Research Article, Int J Cardiovasc Res Vol: 8 Issue: 6
Predictors of Bidirectional Glenn Shunt Failure and Fontan Completion Rate in Patients with Single Ventricle
Ismail AI1,2, Farouk A1, Dähnert I3 and Kos telka M2
1Pediatric Cardiothoracic Surgery Unit, Assiut University, Assiut, Egypt
2Pediatric Cardiac Surgery Section, Leipzig Heart Centre, Leipzig, Germany
3Pediatric Cardiology Department, Leipzig Heart Centre, Leipzig, Germany
*Corresponding Author: Ismail AI
Pediatric Cardiothoracic Surgery Unit, Assiut University, Assiut, Egypt
E-mail: aimi7878@yahoo.com
Received: September 19, 2019 Accepted: November 08, 2019 Published: November 15, 2019
Citation: Ismail AI, Farouk A,Dähnert I, Kostelka M (2019) Predictors of Bidirectional Glenn Shunt Failure and Fontan Completion Rate in Patients with Single Ventricle. Int J Cardiovasc Res 8:6.
Abstract
Background: The introduction of bidirectional Glenn (BDG) procedure in patients with functional single ventricles improved the clinical outcomes for all candidates undergoing total cavopulmonary connection (TCPC). We tried to evaluate the obstacles that prevent patients to achieve TCPC after BDG.
Methods: All patients who underwent BDG at Leipzig Heart Centre from April 2003 to November 2013 were included in this study, except cases preceded by Norwood procedure.
Results: 82 patients were included in this study. 59 patients underwent TCPC (72%), while 12 patients were waiting for TCPC (14.6%). There were two in-hospital deaths (2.4%) after BDG. In two patients (2.4%), completion of TCPC was not possible, while 6 patients were lost during follow-up (7.3%). One patient had his BDG taken down (1.3%). Therefore the survival rate till completion of Fontan was 72% with another 14.6% waiting for TCPC. A univariate analysis revealed that patients with unbalanced atrioventricular septal defect (AVSD), previous repair of total anomalous pulmonary venous connection (TAPVD), elevated mean pulmonary arterial pressure and long operation time were significant predictors of death, take-down, or failure to progress to Fontan Operation. Thrombosis was found to be the main mechanism for morbidity and mortality.
Conclusion: The staged plan for single ventricle candidates provides excellent clinical results. The main risk factors for death, take-down, or failure to progress to Fontan Operation were elevated pulmonary arterial pressure, unbalanced AVSD, TAPVD and long operation time.
Keywords: Bidirectional Glenn; Fontan; Single ventricle
Introduction
Current surgical approaches for congenital heart disease, characterized functionally as single ventricle, are based on diverting systemic venous return directly to the lungs. The classic Glenn shunt was first performed nearly 50 years ago, and has provided good longterm palliation for complex cardiac malformations associated with low pulmonary blood flow, low pulmonary arterial pressure, and low pulmonary vascular resistance [1,2]. This procedure improves oxygen saturation by increasing effective pulmonary blood flow without increasing total pulmonary blood flow, pulmonary artery (PA) pressure, or cardiac work.
After initial widespread use, disadvantages of this procedure became evident with the discovery of pulmonary arterio-venous fistulae on the side of the Glenn anastomosis, and difficulties in application of the technique in small children [3]. Thus, it was largely replaced by the classic Fontan operation in the mid-seventies of the last century [4]. However, some patients were not ideal candidates for the Fontan operation. To overcome these problems, a modification of the Glenn shunt; bidirectional superior cavo-pulmonary anastomosis has been widely used. Like the classic Glenn shunt, it improves systemic arterial oxygen saturation, volume unloads the ventricle by increasing the effective pulmonary flow, and alters ventricular geometry whether it is of right or left ventricular morphology [5,6].
Recently, superior cavo-pulmonary anastomosis has been included as a part of total right heart bypass in both the intra cardiac and extra cardiac procedures [7,8]. This operation improved efficiency of gas exchange without volume or pressure overload of the ventricle unlike the systemic pulmonary arterial shunt or the pulmonary artery band.
In this report we evaluate a ten years’ experience of Leipzig Heart Centre with bidirectional Glenn (BDG) anastomosis for single ventricle patients, stressing on the factors that may account for its failure to progress to completion Fontan.
Patients and Methods
IRB approval was obtained and consent was waived, as it’s a retrospective observational study. Eighty-two consecutive patients, who underwent BDG at Leipzig Heart Centre from 2003 to 2013, were included in this study. There were 42 males and 40 females. Their weights ranged from 4.5 to 56 kg with a median of 6.75 kg. Patient age ranged from 75 days to 30 years old with a median of 6.26 months. Thirty seven patients (45.12%) were less than six months old, and 2 patients (2.5%) were less than three months old.
Primary diagnosis and associated cardiac anomalies are listed in Tables 1 and 2 respectively. Previous surgeries to the BDG are listed in Table 3. There were 18 cases who undergone BDG without having any previous surgery.
| Primary Diagnosis | No. of Patients |
|---|---|
| Tricuspid atresia | 25 |
| Double inlet ventricle | 19 |
| Heterotaxy syndrome (Asplenia) | 11 |
| Mitral atresia | 7 |
| Unbalanced atrio-ventricular septal defect | 7 |
| Tricuspid valve straddling | 2 |
| Ebestein anomaly | 1 |
| Other complex anomalies | 10 |
Table 1: Primary diagnosis of patients.
| Associated Cardiac Anomalies | No. of Patients |
|---|---|
| Pulmonary stenosis | 32 |
| Malposed great arteries | 28 |
| Transposition of great arteries | 17 |
| Pulmonary atresia | 22 |
| Patent ductus arteriosus | 18 |
| Tricuspid stenosis | 16 |
| Pulmonary artery stenosis | 11 |
| Situs inversus (Dextrocardia) | 15 |
| Situs ambiguus | 10 |
| Myocardial sinusoids with ventriculo-coronary fistula | 10 |
| Aorta coarctation | 8 |
| Atrio-ventricular discordance | 7 |
| Total anomalous pulmonary venous drainage | 3 |
| Mitral valve straddling and overriding | 3 |
| Subaortic stenosis | 3 |
| Aortic incompetence | 3 |
| Double aortic arch | 2 |
Table 2: Associated cardiac anomalies.
| Previous Surgery | No. of Patients |
|---|---|
| Systemic pulmonary Shunt | 46 |
| Pulmonary artery banding | 15 |
| Pulmonary arteries reconstruction | 8 |
| Coarctation repair | 7 |
| Damus-Kay-Stansel procedure with aortic arch reconstruction | 6 |
| Total anomalous pulmonary venous drainage repair | 3 |
| Aortic arch reconstruction | 3 |
Table 3: Previous surgeries before Bidirectional Glenn.
Operative techniques
All patients underwent BDG surgery using median sternotomy and cardiopulmonary bypass with mild to moderate hypothermia (32°C). Operation was done on beating heart in 43 patients, and in 15 patients with ventricular fibrillation. Myocardial protection was used in 24 patients using cold crystalloid cardioplegia (St. Thomas) solution when cardiac arrest was necessary. The superior vena-cava was dissected intra- and extra-pericardial up to the innominate vein junction, after ligation of the azygous vein. Heparin was administered (2 mg/kg). After mobilization, the superior vena cava was clamped proximally and distally, and transected just before the cavo-atrial junction. Care was taken to avoid damaging the sinus node. The cardiac end of the superior vena cava was closed using 6/0 polypropylene sutures. A large side-biting clamp was applied to the right pulmonary artery. A longitudinal incision was made in its superior aspect, and the opening was extended centrally. Bidirectional cavo-pulmonary shunt was performed by direct end-to-side anastomosis between the superior vena cava and the right pulmonary artery with running 6/0 polypropylene sutures. Two sutures were used to avoid the purse-string effect. The clamp was released on completion of the anastomosis. The same procedure was repeated when there was a left superior vena cava on the left pulmonary artery (6 patients).
Concomitant procedures are listed in Table 4. The main pulmonary artery was transected, ligated, ignored (due to presence of Pulmonary Atresia), or included in a prior Damus-Kay-Stansel operation.
| Concomitant Procedure | No. of Patients |
|---|---|
| Systemic-to-pulmonary shunt take-down | 39 |
| Atrial Septectomy | 32 |
| Pulmonary arteries reconstruction | 9 |
| Bilateral Bidirectional Glenn | 6 |
| Tricuspid valve closure | 6 |
| Patent ductus arteriosus ligation | 5 |
| Modified Blalock-Taussig shunt was left open or reconstructed | 3 |
| Ligation of major aorto-pulmonary collateral arteries | 3 |
| Aortoplasty | 2 |
| Partial anomalous pulmonary venous drainage repair | 2 |
| Double aortic arch repair | 2 |
| Damus-Kay-Stansel procedure | 1 |
| Ventricular septal defect enlargement | 1 |
Table 4: Concomitant procedure with Bidirectional Glenn.
Statistical Indicators
All variables in this study were first analyzed using a univariate analysis to determine whether any single factor influencing mortality and morbidity (operative death, late death, takedown of BDG, or cannot proceed to completion of TCPC). A P-value of <0.05 was considered statistically significant. Statistical analysis was performed using SPSS software (IBM SPSS Statistics, Version 21.0. Armonk, NY: IBM Corp).
Results
Arterial oxygen saturation rose from a mean of 77.7% preoperatively (range 55%-95%) to 82.6% postoperatively (range 69%- 95%). Hemodynamics showed an early postoperative superior vena cava mean pressure of 11 mmHg (range 3-30 mmHg). Six patients required re-exploration (2 for Glenn shunt takedown, 2 for heart failure, 1 for tamponade, 1 for ventricular fibrillation required open cardiac massage).
Total ICU stay was a mean of 6.6 days (range 2-45 days). Ten patients required prolonged drainage of more than 7 days, with 5 of those patients due to chylothorax.
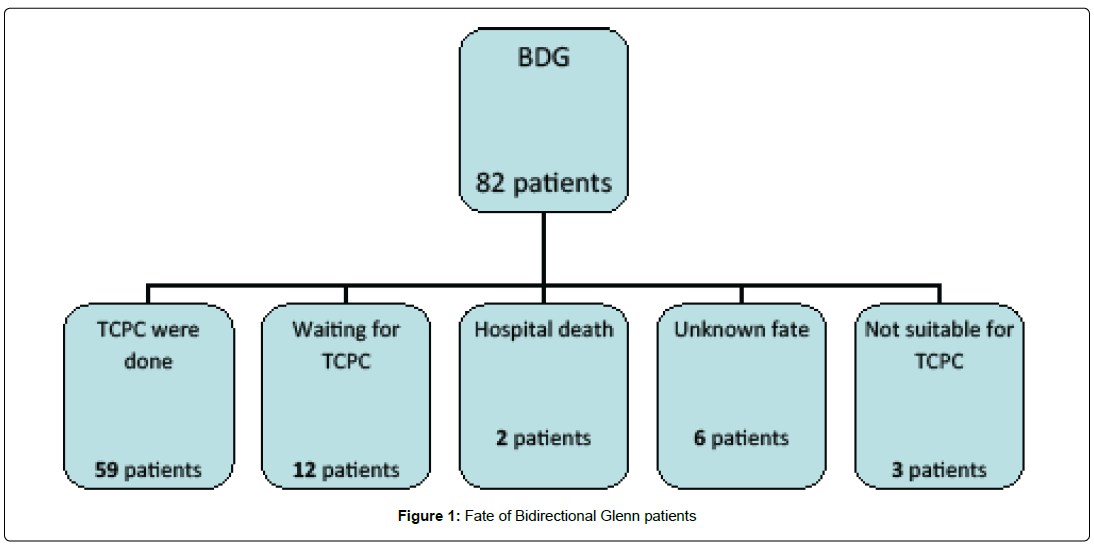
Clinical results following 82 cases of BDG are summarized in the Figure 1. A total of 59 patients (72%) underwent TCPC at a mean duration after BDG of 31.4 months (range 15-56 months). 12 patients were still waiting for TCPC (14.6%). There were two in-hospital deaths (2.4%) after BDG. In two patients (2.4%), completion of TCPC was not possible, while 6 patients were lost during follow-up (7.3%). One patient had his BDG taken down (1.3%).
Following BDG, two patients died in hospital due to hypoxia; one due to low flow through Glenn shunt caused by venous thrombosis and the other due to multiple myocardial infarctions. Two patients underwent Glenn shunt takedown; one due to high pulmonary blood pressure, and the other due to presence of thrombosis. Two patients failed of completion of TCPC; one due to hypoxic ischemic encephalopathy with developmental retardation, and the other due to postoperative cerebral hemorrhage with cerebral infarction and hemiparesis (Figure 1, Table 5).
| Risk Factors | Patients without Problems | Patients with Problem | p -value |
|---|---|---|---|
| ≤ 6 months | 36 | 1 | 0.271 |
| ≤ 5 kg | 4 | 1 | 0.217 |
| Associated anomalies | |||
| Pulmonary atresia with intact IVS | 6 | 0 | 0.999 |
| With tricuspid atresia | 22 | 2 | 0.59 |
| With TAPVD | 1 | 2 | 0.004 |
| Situs ambiguus | 8 | 2 | 0.076 |
| Unbalanced AVSD | 5 | 2 | 0.017 |
| With pulmonary atresia | 21 | 1 | 0.724 |
| Mitral atresia | 6 | 1 | 0.365 |
| Heterotaxy syndrome | 9 | 2 | 0.099 |
| Aortic incompetence | 2 | 1 | 0.092 |
| Previous Surgery | |||
| Pre mBTS | 34 | 2 | 0.856 |
| Pre PA banding | 13 | 2 | 0.217 |
| Pre TAPVD repair | 1 | 2 | 0.004 |
| Pre Aortic repair | 6 | 1 | 0.365 |
| Preoperative Data | |||
| O2 Saturation (%) | 78.08 (65-95) | 72.6 (55-89) | 0.071 |
| Mean PAP (mmHg) | 13.64 (3-39) | 21.2 (11-40) | 0.028 |
| McGoon Index | 2.04 (1.1-3.4) | 2.21 (1.7-3) | 0.432 |
| Nakata Index | 242.8 (122-680) | 263 (173-410) | 0.589 |
| Ventricular end diastolic pressure | 10.03 (2-12) | 7.5 (4-12) | 0.227 |
| AVVR ≥ moderate | 6 | 1 | 0.365 |
| Concomitant Surgery | |||
| Plasty of PA or branches | 8 | 1 | 0.515 |
| Atrial Septectomy | 31 | 1 | 0.385 |
| Intraoperative Data | |||
| OP time (minute) | 161.9 (90-263) | 225.25 (180-280) | 0.01 |
| CPB time | 81.1 (35-155) | 94.5 (59-143) | 0.345 |
Table 5: Univariate relationships between risk factors and complicated cases with mortality and morbidity (In-hospital death, takedown of BDG, or failure to proceed to TCPC).
In our study, the 10 years actuarial survival after BDG was 97.6% (2 deaths out of 82 patients, 2.4%). A univariate analysis revealed that diagnosis of unbalanced atrio-ventricular septal defect (P=0.017), previous repair of total anomalous pulmonary venous connection (P=0.004), high pulmonary arterial pressure (P=0.028) and long operation time (P=0.01) were significant predictors of morbidity and mortality (Table 6).
| Leading cause | End Result | Patient |
|---|---|---|
| Preoperative thrombosis: History of femoral vein and IVC thrombosis (Factor V Leiden mutation=Thrombophilia which high coagulability tending to thrombus formation ) | died | 1st patient |
| Preoperative TAPVD(high PAP): Glenn was done, decrease in O2 saturation due to high PAP, Glenn takedown was done and central AP shunt was implanted. | Glenn Takedown | 2nd patient |
| Preoperative thrombosis: (in old ECHO "1 year before BDG", it was narrow innominate vein and not shown subclavian vein "mostly due to presence of Thrombus"). And because of that, flow was little through Glenn shunt and desaturation, Glenn takedown was done and central AP shunt was put instead. Fulminate course of thrombosis happened after new cAP shunt which led to renal failure, spleen infarctions and lastly multiple myocardial infarctions which it was the cause of death. | Glenn takedown and died | 3rd patient |
| Previous TAPVD repair andclosure of MBTS by thrombus (secondary thrombus): Resuscitation was done and the result was Hypoxic ischemic Encephalopathy with motor retardation and Coordination disturbance. Therefore, we thought that Glenn is sufficient for her and no need to proceed to TCPC). | Not suitable for TCPC | 4th patient |
| Postoperative Glenn shunt stenosis development of veno-venal collateral, Pulmonary infection and recurrent chylothorax. All of those led to O2 desaturation, brain infarction. It was followed by IVC thrombus formation(Secondary thrombosis) and brain haemorrhage (due to thrombocytic aggregation inhibitors intake after brain infarction happened). | Not suitable for TCPC | 5th patient |
Table 6: Patients with complications (in details).
Discussion
In patients with single ventricle physiology, the surgical protocol accepted in most centers is a staging approach and includes Bidirectional Glenn shunt with later Fontan completion. In spite of the complexity of many of our cases, our results showed that 72% of the patients with Bidirectional Glenn Shunt achieved completion of Fontan pathway, with 14% still waiting. Tanoue and colleagues examined the outcomes of 333 patients operated between 1992 and 2004 [9]. Their interim mortality was 7%. They expected their Fontan completion rate to be 89%, but at the time of their publication only 69% had received a Fontan [9].
Good patient’s selection and appropriate surgical interventions before or during the BDG procedure may have contributed to our favorable results. Many studies [5,6] showed that BDG provides adequate oxygen saturation and does not impair ventricular or AV valve functions. It may yield long-lasting adequate palliation and may even be considered as a potential definitive palliation, particularly in high risk Fontan patients [10].
The main causes for death, takedown or failure of completion of Fontan, in our study, were elevated pulmonary arterial pressure in one patient and thrombosis in 4 patients. Kogon et al. [11] estimated that the results of bidirectional Glenn procedure were adversely affected primarily by elevated central venous pressure, elevated trans pulmonary gradient, right ventricular morphology and prolonged CPB time. On the other hand, Friedman et al. [12] mentioned that Pre- BDG atrioventricular valve regurge (AVVR), age ≤ 3 months at time of BDG, and prolonged hospitalization after first stage of palliation are independently associated with decreased successful progression of staged palliation in midterm follow-up after BDG.
We noticed that the incidence of thrombosis was the main mechanism that prevented our patients to proceed into the Fontan Pathway. Various causes for Thrombosis were identified such as primary thrombosis (e.g. Factor V Leiden mutation) or secondary Thrombosis (e.g. synthetic grafts, pulmonary infection or sepsis). Other studies attributed most failures to progressive deterioration in ventricular function [13]. To really know how much caval thrombosis can influence the failure of those patients to proceed to completion Fontan needs more investigations.
In our study, the 10 years actuarial survival after BDG was 97.6%. This confirms the results noted in other series in which patients were systematically entered in a staging to Fontan policy, In Tanoue’s report, among 333 patients who had BDG, the actuarial survival was 90% at 10 years, and In the Melbourne experience which includes 212 patients with BDG survival was 89% at 15 years [14]. In these two series, the Fontan completion rate was 69 and 66%, respectively, whereas it was 72% in our experience (with 14.6% waiting for TCPC). These results support our approach of management of patients with single ventricle, who were scheduled for staged operation, which ultimately ended with completion Fontan.
Limitations
Our study is retrospective, and we studied a relatively small population in a single center. A prospective study, multi central may yield more comprehensive results.
Conclusion
The staged strategy for single ventricle patients has accepted clinical results. In our Study, the mechanisms for complications were elevated pulmonary arterial pressure and thrombosis. The diagnosis of unbalanced Atrioventricular septal defect, previous repair of total anomalous pulmonary venous connection in previous surgery, high mean pulmonary arterial pressure and long operation time were significant predictors of death, shunt takedown, or failure of proceeding to TCPC. We recommend good preoperative evaluation of the risk factors of post-operative thrombosis, which may help in the selection criteria for patients undergoing Fontan pathway.
References
- Glenn WWL (1989) Superior vena cava-pulmonary artery shunt. Ann Thorac Surg 47: 62-64.
- Castaneda AR (1992) From Glenn to Fontan: A Continuing Evolution. Circulation 86: 1180-1184.
- Cloutier A, Ash JM, Smallhorn JF, Williams WG, Trusler GA, et al. (1985) Abnormal distribution of pulmonary blood flow after the Glenn shunt or Fontan procedure: Risk of development of arteriovenous fistulae. Circulation 72: 471-479.
- Fontan F, Baudet E (1971) Surgical repair of tricuspid atresia. Thorax 23: 240-248.
- Hopkins R, Armstrong B, Serwer G, Peterson R, Oldham HJ (1985) Physiological rationale for a bidirectional cavopulmonary shunt. A versatile complement to the Fontan principle. J Thorac Cardiovasc Surg 90: 391-398.
- Bridges ND, Jonas RA, Mayer JE, Flanagan MF, Keane JF, et al. (1990) Bidirectional cavopulmonary anastomosis as interim palliation for high-risk Fontan candidates. Early results. Circulation 82: 170-176.
- Pearl JM, Laks H, Stein DG, Drinkwater DC, George BL, et al. (1991) Total cavopulmonary anastomosis versus conventional modified Fontan procedure. Ann Thorac Surg 52: 189-196.
- Kreutzer J, Keane JF, Lock JE, Walsh EP, Jonas RA, et al. (1996) Conversion of modified Fontan procedure to lateral atrial tunnel cavopulmonary anastomosis. J Thorac Cardiovasc Surg 111: 1169-1176.
- Tanoue Y, Kado H, Boku N, Tatewaki H, Nakano T, Fukae K, et al. (2006) Three hundred and thirty-three experiences with the bidirectional Glenn procedure in a single institute. Interact Cardiovasc Thorac Surg 6: 97-101.
- Day RW, Etheridge SP, Veasy LG, Jenson CB, Hillman ND, et al. (2006) Single ventricle palliation: Greater risk of complications with the Fontan procedure than with the bidirectional Glenn procedure alone. Int J Cardiol 106: 201-210.
- Kogon BE, Plattner C, Leong T, Simsic J, Kirshbom PM, et al. (2008). The bidirectional Glenn operation: A risk factor analysis for morbidity and mortality. J Thorac Cardiovasc Surg 136: 1237-1242.
- Friedman KG, Salvin JW, Wypij D, Gurmu Y, Bacha EA, et al. (2011) Risk factors for failed staged palliation after bidirectional Glenn in infants who have undergone stage one palliation. Eur J Cardio-Thoracic Surg 40: 1000-1006.
- Gatzoulis MA (2000) Definitive palliation with cavopulmonary or aortopulmonary shunts for adults with single ventricle physiology. Heart 83: 51-57.
- Tan AM, Iyengar AJ, Donath S, Bullock AM, Wheaton G, et al. (2010) Fontan completion rate and outcomes after bidirectional cavo-pulmonary shunt. Eur J Cardio-Thoracic Surg 38: 59-65.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi