Research Article, J Polym Sci Appl Vol: 1 Issue: 1
Processing of Epoxy/Graphene Nanocomposites: Effects of Surfactants
| Jiacheng Wei and Fawad Inam* | |
| Northumbria University, Faculty of Engineering and Environment, Department of Mechanical and Construction Engineering, Newcastle upon Tyne NE1 8ST, UK | |
| Corresponding author : Fawad Inam Northumbria University, Faculty of Engineering and Environment, Department of Mechanical and Construction Engineering, Newcastle upon Tyne NE1 8ST, United Kingdom Tel: +44 1912273741 E-mail: fawad.inam@northumbria.ac.uk |
|
| Received: February 02, 2017 Accepted: March 09, 2017 Published: March 14, 2017 | |
| Citation: Wei J, Inam F (2017) Processing of Epoxy/Graphene Nanocomposites: Effects of Surfactants. J Polym Sci Appl 1:1. |
Abstract
The use of graphene has attracted lots of attention in recent years because of its excellent performance in mechanical, electrical and thermal applications. Graphene can significantly improve the properties of epoxy at extremely low loadings. Herein, epoxy/grapheme nanocomposites have been prepared to explore the properties enhancement of graphene in epoxy matrix. However, due to the strong van der Waals force on separately dispersed graphene surface, graphene tends to re-aggregate in epoxy matrix. In this work,Sodium Dodecyl Sulphate (SDS) and Gum Arabic (GA)have been selected to disperse graphene.Tensile properties, flexural properties and hardness, etc have been tested to evaluate the effectiveness. After introduced SDS, the tensile strength of nanocomposites increased to 70.4 MPa, the glass transition temperature increased to 76.96°C, compare to that of 64.46 MPa and 69.28°C for 0.3 wt% epoxy/graphene samples and 57.23 MPa and 66.08°C for neat epoxy samples. Nanocomposites prepared with GA also showed moderate increment. The results show that with the incorporation of graphene, the properties of epoxy improved significantly.Moreover, SDS and GA further increased the properties of nanocomposites. SDS shows better dispersion effect than GA.
Keywords: Epoxy; Graphene; Dispersion; Sodium Dodecyl Sulphate (SDS); Gum Arabic (GA)
Keywords |
|
| Epoxy; Graphene; Dispersion; Sodium dodecyl sulphate (SDS); Gum Arabic (GA) | |
Introduction |
|
| Graphene is a single layered carbon sheet, with high thermal conductivity, superior mechanical strength, and excellent electronic conductivity [1-3]. Graphene shows good potential for the fabrication of high performance polymer nanocomposites because it combines the advantages of both the layered-structure and graphitized-structure [4-6]. Figure 1 shows graphene as the building block of all graphitic carbon allotropes with different dimensionalities [7]. For composites, graphene has a two-dimensional (2D) planar structure and large specific surface area, which allows a bulky surface contact area with polymer and thus lead to improvement of the composites properties [8]. Heretofore, many efforts have been dedicated to employ graphene as reinforcement in polymers [9,10]. | |
| Figure 1: Graphene, the building block of all graphitic forms [7]. | |
| Epoxy resins are an important class of thermoset polymer with excellent mechanical properties, thermal stability, tribological behavior, chemical resistances and other properties [11-13]. It exhibits a wide application due to excellent chemical and corrosion resistance, outstanding adhesion properties, low shrinkage, and low price [14,15]. Hence, epoxy-based resins are extensively applied in the felids of cryogenic fuel tanks, space shuttle, adhesives, coatings, electrical automobiles, and in the aerospace industry for marine, armor, and insulating materials [16-19]. | |
| Recently, the use of graphene to modify epoxy has attracted great interest from both industrial and academic research studies [20-23]. It was found that graphene can significantly improve the properties of epoxy at extremely low loading [24], obtaining a good dispersion state of graphene in the matrix is the key in the preparation of epoxy/ graphene nanocomposites [25,26]. A well dispersed state ensures availability of maximum surface area of filler, which will affect the neighboring polymer chains and, consequently, the properties of the whole nanocomposite [27]. However, in practical terms, graphene is not suitable to disperse in epoxy just by simple mixing. This is due to graphene’s pronounced tendency to re-aggregate in the matrix due to the strong van der Waals force between separately dispersed graphene sheets [28]. | |
| Therefore, surface functionalization of graphene has been widely adopted to resolve this problem [29]. For example, Yang et al. [30] covalently grafted 1-(3-aminopropyl)-3-methylimidazolium bromide onto the surface of graphene sheets. The modified graphene shows enhanced solubility in water, N,N-Dimethylformamide (DMF) and dimethyl sulfoxide (DMSO) at various concentrations and formed stable dispersions. However, covalent functionalization involves in complexities and multi-step processing [31,32], besides this, covalent functionalization could also causes serious damage to the graphene surface structure and thus weakens the physical barrier effect of graphene [33], therefore, non-covalent functionalization of graphene by using surfactants becomes a solution. Non-covalent functionalization helps in connecting the functional molecules without actually forming chemical bonds [34], only requires physical adsorption of the molecules on the graphene surface [35] and could be easily removed if needed [36], which involves both effectiveness and easy process ability. | |
| As the most commonly used amphiphilic water-soluble dispersants, Sodium Dodecyl Sulphate (SDS) and Gum Arabic (GA) show good potential to de-bundle nanofillers from their aggregates. For example, SDS has already been reported to disperse montmorillonite [37], zinc oxide [38] and iron oxide [39] in polymeric matrix, and the final composites show enhanced properties. For SDS, the negatively charged sulphate groups coat on the nanofiller and provide electrostatic repulsion, and thus prevent aggregation [40,41]. For GA, the long polymer chains of GA physically adsorbed by graphene and disperse them by steric repulsion [42]. In this work, graphene has been used as filler to improve the properties of epoxy, nanocomposites have been prepared, SDS and GA have been selected to improve the dispersion. Mechanical properties, glass transition temperature (Tg) and fractured surface morphology have been measured to test the dispersion effect and compare the effect of SDS and GA on the properties of epoxy/graphene nanocomposites. | |
Experimental |
|
| Materials | |
| The epoxy matrix used in this study consists of EPOPHEN EL5 bisphenol A based liquid epoxy and EPOPHENEHA 57 diamine hardener, purchased from Polyfibre UK Ltd. To prepare solid epoxy, the mix proportions are 50 parts by weight of hardener to 100 parts by weight of liquid epoxy. This epoxy system is a multipurpose resin offering good all-round properties. Graphene nanoplatelets were purchased from Graphene Laboratories Inc., USA with an average lateral size of 4.5 μm and thickness of 12 nm. SDS and GA were purchased from Sigma-Aldrich with analytical grades. | |
| Sample preparation | |
| According to our previous research [43], 0.3 wt% epoxy/graphene nanocomposites show the best mechanical properties, therefore, 0.3 wt% nanocomposites were prepared for the test. One set of sample was prepared with unmodified graphene, marked as G-0.3. Two sets of samples were prepared by SDS-graphene and GA-graphene, respectively, marked as SDS samples and GA samples. All sets of nanocomposites constitute of 0.45 g graphene and 149.55 g epoxy resin. Another one set of sample was prepared with neat epoxy as reference. | |
| For samples prepared with unmodified graphene, graphene was first dispersed in liquid epoxy by bath sonication for 30 minutes at room temperature. Then the suspensions were mixed with hardener by the ratio of epoxy:hardener of 2:1. Following thorough hand mixing for 10 min, vacuum degassing was carried out to remove the entrapped air. The mixtures were then mould cast and cured at room temperature for 6 h followed by post-curing at 80°C for 6 h. | |
| For surfactants prepared nanocomposites, firstly, 0.225 g SDS and GA were dissolved in 100 ml de-ionized water respectively in a beaker. After the surfactants were fully dissolved, graphene was added into the solution. After 30 min sonication, the solutions were transferred into an oven and heated to 95°C overnight to fully remove the water. The subsequent products were marked as SDS-graphene and GAgraphene, respectively. Then the SDS-graphene and GA-graphene were used to prepare nanocomposites according to the same method of G-0.3 samples. | |
| Characterization | |
| Tensile, three-point bend, and fracture toughness tests were conducted by Universal Testing Machine (Instron 3382); the crosshead speed was kept at 2 mm/min for all tests. Tensile properties were measured according to ASTMD638 (Type V geometry) with specimen thickness 4 mm. Three-point bend test was conducted according to ASTM D790 with specimen dimensions of 3 × 12.7 × 48 mm. A single-edge-notch three-point bending (SEN-TPB) specimen was used to determine mode-I fracture toughness (K1C) according to ASTM D5045, the specimen dimensions were 3 × 6 × 36 mm with a crack of length 3 mm. The K1C was calculated using Equation (1), | |
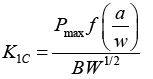 (1) (1) |
|
| Where, Pmax is the maximum load of load-displacement curve, f(a/w) is constant related to the sample geometry and was calculated using Equation (2), B is sample thickness (mm), W is sample width (mm), and a is crack length (kept between 0.45 W and 0.55 W). The critical strain energy release rate (G1C) was calculated using Equation (3) where E is the Young’s modulus obtained from the tensile tests (MPa), and v is the Poisson’s ratio of the polymer, taken to be 0.35. | |
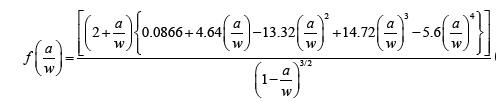 (2) (2) |
|
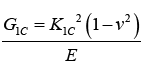 (3) (3) |
|
| Vickers micro hardness was tested by Buehler Micromet II, a load of 200 g was applied for 10 seconds on each sample. Six specimens were tested for all sets of samples. | |
| Dynamic Mechanical Analyzer (DMA) (Model 8000, Perkin Elmer) was used to determine the storage modulus (E’) and loss factor tan δ. Rectangular specimens with dimensions of 2.5 × 8 × 30 mm were tested in single cantilever mode. All tests were carried out by temperature sweep method (temperature ramp from 30 to 150°C at 5°C/min) at a constant frequency of 1 Hz. The glass transition temperature (Tg) was taken as the temperature value at the peak of tan δ curves. Scanning electron microscopy (SEM) analysis was carried out by a FEI Quanta 200 electron microscope on the fractured surface of nanocomposites to evaluate the fracture modes in the samples. A layer of gold with 10 nm thickness was applied on the fractured surface using Emscope sputter coater model SC500A. | |
Results and Discussion |
|
| Tensile properties of nanocomposites | |
| Tensile test is a fundamental test in which a sample is subjected to a controlled tension until failure. The tensile properties of nanocomposites are shown in Figure 2. | |
| Figure 2: Tensile properties of nanocomposites: (A) Tensile strength; (B) Tensile modulus. | |
| As can be seen from Figure 2A, Epoxy shows the tensile strength of 57.23 MPa. After introduced graphene, G-0.3 samples show the tensile strength of 64.64 MPa. Samples prepared with SDS and GA show higher values in the tensile strength. GA samples show medium increase in the tensile strength, which is 67.2 MPa. The maximum increase in the tensile strength is shown by SDS samples, which is 70.40 MPa. The tensile modulus of the nanocomposites is shown in Figure 2B. Epoxy shows the lowest tensile modulus with 0.87 GPa, G-0.3 samples show the tensile modulus of 1.17 GPa. Medium increase in the tensile modulus is observed for GA samples with 1.21 GPa, SDS samples show highest tensile modulus, which is 1.29 GPa. | |
| The results show that after introduced graphene, the 0.3 wt% epoxy/graphene nanocomposites show higher properties than neat epoxy. However, after introduced surfactants, the tensile properties of nanocomposites further increased. This increase is due to the fact that surfactants improved the dispersion of graphene. Uniformly dispersed graphene could shorten the distance among cross linking points, and thus increases the cross linking density of the resultant network, and consequently enhance the mechanical properties of nanomaterial. | |
| In general, 0.3 wt% epoxy/graphene nanocomposites show higher tensile properties than neat epoxy. After introduced surfactants, the properties further increased. However, SDS samples show even higher tensile properties than GA samples, which means SDS has a better dispersion effect than GA. | |
| Flexural properties of nanocomposites | |
| The three-point bending flexural test is most frequently employed, in which a specimen having a rectangular cross-section is bent until fracture. This test provides values for the flexural strength and flexural modulus. | |
| As compare to tensile properties, similar trend has been observed in flexural properties for nanocomposites. From Figure 3A, it can be seen that G-0.3 samples show higher flexural properties than neat epoxy. The flexural strength and flexural modulus increase with the usage of SDS and GA. Epoxy shows the flexural strength of 88.32 MPa, G-0.3 shows the flexural strength of 97.1 MPa. The maximum increment in flexural strength is obtained in case of SDS samples with the value of 110.89 MPa. The flexural strength for GA samples also show increase because of the improved dispersion of graphene in the epoxy matrix, with the value of 102.53 MPa. The flexural modulus of nanocomposites is shown in Figure 3B. Epoxy shows the flexural modulus of 1.72 GPa. Samples prepared with 0.3 wt% graphene show the flexural modulus of 2.08 GPa. After introduced SDS, the maximum flexural modulus is obtained at 2.24 GPa. GA samples also show increased flexural modulus with the value of 2.14 GPa. | |
| Figure 3: Flexural properties of nanocomposites: (A) Flexural strength; (B) Flexural modulus. | |
| In general, graphene could significantly improve the flexural properties of epoxy matrix. After introduced surfactants, the flexural properties have been further improved. This improved flexural strength and flexural modulus are the results of the improved dispersion of graphene in epoxy. For these two surfactants, it is clear that SDS disperse graphene more efficiently as compared to GA. | |
| Fractural properties of nanocomposites | |
| Fracture toughness is a property which describes the ability of a material's resistance to brittle fracture when a crack is present, the strain energy release rate is the energy dissipated during fracture per unit of newly created fracture surface area. Different toughening mechanisms are reported for epoxy/graphene nanocomposites: crack deflection at the epoxy/graphene matrix interface; crack bridging by graphene and graphene shear band collapse. However, no matter which mechanism it is, for optimizing the effectiveness of these mechanisms, it is necessary to homogenously distribute the graphene in the polymeric matrix. | |
| The variation in K1C is shown in Figure 4A. Epoxy shows the K1C value of 0.688 MPa∙m1/2 while G-0.3 samples show the K1C of 0.832 MPa∙m1/2. The maximum K1C increases to 0.88 MPa∙m1/2 with the usage SDS. GA samples show the K1C of 0.862 MPa∙m1/2. The variation of G1C is shown in Figure 4B; the lowest value is observed in epoxy samples, which is 0.172 KJ∙m-1, G-0.3 samples show the value of 0.208 KJ∙m-1 GPa. In case of SDS samples it can be seen that the G1C increases to 0.22 KJ∙m-1 GPa, shows the maximum improvement. GA samples show relative lower G1C value of 0.215 GPa compare to that of SDS samples. | |
| Figure 4: Fracture properties of nanocomposites: (A) Fracture toughness (K1C); (B) Critical strain energy release rate (G1C). | |
| In general, graphene improves the fracture toughness and critical strain release rate of epoxy. As compare to nanocomposites prepared by unmodified graphene, nanocomposites prepared by surfactants show increased fracture properties. This is due to the enhanced dispersion of graphene in epoxy matrix. The uniformly dispersed graphene improves the energy absorbing capacity, as a result improves the fracture toughness of nanocomposites. | |
| Hardness of nanocomposites | |
| Surface hardness is investigated as this is a very important factor that related to the wear and abrasion resistance of materials. Hardness measures how resistant a material is to the shape change when applying compressive loads. To achieve a high hardness for epoxy/graphene nanocomposites, a good dispersion of graphene is a prerequisite, therefore, the Vickers hardness were measured to evaluate the effectiveness of the graphene and surfactants on the properties of epoxy matrix. | |
| As it can be seen from Figure 5, Epoxy shows the hardness of 0.216 GPa. Samples prepared with unmodified graphene show the hardness of 0.235 GPa. A higher surface hardnesscan be observed when SDS was used for dispersing graphene, which is 0.247 GPa. Such an improved hardness indicates better homogenization and debundling of graphene in epoxy. Samples prepared with GA have a surface hardness of 0.244 GPA compare to the equivalent composite prepared with SDS. | |
| Figure 5: Hardness of nanocomposites. | |
| Good dispersion of graphene in the epoxy matrix attributed to the increment in hardness. As described above, homogeneously distributed graphene could shorten the distance among cross linking points, thus increase the crosslinking density of the matrix, and plays a positive role to improve the mechanical properties. | |
| DMA results of nanocomposites | |
| Figure 6A shows the storage modulus (E’) of epoxy/graphene nanocomposites. As shown in the figure, epoxy shows the lowest storage modulus with the value of 1.92 GPa. The storage modulusfor G-0.3 samples have a 22.4% increment, which is 2.35 GPa. Furthermore, the storage modulus shows even higher increment by using SDS and GA, which shows a value of 2.71 GPa and 2.44 GPa, respectively. | |
| Figure 6: DMA results of nanocomposites: (A) Storage Modulus; (B) Tan δ. | |
| Glass transition temperature (Tg) characterizes the segmental motion of the polymer and is taken as the temperature value at the peak of tan δ curves and is shown in Figure 6B. In the figure it is shown that tan δ peak is observed at 66.08°C for neat epoxy and 69.28°C for samples prepared with unmodified graphene. For samples prepared with SDS and GA, Tg shifts to higher temperature. This is attributed to the fact that homogeneously distributed graphene could restrict the molecular mobility of the epoxy matrix, thus lead to higher Tg values. Among all these samples, SDS samples show maximum increment in the Tg with the value of 76.96°C, which is about 11°C higher than neat epoxy and 7°C higher than that of G-0.3 samples. The Tg value for GA samples is 72.19°C, which is a 6°C increment compare to that of neat epoxy. The reason for this increment is attributed to the effect of graphene on the cross-linking structure of the nanocomposites. In general, the cross linking density shows the density of cross-linked bonds per volume. As for typical polymer nanocomposites, the higher cross linking density is, the stronger the polymer chains bond each other, thus lead to a higher Tg. | |
| SEM images of nanocomposites | |
| The fracture surfaces of nanocomposites were studies by SEM and are shown in Figure 7. As can be seen from Figure 7A, sharp river-like fractured patterns can be observed on the epoxy surface, which shows the brittle nature of the material. | |
| Figure 7: SEM images of fracture surfaces of (A) Epoxy; (B) G-0.3; (C) SDS samples and (D) GA samples. | |
| For G-0.3 samples, as shown in Figure 7B, rough surface with large cracks can be seen, which shows poor resistance to crack initiation and propagation. These cracks were caused by the nonuniform dispersion of graphene. In general, the graphene aggregates form defects in the matrix, those defects act as stress concentration center and causes localized weakness, therefore lead to large cracks and cause decrease in the properties. As for improved dispersion, SDS and GA samples show uniform surface, as can be seen in Figures 7C and 7D. These uniform and relative smoother surfaces testified sheet/sheet delamination as the fracture mechanism for the nanocomposites and revealed that the usage of surfactants is able to generate better dispersion of graphene. The uniformly dispersed graphene in the matrix can form a continuous network, which could support the network of the matrix and release stress concentration. Besides that, uniformly dispersed graphene could bridge growing cracks, thus stabilizing and stopping it from developing into larger and harmful crack, thus enhance the properties of nanomaterials. | |
Conclusion |
|
| In this work, epoxy/graphene nanocomposites have been prepared; SDS and GA have been selected as dispersant to disperse graphene in epoxy matrix. Those two surfactants are able to de-bundle graphene from their aggregates by electrostatic and steric repulsions, such repulsions are responsible for improved dispersion of graphene in epoxy matrix. Mechanical properties, glass transition temperature and fractured surface morphology have been tested to evaluate their dispersing effectiveness. | |
| Neat epoxy shows the lowest mechanical properties, storage modulus and Tg. After introduced graphene, the properties of nanocomposites show significant improvement as compare to neat epoxy. However, nanocomposites prepared with simple graphene have large cracks on the fractured surface, which means non-uniformity in the samples. After introduced surfactants, it is clear that SDS and GA are able to produce fine and homogenous graphene dispersions. The mechanical properties and glass transition temperature of SDS and GA samples are also higher than that of simple graphene samples. The results show that both SDS and GA are able to disperse graphene more evenly. However, it should be noted that nanocomposites prepared with SDS possess higher mechanical properties and Tg as compare to GA samples, hence it is concluded that SDS is a better dispersing agent than GA for graphene in epoxy matrix. | |
| This work has developed a new, environmental friendly and industrial scalable method to produce epoxy/graphene nanocomposites. This research gives guidelines in the usage of SDS and GA in the preparation of epoxy/graphene nanocomposites, and could also be referred for other polymer composites where using of surfactants is applicable. | |
Acknowledgment |
|
| The authors would like to thank the Department of Mechanical and Construction Engineering, Northumbria University for the provision of research facilities, without which the collection and analysis of relevant data was not possible. | |
References |
|
|
|
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi