Research Article, Int J Cardiovasc Res Vol: 7 Issue: 1
Procedural and Clinical Results of the New MitraClip® NT after Percutaneous Edge to Edge Repair of Mitral Valve Regurgitation
Mirjam Keßler, Julia Seeger, Jochen Wöhrle, Wolfgang Rottbauer, Sinisa Markovic*
Department of Internal Medicine II, University of Ulm, Ulm, Germany
*Corresponding Author : Dr. Sinisa Markovic
Department of Internal Medicine II, University of Ulm Albert-Einstein llee 23, 89081 Ulm, Germany
Tel: 0049-731 500 45001
Fax: 0049-731-500 45029
Email: sinisa.markovic@uniklinik-ulm.de
Received: January 17, 2018 Accepted: February 02, 2018 Published: February 07, 2018
Citation: Keßler M, Seeger J, Wöhrle J, Rottbauer W, Markovic S (2018) Procedural and Clinical Results of the New MitraClip® NT after Percutaneous Edge to Edge Repair of Mitral Valve Regurgitation. Int J Cardiovasc Res 7:1. doi: 10.4172/2324-8602.1000340
Abstract
Objective: The MitraClip® NT (MC-NT) as an updated version aim to facilitate improved maneuvering of the guide catheter and more efficient leaflet capture due to technical improvements of the delivery system and clip material. We evaluate procedural results and 12-month clinical outcomes of the new MC-NT device and compare them with the previous MitraClip® MC.
Methods: We analyzed a total population of 231 patients from our Ulm - Transcatheter Mitral Valve Repair registry. To adjust for differences of baseline characteristics a propensity-score matching was performed (N=142). 30-day endpoints were analyzed in accordance with the Mitral Valve Academic Research Consortium (MVARC). For both devices 12-month clinical results (MACCE, mortality, and heart failure rehospitalization) were evaluated.
Results: Acute technical success was achieved in 100% with both device groups. Device, procedure and fluoroscopy time were comparable in both treatment groups. In-hospital mortality was 2.8% in the MC-NT group and 4.2% in the MC group (p=0.65). After 30 days single-leaflet clip detachment was observed in 2.8% in the MC-NT group compared to 1.4% (p=0.56). Mitral regurgitation was reduced to the same extent in both treatment groups (1.6 ± 0.6 vs. 1.6 ± 0.5 in MC-NT group, p=0.57) with comparable rates of recurrent moderate to severe mitral regurgitation after 30 days and during 12-month follow-up. Clinical results up to 12 months and improvement of New York Heart Association functional class were comparable in both device groups (3.1 ± 0.7 to 2.1 ± 0.9 vs. 3.2 ± 0.6 to 2.1 ± 0.9, p=0.75).
Conclusion: The new MitraClip® NT as an updated version presents itself as an efficacious and safe device, but all in all failed to prove superiority compared to the original MitraClip® in terms of procedural and clinical results.
Keywords: MitraClip NT; MVARC; Device endpoints; Clinical endpoints
Introduction
The transcatheter edge-to-edge mitral valve repair by MitraClip (MitraClip®, Abbott Vascular, Menlo Park, California, USA) implantation emerged as a thankful treatment option for patients presenting with a symptomatic high-grade mitral regurgitation (MR) and high surgical risk or inoperability. The procedure has been proved to be safe and effective in several trials, demonstrating similar improvement in clinical outcomes compared to surgery [1-4].
In the first half of 2016, the MitraClip® NT (MC-NT) as an updated version of the original MitraClip® (MC) device was introduced. It features improved maneuvering of the guide catheter and facilitates more efficient leaflet capture due to technical improvements of the delivery system and, in particular, of the clip material. However, procedural results and clinical outcomes of the MC-NT have not been systematically compared with its predecessor device MC in clinical trials.
Therefore we aimed to compare the procedural results and clinical outcomes of the new MC-NT device with the previous MC device in a 12-month follow-up.
Methods
We analyzed a total population of 231 patients from our Ulm - Transcatheter Mitral Valve Repair registry undergoing percutaneous edge-to-edge mitral valve repair at our high-volume center [5,6] between January 2015 and December 2016. The MC-NT has been used at our center since June 2016.
For the registry, the baseline clinical characteristics and data from invasive and non-invasive studies were prospectively collected and were recorded in a database. Follow-up data were acquired by clinical routine visits or phone contact at 3 months and 12 months after MitraClip implantation. There exist no exclusion criteria for this registry. Written informed consent was obtained from all patients. The study was approved by the ethics committee of the University of Ulm and complies with the principles of the Declaration of Helsinki.
All patients suffered from symptomatic high-grade MR documented by echocardiography according to the “Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging” [7]. Pre-procedural transesophageal, and transthoracic echocardiography and invasive hemodynamic measurement by cardiac catheterization were performed prior to MitraClip implantation procedure. All procedures were performed in accordance with our in-house standard operating procedure (SOP) referring to the manufacturer’s Instruction for Use and described elsewhere [5,8] and was not altered during the study period. The operator team remained unchanged throughout the study period. In conformity with the MVARC criteria [9] 30-day mortality, neurological events, residual MR grade after MitraClip implantation and single leaflet clip detachment were analyzed. Patients received oral anticoagulation and acetylsalicylic acid for 4 weeks after the MitraClip implantation. After 4 weeks of dual therapy, patients received either acetylsalicylic acid or oral anticoagulation if indicated for comorbidities.
Statistical analysis
Baseline characteristics, procedural data and post-procedural outcomes were compared between 2 groups of patients: Patients treated with the new MC-NT or the original MC device implantation. A value of p<0.05 was considered statistically significant. To adjust for differences of baseline characteristics a propensity-score matching was performed. The variables STS Score, NYHA class, atrial fibrillation, MR grade before MitraClip implantation, mitral valve prolapse and severe tricuspid regurgitation were identified as potential confounders and were employed in propensity-score matching. A one-to-one matched analysis was performed. Patients were eligible for matching, if the difference of the estimated propensity score between MC and MC-NT group was within the caliper radius of 0.10* sigma.
Primary endpoint of interest was device success (measured at 30 days) defined as successful delivery of the MitraClip device, achieving a reduction of the mitral regurgitation of more than two degrees, freedom from emergency surgery or reintervention and absence of procedural mortality or stroke. Secondary endpoints were device time, procedural time, and rate of clip detachment. Furthermore following clinical endpoints were collected during the 12 month follow up were collected: rehospitalization for heart failure, reintervention of the mitral valve and composite endpoint of major cardiovascular and cerebral events (MACCE) including rehospitalization due to heart failure, neurological events or bleeding, further reintervention on the mitral valve, need for left ventricular assist device and mortality. Categorical parameters are presented as counts and percentages. Comparison of proportions was done with χ2 test. Continuous variables are presented as mean ± SD. Continuous variables for two groups were compared with the unpaired U-test. Time-to-event analyses for 12-month follow-up were performed using Kaplan-Meier estimates and were compared with the log-rank test. Kaplan-Meier survival curves were generated for time to event outcomes. Propensity-score matching was performed with XLSTAT (XLSTAT- Premium, Addinsoft, New York, USA). Statistical analyses were calculated with Statistica release 7.1 (StatSoft, Inc., Tulsa, OK, USA).
Results
Between January 2015 and December 2016 a total of 231 patients were treated with percutaneous edge-to-edge mitral valve repair by MitraClip implantation at our high-volume center and were included in the Ulm - Transcatheter Mitral Valve Repair registry. Starting in June 2016, 79 patients were treated with the new MC-NT device. Baseline characteristics and anatomical findings are reported in Table 1.
| Total | MC | MC-NT | P-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 231 | 152 | 79 | |||||||||
| Male, N (%) | 140 (60.6) | 88 (57.9) | 52 (65.8) | 0.24 | ||||||||
| Age > 75 years | 163 (70.6) | 103 (67.8) | 60 (70.0) | 0.07 | ||||||||
| Height | 168.5±9.1 | 168.3±8.9 | 169.1±9.4 | 0.41 | ||||||||
| Weight | 73.9±14.9 | 74.1±14.9 | 73.8±14.9 | 0.56 | ||||||||
| NYHA class | 3.1±0.6 | 3.0±0.6 | 3.2±0.6 | 0.03 | ||||||||
| Logistic Euro SCORE | 7.1±5.9 | 7.3±6.6 | 7.3±5.0 | 0.28 | ||||||||
| STS Score of mortality | 4.7±5.4 | 23.6±9.8 | 30.1±13.6 | <0.001 | ||||||||
| ASA Score | 3.7±0.5 | 3.7±0.5 | 3.7±0.5 | 0.95 | ||||||||
| non ischemic cardiomyopathy | 45 (19.5) | 32 (21.0) | 13 (16.5) | 0.40 | ||||||||
| ischemic cardiomyopathy | 186 (80.5) | 120 (79.0) | 0.40 | |||||||||
| AFib / AFlu | 157 (68.0) | 100 (65.8) | 0.21 | |||||||||
| NT-pro BNP (pg/ml) | 5383.7±5996 | 5327.5±6366 | 0.25 | |||||||||
| chronic obstructive pulmonary disease | 30 (13.0) | 20 (13.2) | 0.91 | |||||||||
| Echocardiographic parameters | ||||||||||||
| Etiology of mitral valve regurgitation | ||||||||||||
|
116 (50.2) | 72 (47.4) | 44 (55.7) | 0.23 | ||||||||
|
115 (49.8) | 80 (52.6) | 35 (44.3) | 0.23 | ||||||||
|
55 (23.8) | 42 (27.6) | 13 (16.5) | 0.05 | ||||||||
|
75 (32.5) | 47 (30.9) | 28 (35.4) | 0.49 | ||||||||
| Pre-procedural transmitral gradient (mmHg) | 1.5±0.9 | 1.5±0.9 | 1.5±0.9 | 0.63 | ||||||||
| EROA of MR before clip (cm2) | 0.4±0.4 | 0.4±0.4 | 0.4±0.3 | 0.47 | ||||||||
| Grade of MR before clip | 3.5±0.6 | 3.6±0.5 | 3.2±0.7 | <0.01 | ||||||||
| Severe tricuspid regurgitation | 85 (36.8) | 68 (44.7) | <0.01 | |||||||||
| LA diameter before clip (mm) | 54.6±8.8 | 54.6±9.5 | ±7.5 | 0.98 | ||||||||
| LVEDd (mm) | 60.1±11.4 | 60.5±12.3 | 0.83 | |||||||||
| Max. transtricuspid gradient (mmHg) | 45.6±14.6 | 45.0±14.2 | ±15.3 | 0.62 | ||||||||
| Cardiac catheterization | ||||||||||||
| Mean LA pressure (mmHg) | 17.0±9.2 | 15.9±9.5 | ±7.6 | <0.01 | ||||||||
| v-wave (mmHg) | 30.3±16.6 | 27.8±16.5 | ±14.2 | <0.01 | ||||||||
| Mean pulmonary artery pressure (mmHg) | 32.1±11.6 | 31.4±10.9 | ±13.1 | 0.35 | ||||||||
| LVEF < 35% | 87 (37.8) | 56 (36.8) | 0.55 | |||||||||
| Cardiac output (l/min) | 3.7±1.1 | 3.7±1.1 | ±1.1 | 0.55 | ||||||||
NYHA: New York Heart Association, SCORE: System for Cardiac Operative Risk Evaluation STS: Society of Thoracic Surgeons; ASA Score according to the physical status protocol; AFib: atrial fibrillation; AFlu: atrial flutter; NT-pro-BNP: Nâ€Âterminal fragment Bâ€Âtype natriuretic peptide; EROA: Effective regurgitant orifice area MR: mitral regurgitation; LA: left atrium; LVEDd left ventricle end-diastolic diameter; LVEF: left ventricular ejection fraction;
Table 1: Baseline characteristics.
After adjusting for relevant baseline characteristics with propensity-score matching, 71 patients in each MitraClip device group were assembled with similar baseline characteristics, anatomical and functional findings as detailed in Table 2. The adequacy of the propensity score given by the area under the curve was fair (C-statistic 0.74).
| Total | MC group | MC-NT group | P-Value | ||||
|---|---|---|---|---|---|---|---|
| Number of patients | 142 | 71 | 71 | ||||
| Male, N (%) | 85 (59.9) | 40 (56.3) | 45 (63.4) | 0.39 | |||
| Age > 75 years | 103 (72.5) | 48 (67.6) | 55 (77.5) | 0.19 | |||
| Height | 168.0±9.1 | 167.6±8.6 | 168.9±9.5 | 0.37 | |||
| Weight | 72.9±14.5 | 73.0±14.4 | 73.6±14.7 | 0.85 | |||
| NYHA class | 3.1±0.6 | 3.1±0.7 | 3.2±0.6 | 0.43 | |||
| Logistic Euro SCORE | 7.5±6.0 | 8.2±7.2 | 6.7±4.4 | 0.56 | |||
| STS Score of mortality | 5.4±6.2 | 4.5±3.8 | 6.2±7.8 | 0.23 | |||
| ASA Score | 3.7±0.5 | 3.7±0.5 | 3.7±0.5 | 0.68 | |||
| non ischemic cardiomyopathy | 29 (20.4) | 17 (23.9) | 12 (16.9) | 0.30 | |||
| ischemic cardiomyopathy | 113 (79.6) | 54 (76.1) | 53 (83.1) | 0.30 | |||
| NT-pro-BNP (pg/ml) | 5526.3±6256 | 5716.0±6985 | 5336.6±5482 | 0.75 | |||
| AFib / AFlu | 96 (67.6) | 46 (64.8) | 50 (70.4) | 0.47 | |||
| chronic obstructive pulmonary disease | 17 (12.0) | 8 (11.3) | 9 (12.7) | 0.80 | |||
| Echocardiographic parameters | |||||||
| Etiology of mitral valve regurgitation | |||||||
|
81 (57.0) | 40 (56.3) | 41 (57.8) | 0.80 | |||
|
61 (43.0) | 31 (43.7) | 30 (42.3) | 0.87 | |||
|
28 (19.7) | 16 (22.5) | 12 (16.9) | 0.40 | |||
|
48 (33.8) | 25 (35.2) | 23 (32.4) | 0.72 | |||
| Pre-procedural transmitral gradient (mmHg) | 1.5±0.9 | 1.5±0.8 | 1.5±0.9 | 0.81 | |||
| EROA of MR before clip (cm2) | 0.4±0.3 | 0.3±0.3 | 0.4±0.3 | 0.54 | |||
| Grade of MR before clip | 3.4±0.6 | 3.3±0.5 | 3.3±0.6 | 0.25 | |||
| Severe tricuspid regurgitation | 41 (28.9) | 24 (33.8) | 17 (23.9) | 0.19 | |||
| LA diameter pre clip (mm) | 53.8±8.8 | 52.8±10.0 | 54.7±7.6 | 0.47 | |||
| LVEDd (mm) | 60.7±11.7 | 61.9±13.8 | 59.6±9.4 | 0.58 | |||
| Max. transtricuspid pressure (mmHg) | 43.8±15.0 | 41.8±14.3 | 45.6±15.5 | 0.22 | |||
| Cardiac catheterization | |||||||
| Mean LA pressure (mmHg) | 18.2±9.6 | 16.7±10.5 | 20.3±7.9 | 0.04 | |||
| v-wave (mmHg) | 31.5±15.8 | 26.7±14.5 | 39.6±14.6 | <0.01 | |||
| Mean pulmonary artery pressure (mmHg) | 31.7±10.2 | 31.2±10.9 | 32.4±9.2 | 0.41 | |||
| LVEF <35% | 61 (43.0) | 30 (42.3) | 31 (43.7) | 0.87 | |||
| Cardiac output (l/min) | 3.7±1.1 | 3.6±1.1 | 3.8±1.1 | 0.47 | |||
NYHA: New York Heart Association, SCORE: System for Cardiac Operative Risk Evaluation STS: Society of Thoracic Surgeons; ASA Score according to the physical status protocol; AFib: atrial fibrillation; AFlu: atrial flutter; NT-pro-BNP: Nâ€Âterminal fragment Bâ€Âtype natriuretic peptide; EROA: Effective regurgitant orifice area MR: mitral regurgitation; LA: left atrium; LVEDd left ventricle end-diastolic diameter; LVEF: left ventricular ejection fraction;
Table 2: Baseline characteristics matched population.
73% of the matched population (N=142) were older than 75 (67.6% vs. 77.5% in the MC-NT group, p=0.19). Patients had severe symptoms of heart failure with at least one inpatient stay in 55.6% of the cases in the year before clip implantation. The median New York Heart Association (NYHA) functional class at baseline was 3.1 ± 0.6 (3.1 ± 0.7 vs. 3.2 ± 0.6, p= 0.43). Mean STS Score was 5.4 ± 6.2 (4.5 ± 3.8 vs. 6.2 ± 7.8 in the MC-NT group, p=0.23). In 57% of the cases a functional etiology of the mitral insufficiency was identified (56.3% vs. 57.8%, p=0.80). The left ventricular ejection function was severely impaired in 43% of the cases (42.3% vs. 43.7% in MC-NT group, p= 0.87).
Acute technical success measured at exit from the catheterization laboratory was 100% (no periprocedural death, successful access, delivery and retrieval of the device delivery system, successful clip implantation, and freedom from emergency surgery or reintervention). Device success measured at thirty days was achieved in 86.6% (83.1% for the MC group vs. 90.1% in MC-NT group, p=0.22). Device failure was mainly attributed to in-hospital death or stroke and inability to achieve a reduction of mitral regurgitation to an acceptable level. No intra-procedural death was observed, but 5 patients (3.5% in total, 4.2% in MC group vs. 2.8% in MC-NT group, p=0.65) died within 30 days of the intervention and thus are considered as in-hospital deaths. Four patients (2.8% in both MitraClip device groups) required cardiopulmonary resuscitation during the procedure. Two cases of periprocedural stroke were observed (2.8% in MC group vs. 0% in MC-NT group, p=0.09). Residual or recurrent moderate to severe mitral regurgitation (Grade III or IV) at thirty days was observed in 5 cases (3.5% in total, 5.6% in MC group compared to 1.4% in MC-NT group, p=0.16). Single leaflet clip detachment was observed in 2.1% (1.4% in the MC group compared to 2.8% in the MC-NT group, p= 0.56). Device and procedure time were slightly prolonged in the MCNT group (45 min and 133 min vs. 51 min and 140 min, p=0.26 and 0.41, respectively), while fluoroscopy time was marginally shorter in the MC-NT group (p=0.15). All in all, device success achieved with the MC-NT was comparable to the original MC device (see Table 3).
| Total | MC group | MC-NT group | P-Value | |
|---|---|---|---|---|
| Number of patients | 142 | 71 | 71 | |
| Device success (MVARC) | 123 (86.6) | 59 (83.1) | 64 (90.1) | 0.22 |
| In-hospital mortality | 5 (3.5) | 3 (4.2) | 2 (2.8) | 0.65 |
| Neurological events (TIA or stroke) | 2 (1.4) | 2 (2.8) | 0 (0.0) | 0.09 |
| In-hospital mortality /stroke | 7 (4.9) | 5 (7.0) | 2 (2.8) | 0.24 |
| Single leaflet clip detachment | 3 (2.1) | 1 (1.4) | 2 (2.8) | 0.56 |
| Residual MR Grade III or IV | 5 (3.5) | 4 (5.6) | 1 (1.4) | 0.16 |
| Need for resuscitation during procedure | 4 (2.8) | 2 (2.8) | 1.00 | |
| Grade of MR after clip | 1.6±0.5 | 1.6±0.6 | ±0.5 | 0.57 |
| Transmitral gradient mean post (mmHg) | 3.7±1.6 | 3.6±1.6 | 0.34 | |
| Number of implanted clips | 1.3±0.5 | 1.2±0.5 | 1.3±0.5 | 0.11 |
| Procedure time (min) | 136.2±42 | 133.4±41 | 139.0±43 | 0.41 |
| Device time (min) | 47.6±30 | 44.5±25 | 50.6±34 | 0.26 |
| Fluoroscopy time (min) | 28.7±16 | 32.2±20 | 25.7±10 | 0.15 |
| Periprocedural MI | 0 (0.0) | 0 (0.0) | 0 (0.0) | -- |
| Access and vascular complications | 9 (6.3) | 3 (4.2) | 6 (8.5) | 0.30 |
| Bleeding (MVARC ≥ 2) | 3 (2.1) | 1 (1.4) | 2 (2.8) | 0.56 |
| Time in hospital before clip (days) | 5.5±6.7 | 5.3±6.7 | 5.6±6.7 | 0.56 |
| Time in hospital after clip (days) | 6.8±5.6 | 7.4±5.9 | 6.3±5.3 | 0.008 |
MVARC: Mitral Valve Academic Research Consortium; TIA: transient ischemic attack; MR: mitral regurgitation; MI: myocardial infarction;
Table 3: Measures of technical /device results.
Clinical endpoints
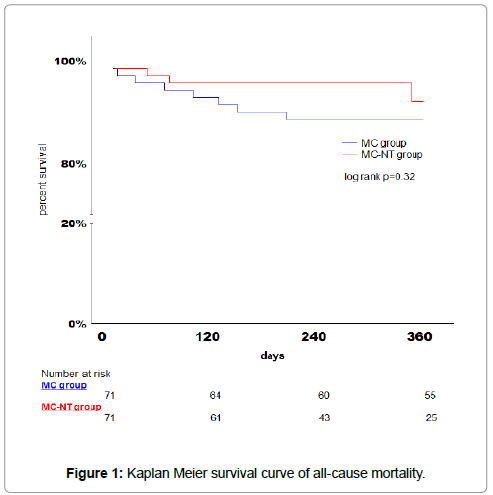
The median clinical follow-up was 365 days (296 ± 106 days). Effectiveness of the MitraClip implantation was reflected by an improvement of the New York Heart Association (NYHA) functional class in both groups (2.1 ± 0.9 vs. 2.1 ± 0.9, p=0.75) and a reduced rate of rehospitalization due to heart failure (12.4 % vs. 20.9%, p=0.13) during 12-months follow-up. The rate of recurrent severe mitral regurgitation during follow up remained slightly lower in the MC-NT group (MR Grad III: 4.2% vs. 1.4% for the MC-NT, MR Grad IV: 1.4% vs. 0% for the MC-NT, p=0.30 and 0.24). Kaplan-Meier estimates for all-cause mortality was numerically lower in the MC-NT group compared to the MC device group (7.5% vs. 11.5%, p=0.32, (Figure 1). Analogously, cardiovascular mortality was lower in the MC-NT group (7.5% vs. 8.9%, p=0.32). MACCE was 30% in the MC-NT group compared to 26 % in the MC group (p=0.74), mainly caused by higher rates of rehospitalization due to heart failure in the MCNT group. Detailed results of the clinical outcomes during 12-month follow-up are listed in Table 4.
| Total | MC group | MC-NT group | P-Value | |
|---|---|---|---|---|
| 12 month FUP | 12 month FUP | 12 month FUP | ||
| MACCE | 38 (26.8) | 26.0 | 30.0 | 0.74 |
| All cause death, N (%) | 12 (8.5) | 11.5 | 7.5 | 0.32 |
| Cardiovascular death | 10 (7.0) | 8.9 | 7.5 | 0.65 |
| Non-cardiovascular death | 2 (1.4) | 2.9 | 0.0 | 0.16 |
| Heart failure rehospitalization, N (%) | 21 (14.8) | 12.4 | 20.9 | 0.13 |
| Mitral valve reintervention, N (%) | 1 (0.7) | 1.4 | 0.0 | 0,24 |
| NYHA class post procedure | 2.1±0.9 | 2.1±0.9 | 2.1±0.9 | 0.75 |
MACCE: Major Adverse Cardiac and Cerebrovascular Events, NYHA: New York Heart Association,
Table 4: Kaplan Meier estimates of clinical events.
Discussion
The percutaneous edge-to-edge mitral valve repair by MitraClip® implantation emerged as a safe and effective treatment option for patients presenting with a symptomatic high-grade mitral regurgitation (MR) and high surgical risk or inoperability.
The MC-NT device as an updated version of the original MC was lanced in 2016 at our center. Several technical alterations of the new MC-NT device were implemented by the manufacturer, aiming to improve the precision and handiness of the entire system. The key material of the clip delivery system has been changed from stainless steel to Nylon allowing an improved and more precise straddling of the clip in the left atrium. The modified steerable sleeve allows an accurate response of the M-knob and causes a reduced anterior movement. Furthermore, the gripper material has been changed from Elgiloy to Nitinol allowing an increase of the gripper drop angle from 85° to 120°, providing a greater grasping arm angle and a deeper leaflet insertion. All in all, the modifications of the MC-NT aim to facilitate the delivery of the MitraClip device and are supposed to increase the safety of the grasping.
However, procedural results and clinical outcomes of the new MCNT device have not been systematically compared with its predecessor device MC. Therefore we compared the procedural results and clinical outcomes of the new MC-NT device with the previous MC device in a 12-month follow-up. To adjust for potential confounders between subjects treated with the MC-NT or MC a propensity-score matching was performed.
In our propensity-score matched population with adjusted baseline clinical and anatomical characteristics device success for the new MC-NT was comparable to the original MC. Device success was achieved in 86.6% in total in our cohort, with 90.1% success in the MC-NT group and 83.1% in the control group (p=0.22), not reaching statistical significance supposedly due to the limited number of patients. Analogously, periprocedural mortality and stroke rate (2.8% vs. 7.0%, p=0.24) at 30 days follow-up were lower in the MC-NT group without reaching statistical significance. Notably, mitral regurgitation was reduced to the same extent in both treatment groups (1.6 ± 0.6 vs. 1.6 ± 0.5 in MC-NT group, p=0.57) with comparable rates of recurrent moderate to severe mitral regurgitation after 30 days and during 12-month follow-up (p=0.22 and 0.24). The determination of measurable benefits in grasping by the MC device is difficult, but rate of single-leaflet detachment and device time might serve as useful measures to evaluate grasping. However, the rate of single-leaflet clip detachment in our cohort was not lower in the MC-NT group (p=0.56). Moreover, device and procedural time were numerically longer in the MC-NT group, even though the operator team and the SOP remained unchanged throughout the study period. In summary, the modifications of the MC-NT device were not reflected in improved technical performance measured by device success and clinical endpoints at 30 days compared to the previous MC device.
In the pivotal EVEREST trial [10], in which the original MitraClip device was used, periprocedural death rate was 7.7%, and 12-month death rate was 24.4%. In a recent meta-analysis published by Chiarito and colleagues [11] one year mortality was estimated to 16%. In a single center cohort with 194 patients presented by Schau and colleagues [12] the overall mortality was 24% after successful MitraClip procedure. Lesevic et al. published in 2015 [13] a cohort with 136 patients undergoing a MitraClip procedure, observing a 12-month mortality rate of 17% among those patients with severe reduction of ejection fraction. Capodanno and colleagues published a cohort with 304 patients, where the all-cause mortality was quantified to 10.8% after one year [14]. All this data mirror our results. Regarding clinical events in the 12-month follow-up, mortality was 8.5% and rate of rehospitalization was 14.8%, all in all with comparable results in both groups (MACCE rate 26.0% in MC compared to 30% in MC-NT group, p=0.74).
Strength and Limitations
This study was a single-center trial with all of its inherent limitations. We included 231 consecutive patients within 2 years and thoroughly analyzed procedural and 12-month follow-up clinical outcome of all patients. The clinical outcome and procedural success of our cohort is comparable to those of other registries and trials.
Conclusion
Several technical alterations of the new MitraClip® NT device were implemented, aiming to facilitate the delivery of the MitraClip device and to increase the safety of the grasping. However, the technical modifications of the new MC-NT device were not reflected in improved technical performance measured by device success according to MVARC criteria and clinical endpoints in the 12-month follow-up compared to the previous MitraClip® device in our cohort.
Disclosure statement: The authors report no financial relationships or conflicts of interest regarding the content herein.
References
- Maisano F, Torracca L, Oppizzi M, Stefano PL, D'Addario G, et al. (1998) The edge-to-edge technique: a simplified method to correct mitral insufficiency. Eur J Cardiothorac Surg 13: 240-245.
- Nickenig G, Estevez-Loureiro R, Franzen O, Tamburino C, et al. (2014) Percutaneous mitral valve edge-to-edge repair: in-hospital results and 1-year follow-up of 628 patients of the 2011-2012 Pilot European Sentinel Registry. J Am Coll Cardiol 64: 875-884.
- Sorajja P, Mack M, Vemulapalli S, Holmes DR Jr, Stebbins A, et al. (2016) Initial Experience With Commercial Transcatheter Mitral Valve Repair in the United States. J Am Coll Cardiol 67: 1129-1140.
- Feldman T, Kar S, Elmariah S, Smart SC, Trento A, et al. (2015) Randomized Comparison of Percutaneous Repair and Surgery for Mitral Regurgitation: 5-Year Results of EVEREST II. J Am Coll Cardiol 66: 2844-2854.
- Seeger J, Müller P, Gonska B, Scharnbeck D, Markovic S, et al. (2017) Percutaneous Mitral Valve Repair With the MitraClip in Primary Compared With Secondary Mitral Valve Regurgitation Using the Mitral Valve Academic Research Consortium Criteria. J Invasive Cardiol 29: 145-150.
- Wöhrle J, Karakas M, Trepte U, Seeger J, Gonska B, et al. (2015) Midregional-proAtrial Natriuretic Peptide and High Sensitive Troponin T Strongly Predict Adverse Outcome in Patients Undergoing Percutaneous Repair of Mitral Valve Regurgitation. PLoS One 10: e0137464.
- Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, et al. (2013) Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 14: 611-644.
- Feldman T, Foster E, Glower DD, Kar S, Rinaldi MJ, et al. (2011) Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 364: 1395-1406.
- Stone GW, Vahanian AS, Adams DH, Abraham WT, Borer JS, et al. (2015) Clinical Trial Design Principles and Endpoint Definitions for Transcatheter Mitral Valve Repair and Replacement: Part 2: Endpoint Definitions: A Consensus Document From the Mitral Valve Academic Research Consortium. J Am Coll Cardiol 66: 308-321
- Whitlow PL, Feldman T, Pedersen WR, Lim DS, Kipperman R, et al. (2012) Acute and 12-month results with catheter-based mitral valve leaflet repair: the EVEREST II (Endovascular Valve Edge-to-Edge Repair) High Risk Study. J Am Coll Cardiol 59: 130-139.
- Chiarito M, Pagnesi M, Martino EA, Pighi M, Scotti A, et al. (2015) Outcome after percutaneous edge-to-edge mitral repair for functional and degenerative mitral regurgitation: a systematic review and meta-analysis Heart 104: 306-312.
- Schau T, Isotani A, Neuss M, Schöpp M, Seifert M, et al. (2016) Long-term survival after MitraClip((R)) therapy in patients with severe mitral regurgitation and severe congestive heart failure: A comparison among survivals predicted by heart failure models. J Cardiol 67: 287-294.
- Lesevic H, Sonne C, Braun D, Orban M, Pache J, et al. (2015) Acute and Midterm Outcome After MitraClip Therapy in Patients With Severe Mitral Regurgitation and Left Ventricular Dysfunction. Am J Cardiol 116: 749-756.
- Capodanno D, Adamo M, Barbanti M, Giannini C, Laudisa ML, et al. (2015) Predictors of clinical outcomes after edge-to-edge percutaneous mitral valve repair. Am Heart J 170: 187-195.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi