds, which is consistent with the study of Sir Elkhatim, et al. in which grapefruit and orange have higher vitamin C content [13]. As compared to the results of Sir Elkhatim, et al. in the current study, vitamin C content was less in the sweet orange than lemon group. In contrast to the findings, de Moraes-Barros, et al. reported that the vitamin C content of the pulp of various commercial citrus fruits from Brazil was higher than that of the peels [14]. The variations in cultivars, maturity phases, and other environmental conditions may be the cause of these variations in results. In this study, vitamin C content from the fresh juice of the mandarin kinnow cultivar was in third number after grapefruit and lime, which matches with the study results of Perez, et al. in which mandarin fruits showed maximum vitamin C content in fresh juice and less in stored fruit [15]. Kaghzi lime of the lime group showed more vitamin C content than cultivars of the lemon group, which is in harmony with the results of Manuha, et al. which revealed that from lime and lemon cultivars of Sri Lanka, lime has more vitamin C content [16]. It leads to the hypothesis that there is a close relation between ascorbic acid content and storage conditions, as a study on strawberry fruit reported good retention of vitamin C and even showed a small initial rise after 10 days at 2°C.
Content of total phenolics, antioxidants, and anthocyanin: TPC is always much higher in all citrus fruits. The TPC was higher in most cultivars of sweet orange such as in succari followed by seedless kinnow of mandarin as compared to the cultivars of the lemon group. The results are the same when compared with the previous findings by Xu, et al. explaining that oranges have a higher TPC than grapefruits and mandarins [17]. In this study, non-significant variation with less TPC was observed among cultivars of grapefruits and lime. However, the opposite study showed that grapefruit has a higher TPC followed by lemon and orange. According to the results of the current study, it can be inferred that the variations in TPC might be due to the variety diversity or accumulation of phenolic acid in different parts of fruits, as the TPC was calculated using the inner part of the fruit (juice, pulp, and seeds). Although, in comparison to the pulp and seeds, the peel of citrus fruits contains higher phenolic compounds. Additionally, compared to fresh citrus fruits, their waste portions have a higher phenolic content.
Antioxidants and anthocyanin are the phytochemicals that are highly affected by preharvest and postharvest factors. In the present study, flame, shamber, and star ruby cultivars from the grapefruit group showed the highest antioxidants, however, the anthocyanin production in grapefruit cultivars was lower than in other citrus groups. This variation could be that the content of flavonoids, phenolic acid, and anthocyanin is impacted by the temperature variations between day and night, which in turn affects the antioxidant potential of citrus fruits. The pine apple and at wood early navel cultivars from the sweet orange group and M-H-R. 173 and frost lisbon from the lemon group also showed a maximum percentage of antioxidants. The results are in harmony with the results of Shehata, et al. where the extract of sweet orange, lemon, and citrus peel showed maximum antioxidant percentage [18]. Antioxidants were minimal in the seedless kinnow cultivar; however, the amount was maximum in other cultivars of the mandarin group. As compared to others, kaghzi lime and Persian lime from the lime group also showed fewer antioxidants. This variation in results could be due to environmental factors as they affect the content of antioxidants in citrus fruits, such as soil moisture, temperature fluctuations, solar radiation, and climatic conditions within a geographic area.
The anthocyanin percentage was higher in the cultivar of the sweet orange group (At wood early navel), lemon group (M-H-R. 173), and lime group (Persian lime) but less in other cultivars of the same groups. It leads to the hypothesis that postharvest treatment of citrus peel with methyl jasmonate and ethanol increased the ethylene hormone, which enhanced levels of total phenolics, flavonoids, and anthocyanin in these fruits. Furthermore, the over-ripening of fruits also enhances anthocyanin production. The cultivars from mandarin and grapefruit showed a minimum concentration of anthocyanin and the other group’s cultivars also showed variations with less anthocyanin growth. These alterations could be due to light intensity and temperature as the high temperature decreased anthocyanin content in the skin of apples and grapevine by regulating the expression of anthocyanin biosynthesis-related MYB genes. The cultivars of different groups showed variations in anthocyanin content which might be due to preharvest use of chemicals, plant growth regulators, and genetic diversity which leads to an imbalance concentration of antioxidants and anthocyanin in horticultural crops.
Sugar (sucrose and fructose): Sugars are the most abundant carbohydrates in citrus fruits, accounting for the majority of the TSS in the juice. The highest sugar content (sucrose and fructose) was observed in the navelina cultivar of sweet orange, which is in favor with the study of Kelebek, et al. with a total sugar content of 120.19 gL−1 [19]. The findings explained that the higher sugar content in kozan orange juice could be due to a large amount of sucrose. In mandarin, kinnow cultivar, and in grapefruit, shamber cultivar showed maximum sucrose and fructose content. The high sugar content in sweet oranges, mandarin, and grapefruit can be due to the high level of fructose and sucrose and the low level of citric acid. Furthermore, the variation of sugar content could be affected by genetic factors of different varieties. In the lemon and lime groups, the sugar content was less as compared to the sweet orange, grapefruit, and mandarin groups. The reason might be the higher citric acid concentration and lower fructose concentration. Acidic lemon and lime vacuoles store a significant amount of citric acid. The vacuolar H+-ATPase (V-ATPase) regulates a significant protons inflow that occurs concurrently with this buildup of protons. This increase in protons lowers the pH of the vacuoles and acts as a catalyst for further citric acid absorption [20].
Conclusion
In conclusion, there were substantial differences between cultivars of the five citrus groups for the physiochemical properties of citrus fruits. When establishing a nursery for a citrus orchard, potential cultivars that exhibit the desired fruit yield and quality must be taken into account. The current investigation demonstrated the variances among many physiochemical features; nonetheless, among the groups of sweet oranges, rhode red valencia, and from the mandarin group, minniola, and honey cultivars performed best. Reed and frost marsh and mozero lemon displayed the best performance in grapefruit and lemon, respectively. Persian lime performed better than the kaghzi lime cultivar. Based on the bioactive compound, cultivars from the sweet orange group performed better as compared to other citrus groups in terms of fruit quality parameters, including the maximum value of juice pH range (3.75-5.23), TSS/TA (5.49-101.82), TPC (150-312 GAE mg/100 g), antioxidants (82.18-54.58%), sucrose (55-112 mg/100 g) and fructose (29-262 mg/100 g). Therefore, to improve fruit output and quality, the aforementioned cultivars with the greatest performance can be recommended. The results of this study will provide guidelines for selecting promising cultivars for breeding in tropical and subtropical agroclimatic environments to increase the quality of citrus crops.
Author Contributions
Muhammad Aqib Shah: Conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing–original draft, review, and editing. Hafiz Saif Ur Rehman Shah: Methodology, data curation, review, and editing. Ume Habiba and Muhammad Amjad Farooq: Methodology. Irsa Ejaz, Ume Hani: Data analysis and review. Saqib Hussain Bangash, Daniel Adjibolosoo: Data curation. Tahir Abbas Khan, Iftikhar Ali Ahmad, Raheela Yasin, and Ume Laila: Review and editing. Iza Fatima: Conceptualization, data curation, validation, writing–original draft and writing–review and editing.
Acknowledgments
We acknowledge the staff of citrus germplasm unit, Arifwala, Punjab, Pakistan, and the Department of Horticulture, Muhammad Nawaz Shareef University of Agriculture Multan, Pakistan for their support in conducting this research.
Disclosure Statement
The authors report there are no competing interests to declare.
References
- Ishfaq M, Ahmad S, Awan MZ, Nasir MA (2007) Performance of grape fruit cultivars under agro-climatic conditions of Chakwal. Pak J Agri Sci 44: 472-472.
- Bermejo A, Cano A (2012) Analysis of nutritional constituents in twenty citrus cultivars from the Mediterranean area at different stages of ripening. Food Nutr Sci 3: 639-650.
- Usman M, Rehman W, Fatima B, Shahid M, Saggu AH, et al. (2020) Fruit quality assessment in pigmented grapefruit (Citrus paradisi macf.) for varietal diversification. Pak J Agri Sci 57: 1029-1034.
- Horwitz W (1964) The Association of Official Agricultural Chemists (AOAC). In: Gunther, F.A. (eds) Residue Reviews/Rückstands-Berichte. Reviews of Environmental Contamination and Toxicology, Springer, New York, USA. 7: 37-60.
- Ruck JA (1963) Chemical methods for the analysis of fruits and vegetables. Contribution No. B7, Research Station, Summerland, B.C: Canada, Department of Agriculture, Research Branch, Ottawa, Canada 47: 1154.
- Brand-Williams W, Cuvelier ME, Berset CLWT (1995) Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 28: 25-30.
- Giusti MM, Wrolstad RE (2001) Characterization and measurement of anthocyanins by UVâ?ÂÂÂvisible spectroscopy. Curr Protoc Food Analyt Chem 1: 1-13.
- Ali MM, Yousef AF, Li B, Chen F (2021) Effect of environmental factors on growth and development of fruits. Trop Plant Biol 14: 226-238.
- Tounsi MS, Wannes WA, Ouerghemmi I, Jegham S, Njima YB, et al. (2011) Juice components and antioxidant capacity of four Tunisian citrus varieties. J Sci Food Agric 91: 142-151.
[Crossref] [Google Scholar] [PubMed]
- Irkin R, DoÄ?an S, DeÄ?irmencioÄ?lu N, Diken ME, GüldaÅ? M (2015) Phenolic content, antioxidant activities and stimulatory roles of citrus fruits on some lactic acid bacteria. Arch Biol Sci 67: 1313-1321.
- Baswal AK, Rattanpal HS, Sidhu GS (2016) Varietal assessment and variability studies in grapefruit (Citrus paradisi Mac Fadyen) genotypes in subtropical zones of Punjab, India. Bioscan 11: 1369-1371.
- Gloria PM, Egid BM, Chande CO (2010) Postharvest changes in physico-chemical properties and levels of some inorganic elements in off vine ripened orange (Citrus sinensis) fruits cv (Navel and Valencia) of Tanzania. Afr J Biotechnol 9: 1809-1815.
- Sir Elkhatim KA, Elagib RAA, Hassan AB (2018) Content of phenolic compounds and vitamin C and antioxidant activity in wasted parts of Sudanese citrus fruits. Food Sci Nutr 6: 1214-1219.
[Crossref] [Google Scholar] [PubMed]
- de Moraes-Barros HR, de Castro-Ferreira TAP, Genovese MI (2012) Antioxidant capacity and mineral content of pulp and peel from commercial cultivars of citrus from Brazil. Food Chem 134: 1892-1898.
[Crossref] [Google Scholar] [PubMed]
- Perez AG, Luaces P, Oliva J, Rios JJ, Sanz C (2005) Changes in vitamin C and flavour components of mandarin juice due to curing of fruits. Food Chem 91: 19-24.
- Manuha DMI, Paranagama PPA, Nageeb DBM (2019) Quantitative analysis of vitamin C in lime and lemon in vitro: Verification of vitamin C on the impairment of obesity. Int J Adv Sci Res Eng 5: 157-161.
- Xu G, Liu D, Chen J, Ye X, Ma Y, et al. (2008) Juice components and antioxidant capacity of citrus varieties cultivated in China. Food Chem 106: 545-551.
- Shehata MG, Awad TS, Asker D, El Sohaimy SA, Abd El-Aziz NM et al. (2021) Antioxidant and antimicrobial activities and UPLC-ESI-MS/MS polyphenolic profile of sweet orange peel extracts. Curr Res Food Sci 4: 326-335.
[Crossref] [Google Scholar] [PubMed]
- Kelebek H, Selli S, Canbas A, Cabaroglu T (2009) HPLC determination of organic acids, sugars, phenolic compositions and antioxidant capacity of orange juice and orange wine made from a Turkish cv. Kozan. Microc J 91: 187-192.
- Müller ML, Taiz L (2002) Regulation of the lemon-fruit V-ATPase by variable stoichiometry and organic acids. J Membrane Biol 185: 209-220.
[Crossref] [Google Scholar] [PubMed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi