Research Article, Int J Cardiovasc Res Vol: 6 Issue: 4
Possible Pitfalls of Auto Capture and Automatic Capture Management Algorithms or Trust but Verify- Post Marketing Study
Pavlovic J1,4*, Stritecky J2, Cvancara M3, Majtan B1, Volman H4, Beer M1, Sabolová L1, Lenartova J3 and Machacek T5
1Department of Cardiology, Karlovarská Krajská Nemocnice, BezruÄÂova 1190/19, 360 01, Karlovy Vary, Czech Republic
2Faculty of Medicine in Hradec Králové, Department of Biophysics, Šimkova 870, Hradec Králové 500 03, Czech Republic
3Department of Biomedical Engineering, Karlovarská Krajská Nemocnice, BezruÄÂova 1190/19, 360 01, Karlovy Vary, Czech Republic
4Krajská Zdravotní a.s., Masarykova Nemocnice v Ústí nad Labem, Klinika Kardiologie, Sociální PéÄÂe 3316/12A, 400 11, Czech Republic
5Department of Automated Information Technology, Karlovarská Krajská Nemocnice, BezruÄÂova 1190/19, 360 01, Karlovy Vary, Czech Republic
*Corresponding Author : Pavlovic Jan MD
Department of Cardiology, Karlovarská Krajska Nemocnice, Bezrucova 1190/19, ,and Krajská Zdravotní a.s.
Masarykova Nemocnice v Ústí nad Labem, Klinika Kardiologie, Sociální PéÄÂe 3316/12A, 400 11, Czech Republic
Tel: +420 737 420 893
E-mail: janpavlovic@seznam.cz
Received: July 07, 2017 Accepted: August 04, 2017 Published: August 10, 2017
Citation: Pavlovic J, Stritecky J, Cvancara M, Majtan B, Volman H (2017) Possible Pitfalls of Auto Capture and Automatic Capture Management Algorithms or Trust but Verify- Post Marketing Study. Int J Cardiovasc Res 6:4. doi: 10.4172/2324-8602.1000321
Abstract
Background: Automatic pacing output management has been used for more than 20 years and it has been generally accepted as safe and device longevity prolonging mode of pacing. Despite that, accuracy of these algorithms in traditional VVI(R) and DDD(R) pacemakers on long term basis has not, to our knowledge, been defined. There is evidence that in patients with atrial fibrillation (AF) and low percentage of ventricular pacing (VP) this function might be less suitable.
Methods: We have followed up a population of 559 patients with permanent pacemakers for 3 years and 8 months. 274 of them had the automatic output management (AOM) function activated. We have prospectively searched for inappropriately set pacing output in both subgroups. That is, either too high or too low. We have compared this subgroup to that with fixed output pacing (FOP). Patients with any mechanical complication and those having pacemaker implanted for less than three months were excluded from the study.
Results: We have found 11 patients out of 274 in whom the value of pacing output was inappropriate.
Conclusions: In our study, 99.6% of patients with AOM functions activated always had effective pacing and the percentage of ideal performance of this function was 96%. These numbers confirm safety of AOM functions with only a few caveats.
Keywords: Auto capture; Cardiac pacing; Evoked response; Polarization signal; Threshold management algorithm
Abbreviations
A: Atrial; AC: Autocapture; AOM: Automatic Output Management; AF: Atrial Fibrillation; AP: Atrial Pacing; ER: Evoked Response; ERI: Elective Replacement Indication; FOP: Fixed Output Pacing; HR: Heart Rate; PS: Polarization Signal; PSA: Pacing System Analyser; SJM: St. Jude Medical; SR: Sinus Rhythm; V: Ventricular; VCM: Ventricular Capture Management; VP: Ventricular Pacing
Introduction and Review of Literature
The idea of threshold tracking pacemaker was introduced by Funke, Preston, Bowers and Mugica in the 1970s [1-5]. Research, which was initiated by these authors, led to the first clinically successful automatic algorithm of a pacemaker capable of detecting capture, i.e. evoked response to electric stimulation and adapting pacing output according to measured pacing threshold in a singlechamber pacemaker (Pacesetter Microny, Solna, Sweden in 1994 - Pacesetter - a subsidiary of St. Jude Medical since 1994). In 1999 a dual-chamber pacemaker with this technology (Affinity DR) was presented. Algorithms for automatic management of pacing energy have also been developed by other manufacturers.
Thereafter, there were studies leading to a common sense that AOM algorithms offer, besides other advantages [6-16] enhanced patient´s safety [17,18].
However, other authors [19] conclude that: benefit in some patients with ventricular pacing ≤25%, low stimulation threshold, and/or AF could be questionable, special attention should be paid to the long-term AOM functioning reports, activation of the algorithm should be individualized in each patient and long-term AOM pattern must be checked routinely during follow-up. Authors of this work also point out, that a long-term evaluation of the efficiency of AOM in the real world is lacking.
The aim of our study is to report inadequately set values of pacing output by automatic algorithms with a concise explanation of possible causes and this way contribute to generally better awareness of potential problems and their solutions.
Materials and Methods
We have analysed a population of 559 patients, coming to our hospital for routine pacemaker controls in the period since February 2013 to October 2016 (3 years and 8 months). Out of these, 274 patients (49%) had the AOM function activated. In this AOM subgroup, there are 130 Medtronic, 133 St. Jude Medical, 6 Biotronik and 5 Vitatron devices. In all automatic algorithms we have left the default (nominal) setting unchanged (once daily measurement in Medtronic and three times in 24 hours in St. Jude Medical devices). The control group with FOP consisted of 285 patients: 184 with Medtronic and 101 with Saint Jude Medical devices. We have included all patients coming for pacemaker controls into our hospital, even those whose pacemaker was implanted in another hospital and those who already underwent generator replacement(s). So, the time from implantation was random, but we have excluded those with pacemaker implanted less than three months ago. Only patients without any mechanical complication (like lead dislodgement, lead damage or perforation) were included into the study.
In both groups, we have prospectively searched for inappropriately set pacing output. In pacemakers without automatic output algorithms it has been common practice to set the output on twice the value of the threshold. Therefore, in our study, we have defined the inappropriate pacing output as either lower than pacing threshold or more than 3 times the value of the pacing threshold but not less than 2.5 V/0.4 ms. In all patients with the automatic capture management activated, we have measured the pacing threshold manually and compared it to that determined automatically and with the pacing output. These measurements were performed within one minute interval. The threshold was considered accurate if it corresponded with the manually measured one. In the following text, the term “pacing threshold” will refer to the manually measured pacing threshold unless stated otherwise. Data collected by authors were verified by the expert analysis of the hospital information system (Akord Stapro s.r.o.). We have compared two groups of patients with regard to the difference between the pacing threshold and pacing output. We have not compared the relative amount of energy depletion, because there are multiple factors influencing the energy consumption and thus it is too complicated to quantify it accurately enough.
Results
In the AOM group, the mean pacing threshold and output were 0.91 V/0.40 ms and 1.67 V/0.40 ms respectively (average difference 0.76 V). We have found 9 patients (3.2%) in whom the pacing output was set too high and 2 patients (0.7%), in whom it was too low. Characteristics of these patients are summarized in Table 1.
| Pt. No. | Age | Pacemaker model, year of first implantation, year of generator replacement(s) | Lead (V/A, type) |
Manually determined pacing threshold (V/ms) | Pacing output set by AOM (V/ms) | Sensing (mV) | VP (%) | AP (%) | Pacing mode | Rhythm | Intrinsic HR | Paced AV delay (ms), in SR intrinsic PR interval (if not high degree AV block), (ms) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 83 | SJM Sustain 2006, 2014 | V/ SJM 1688T | 0.75/0.4 | 5/0.4 | 10.7-12 | 50-55 | NA | VVIR | Permanent AF | 83 | NA |
| 2. | 77 | SJM Zephyr DR 2005, 2010 | A/unknown | 0.75 /0.4 | 3.25/0.4 | 3.3-3.7 | 100 | 1 | DDD | 3rd degree AV block | <30 | 160, NA |
| 3. | 87 | SJM VerityADx XL, 2008 | V/SJM, 1788TC/58 | 1.25 /0.4 | 4.5/0.4 | 12.3-16.3 | 98 | 7 | DDD | SR, 2nd degree AV block | 30-60 | 160, 180 |
| 4. | 78 | SJM Sustain 2003, 2014 |
A/ Medtronic 5076 | 0.5/0.4 | 3/0.4 | 0.6-0.8 | > 99 | 99 | DDDR | SR, 3rd degree AV block | 35-40 | 200, NA |
| 5. | 85 | SJM Zephyr DR, 2011 | V/SJM, 1788TC/58 | 1/0.4 | 5/0.75 | 3.5-4 | 11 | 98 | DDD | SR | 54 | Adaptive,190-200 |
| 6. | 94 | Medtronic Sensia DR, 2012 | A/Unknown | 0.75 /0.4 | 4.5/0.4 | 0 | 100 | 100 | DDDR | 3rd degree AV block | 30-60 | 160, NA |
| 7. | 86 | SJM Accent DR, 2013 | A/SJM, 1888TC - | 0.75/0.4 | 3.25/0.4 | 2.6 | 4-8 | 25 | DDDR | SR | 69-80 | Adaptive, 200 |
| 8. | 75 | SJM Zephyr DR, 2010 | V/SJM, 1788TC/58 | 0.75 /0.4 | 5/0.4 | 12 | 100 | 98 | DDDR | SR, parox. AF | 30 | Adaptive, 220 |
| 9. | 94 | SJM VerityADx XL, 2008 | V/ SJM, 1788TC/58 | 0.75 /0.4 | 4.5/0.4 | 8.3-9.1 | 42 | NA | VVI | Permanent AF | 50-100 | NA |
| 10. | 87 | SJM VerityADx XL 2007 | V/ SJM,1788TC/58 | 1.0/0.4 | 0.375/0.4 | 11.4-16.6 | 30 | NA | VVIR | Permanent AF | 60-70 | NA |
| 11. | 91 | SJM Sustain, 2009, 2014 | V, SJM,1788TC/58 | 1-4/1 | 1.8/1 | 2-2.6 | 98 | 41 | DDDR | SR, complete AV block | <30-32 | 220, NA |
Table 1: Characteristics of patients with automatic output management and inappropriately set pacing output.
In the control group with FOP we have not recorded any case of ineffective pacing. We have seen very few of such cases, but these were due to some mechanical complication, like lead dislodgement or perforation (mainly in the first 3 months after implantation) and these patients were excluded from the study as mentioned above. Mean pacing threshold in the control group was 0.94 V/0.40 ms and average pacing output in this group was 2.23 V/0.40 ms. Average difference between threshold and output in this group was 1.29 V/0.4 ms. Statistical analysis (ANOVA) have confirmed, that in the AOM group the difference between the output and threshold was significantly lower than in the FOP group (P<0.001).
In the control group, we have found 19 patients meeting our criteria of too high output with maximum difference between threshold and output of 2.8 V.
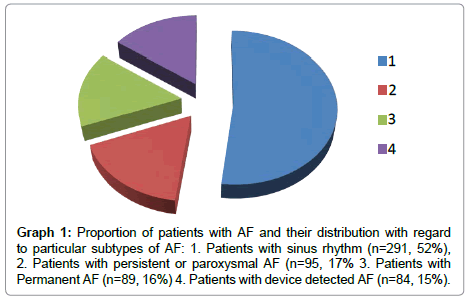
Prevalence of AF in both groups (559 patients), is 47.9%. In 15 % the AF was only subclinical and device detected AF - with low AF burden. Distribution of the particular types of AF is displayed in Graph 1.
In the AOM group with inappropriate output, there are only three patients (27%) with significant proportion of AF or permanent AF and only two patients (18%) with VP<30%. The rest of them had long standing sinus rhythm and high percentage of ventricular pacing.
As shown in Table 1, in 10 of these patients the pacing output was set inappropriately high and, in 2 cases inappropriately low with output set below the pacing threshold. Because this is a feared malfunction of a pacemaker - when it fails to pace - we give detailed information on these two cases.
First one was 87 years old, but mentally and physically capable (self-contained) female patient, with Verity TM ADx XL device with VP (ventricular pacing) of 30% and permanent atrial fibrillation. This patient had suffered multiple episodes of dizziness with falling (it was uncertain, if these were accompanied by losses of consciousness), but bradycardia or loss of capture was not documented. In this patient AC was functioning well for several years before this episode. We have checked the evoked response/polarization signal ratio and it was excellent and stable (evoked response 12-17 mV, polarization 0.39 mV) just like other electrical parameters. Despite that, the automatically measured pacing threshold was falsely stated 0.125 V while in reality it was 1V. The value of automatically set output was 0,375 V. Hence, we have set the device on FOP 2V/0.4 ms but, when writing this article, the patient still reported episodes of dizziness with falling.
The second case of pacing output set below the pacing threshold was a 91- year old man, with SJM SustainTM XL DR and an unstable pacing threshold ranging, according to the records of the device for several months, from 1 to 4 V/1 ms. This patient, who was dependent on the pacemaker, with AC function activated, experienced repeated episodes of ineffective pacing with symptomatic bradycardia and also with syncopes. Since the last (emergent) pacemaker control, it was not even possible to just switch the AC on, because this immediately led to a loss of capture and severe symptomatic bradycardia, resolving only after manual reprogramming it to fixed output. We have interrogated the pacemaker and displayed the leads by chest fluoroscopy, CT scan and transthoracic echocardiography. The only abnormities we have found were unstable pacing threshold – on long term basis – and lower sensing (2.6-2.8 mV) on the ventricular lead. The impedance was stable, around 380 Ohms. This patient´s intrinsic heart rate was under 30 bpm for the most of the time and the sensing was only measurable during the last session, thus it did not prompt any physician or technician to switch the AC off. The device has been put on high FOP - 7V/1ms (with regard to the patients age and comorbidities we have decided to wait with new lead implantation until ERI) and the patient had no syncope since then (when writing this article – for two months).
Discussion
In the literature, we have found only one case report [8] of pacing output set by AOM algorithm lower than threshold. Despite the fact, that the cause of this was identified and resolved, this case report has illustrated the need for close post-marketing surveillance of this technology. We have found two patients with pacing output lower than pacing threshold. In one of these ineffective pacing was documented. 2 patients in the group of 274 patients is so low and insignificant number, that it is actually not suitable for any statistical analysis. But these cases have taught us an important lesson on how to manage patients with these algorithms activated. In the first case, the intrinsic rhythm of the patient on follow up was 60-70 bpm, so the algorithm most probably interpreted patient´s own beats as an evoked response. It was a SJM device with beat to beat verification of ER and we have not documented ineffective pacing in this case. So it is very unlikely, that the syncopes or falls were caused by bradycardia.
In the second reported patient usage of AC function was inconvenient because of too low value of sensing. There is evidence, that auto capture should not be used if the ER amplitude is small (<2.5 mV), the polarization signal amplitude is large (>4 mV), the ER sensitivity/ polarization ratio is <1.7, or the ER amplitude/ER sensitivity ratio is <1.8 (20-21). This also must have been the case of our patient, although Sustain model does not enable manual numeric measurement of polarization and ER/polarization ratio. It seems clear, that this case was an example of a poor cardiac substrate or a suboptimal lead placement. This case demonstrates that in patients with intrinsic heart rate lower than 30 bpm - with unmeasurable i.e. unknown sensing and in whom the value of evoked response is also unknown, auto capture should probably not be activated.
Changes in pacing threshold are described in literature [21-24]. In the study of Burri et al. [25] on Medtronic Concerto CRT-D devices it has been shown, that LV Capture Management algorithm was actually beneficial in patients with threshold fluctuations of more than 1 V, which were those with higher pacing threshold.
It would be unrealistic to expect from any method or algorithm to have 100% reliability. In the real world, the most exact and most reliable methods have over 90% reliability. In our opinion, 99.6% likelihood that the pacemaker will effectively pace with the AOM function on, and only 3.3% probability, that the energy output is higher, than it could be, would be, from that point of view, excellent numbers. Of note, our results are not very different from those, recorded by other authors [16,26,27]. Although the latter two studies were performed on totally different devices and leads: First of them [26] evaluated the performance of automated pacing threshold algorithm on RV ICD leads, working with RV-coil to can sensing of ER. The second study [27] was performed on LV-leads (Boston Scientific CRT-D/P devices) with automatic threshold detection algorithm using four pacing vectors and it demonstrated significantly higher accuracy than we have found in our group of patients.
Our data show that under certain circumstances automatic output algorithms may fail to set optimal value of output and this is the fact which, in our opinion, still prevents routine pacemaker controls to be performed without supervision of an experienced specialist, with good knowledge of all of these algorithms. One of the important limitations of our study is, that it included devices practically only of two manufacturers, thus its conclusions cannot be applied to all AOM algorithms. It is convenient to add, that algorithms are undergoing continuous development and improvements like fusion detection algorithms, e.g. that by Biotronik [28] are expected to further decrease the number of inaccurately working algorithms.
We are not trying to question reliability of automatic algorithms. Our data actually suggest high reliability and it has been reported in PACE [11] that automated output management is safer than a fixed output, which may result in exit block. We would rather like to provide clues on how to interpret and manage output, which is inadequate to a measured threshold. We offer these recommendations: Unstable pacing thresholds with significant circadian fluctuation can be unmasked by setting automatic threshold tests at least 3 times daily. Fusion or pseudo fusion can be revealed by running automatic threshold tests at pacing rate similar to the intrinsic rate. If the automatically set output is too high in chronically inhibited pacemakers and the device does barely have a chance to measure the threshold, the algorithm does not necessarily have to be switched off because in this case the battery longevity is not significantly affected due to very low pacing percentage.
Our first case report indicates, that in patient with atrial fibrillation, predominant intrinsic rhythm, who would have pacemaker without beat to beat verification of capture, ineffective pacing might occur in theory, but these devices, as a rule, operate with higher safety margin and thus the likelihood of such an event is extremely low and will probably be even lower with further extension of fusion detection algorithms.
As indicated in the text, values of ER and PS are crucial for optimal AOM functioning. Yet, these measurements cannot be made with the standard pacing system analysers (PSA) during implantation. As a consequence, approximately 5-7% of patients were found to have inadequate ER signals and AC could not be activated [15,29]. The correlation between the spontaneous R wave as measured with the standard PSA and the ER signal has been reported to be weak to moderate: In the studies of Clarke [6] and Schubert [15] the correlation coefficients were r=0.29 and r=0.44 respectively. Therefore, it should probably be a topic of consideration to develop and implement analysers with such capabilities. And for the same reason, it would be also, in our opinion, probably worth considering to enable direct numeric measurement of these values in all pacemaker models during follow up controls.
We did not compare the influence of both methods of pacing (FOP and AOM) on battery depletion, but it is logical and there is also evidence in the literature [10-14] that the AOM functions are less energy consuming than fixed output pacing.
According to Benezet-Mazuecos et al. [19], suboptimal performance of AOM is most likely in patients with AF and those with VP<25%. Despite that, as logical as it may seem, finding of these authors, is not supported by our investigation.
Conclusion
According to our analysis, the percentage of an ideal performance or accuracy of the AOM function is 96%. Potentially very harmful situation is setting the pacing output under the pacing threshold. This situation did not occur in any patient in the control group and only twice (0.7%) in the “AOM” group. In the first case, low value of pacing output had no clinical significance because the PM was inhibited by patient´s intrinsic heart rate and ineffective pacing did not occur. In the second case, AOM function should not have been activated at all, in accordance with the manufacturer´s recommendation, because of low sensing and very unstable pacing threshold.
Over 20 years, the AOM functions have proved its advantages. However, since the first rule in medicine is “safety first” or “primum non nocere”, on the basis of our findings, we would suggest that the AOM functions be checked during every pacemaker control: Automatically measured pacing threshold should be compared to the manual measurement and the record of pacing thresholds measured by the device between the patient´s visits (or remote controls) should be read. Similarly, the measurements of evoked response and polarization should also be performed on regular basis, if the device enables it. Despite the on-going developments of algorithms and leads, even in the year 2017, the supervision of an educated specialist during pacemaker controls is still necessary.
Financial support and funding
None
Conflict of interest
The authors declare that they have no competing interest.
References
- Funke HD (1972) Der Schwellenwertschrittmacher. Ein neuartiges Prinzip der elektrischen Myokard-Stimulation. Inaugural-Dissertation, University of Bonn, Germany.
- PrestonTA, Bowers DL (1973) Report of a continuous threshold tracking system. In J Hilbert, Th Thalen (eds.): Cardiac Pacing. Assen, Van Gorcum.
- Mugica J, Lazarus B, Buffet J (1973) Pacemaker with automatic adaptation to the pacing threshold. J Hilbert 150-155.
- Preston TA, Bowers DL (1974) The automatic threshold tracking pacemaker. Med Instrument 8: 322-325.
- Preston TA, Bowers DL (1975) Clinical applications of the threshold tracking pacemaker. Am J Cardiol 36: 322-326
- Clarke M, Liu B, Schuller H, Binner L, Kennergren C, et al. (1998) Automatic adjustment of pacemaker stimulation output correlated with continuously monitored capture thresholds: a multicentre study. Pacing Clin Electrophysiology 21: 1567–1575.
- Lau C, Cameron DA, Nishimura SC, Ahern T, Freedman RA, et al. (2000) A Cardiac evoked response algorithm providing threshold tracking: a North American multicentre study. Pacing Clin Electrophysiol 23: 953-959
- Suri R, Harthorne JW, Galvin J (2001) Automatically optimizing pacing output: an excellent idea, but with potentially lethal pitfalls. Pacing Clin Electrophysiol 24: 520–523
- Duru M, Bauersfeld U, Schuller H, Candinas R (2000) Threshold tracking pacing based on beat by beat evoked response detection: Clinical benefits and potential problems. J Interv Card Electrophysiol 4: 511-522
- Koplan BA, Gilligan DM, Nguyen LS, Lau TK, Thackeray LM, et al. (2008) A randomized trial of the effect of automated ventricular capture on device longevity and threshold measurement in pacemaker patients. Pacing Clin Electrophysiol 31: 1467-1474
- Biffi M, Bertini M, Saporito D, Ziacchi M, Martignani C, et al. (2010) Actual pacemaker longevity: The benefit of stimulation by automatic capture verification. Pacing Clin Electrophysiol 33: 873–881
- Boriani G, Rusconi L, Biffi M, Pavia L, Sassara M, et al. (2006) Role of ventricular autocapture function in increasing longevity of DDDR pacemakers: a prospective study. Europace 8: 216–220
- Brockes C, Rahn-Schonbeck M, Duru F, Candinas R, Turina M (2003) Impact of automatic adjustment of stimulation outputs on pacemaker longevity in a new dual-chamber pacing system. J Interv Card Electrophysiol 8: 45-48
- Boriani G, Biffi M, Branzi A, Mininno A, Sigliano R (2000) Benefits in projected pacemaker longevity and in pacing-related costs conferred by automatic threshold tracking. Pacing Clin Electrophysiol 23: 1783-1787
- Schubert A, Ventura R, Meinertz T (2000) Automatic threshold tracking activation without the intraoperative evaluation of the evoked response amplitude. Pacing Clin Electrophysiol 23: 321-324.
- Pecora D, Morandi F, Liccardo M, Pepi P, Orazi S, et al. (2008) Performance of a ventricular automatic-capture algorithm in a wide clinical setting. Pacing Clin Electrophysiol 31: 1546-1553.
- Stambler BS, Ellenbogen KA. (2011) Clinical Cardiac Pacing, Defibrillation, and Resynchronization Therapy, fourth ed., Philadelphia.
- Vijayaraman P, Ellenbogen KA (2011) Clinical Cardiac Pacing, Defibrillation, and Resynchronization Therapy, 5th Edition. Philadelphia.
- Benezet-Mazuecos J, Iglesias JA, Rubio JM, Cortés M, De la Cruz E, et al. (2014) Limitations of the AutoCapture™ pacing system in patients with cardiac stimulation devices. Europace 16: 1469–1475.
- Kay NG, Shepard RB. Stimulation and Excitation of Cardiac Tissues In: Clinical Cardiac Pacing, Defibrillation and Resynchronization Therapy. Philadelphia.
- Lau C, Nishimura SC, Yee R, Lefeuvre C, Philippon F, et al. (2001) Intraoperative study of polarization and evoked response signals in different endocardial electrode designs. Pacing Clin Electrophysiol 24: 1055-1060
- Preston TA, Fletscher RD, Lucochesi BR, Judge RD (1967) Changes in stimulation thresholds: Physiologic and pharmacologic factors in patients with implanted pacemakers. American Heart Journal 74: 235-242
- Grendahl H, Schaanning CG (1970) Variations in pacing thresholds. Acta Med Scand 187: 75-78.
- Kadish A, Kong T, Goldberger J (1992) Diurnal variability in ventricular stimulation threshold and electrogram amplitude. Eur J Card Pacing Electrophysiol. 2: A.
- Burri H, Geritse B, Davenport L, Demas M, Sticherling C (2009) Fluctuations in left ventricular thresholds and required safety margin for left ventricular pacing with cardiac resynchronization therapy. Europace 11: 931-936.
- Gold MR, Dong Y, Greer S, Giudici MC, Haffajee CI, et al. (2012) Acute Performance of a Right Ventricular Automatic Pacing Threshold Algorithm for Implantable Defibrillators PACE 35: 259-268.
- Kalahasty G, Giudici M, Lobban J, Doshi R, Delanay C, et al. (2012) Acute Clinical Evaluation of a Left Ventricular Automatic Threshold Determination Algorithm Based on Evoked Response Sensing. PACE 35: 348-356
- Biffi M, Bertini M, Saporito D, Nelotri G, Quartieri F, et al. (2016) Automatic management of atrial and ventricular stimulation in a contemporary unselected population of pacemaker recipients: the ESSENTIAL Registry. Europace 18: 1551-1560
- Kam RM, Tan CS, Teo WS (2000) Initial experience with an auto capture pacemaker system. Ann Acad Med Singapore 29: 732-734.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi