Research Article, J Plant Physiol Pathol Vol: 7 Issue: 1
Promotive Effect of Ascorbic Acid, Gallic Acid, Selenium and Nano-Selenium on Seed Germination, Seedling Growth and Some Hydrolytic Enzymes Activity of Cowpea (Vigna unguiculata) Seedling
Ibrahim Mohamed Zeid, Fatma Abd El Lateef Gharib, Safia Mohamed Ghazi and Eman Zakaria Ahmed*
Department of Botany and Microbiology, Faculty of Science, Helwan University, Cairo, Egypt
*Corresponding Author : Eman Zakaria Ahmed
Department of Botany and Microbiology, Faculty of Science, Helwan University, Cairo, Egypt
Tel: +20 1145103804
E-mail: em_7891@yahoo.com
Received: March 01, 2018 Accepted: March 13, 2019 Published: March 22, 2019
Citation: Zeid IM, Gharib FAEL, Ghazi SM, Ahmed EZ (2019) Promotive Effect of Ascorbic Acid, Gallic Acid, Selenium and Nano-Selenium on Seed Germination, Seedling Growth and Some Hydrolytic Enzymes Activity of Cowpea (Vigna unguiculata) Seedling. J Plant Physiol Pathol 7:1. doi: 10.4172/2329-955X.1000193
Abstract
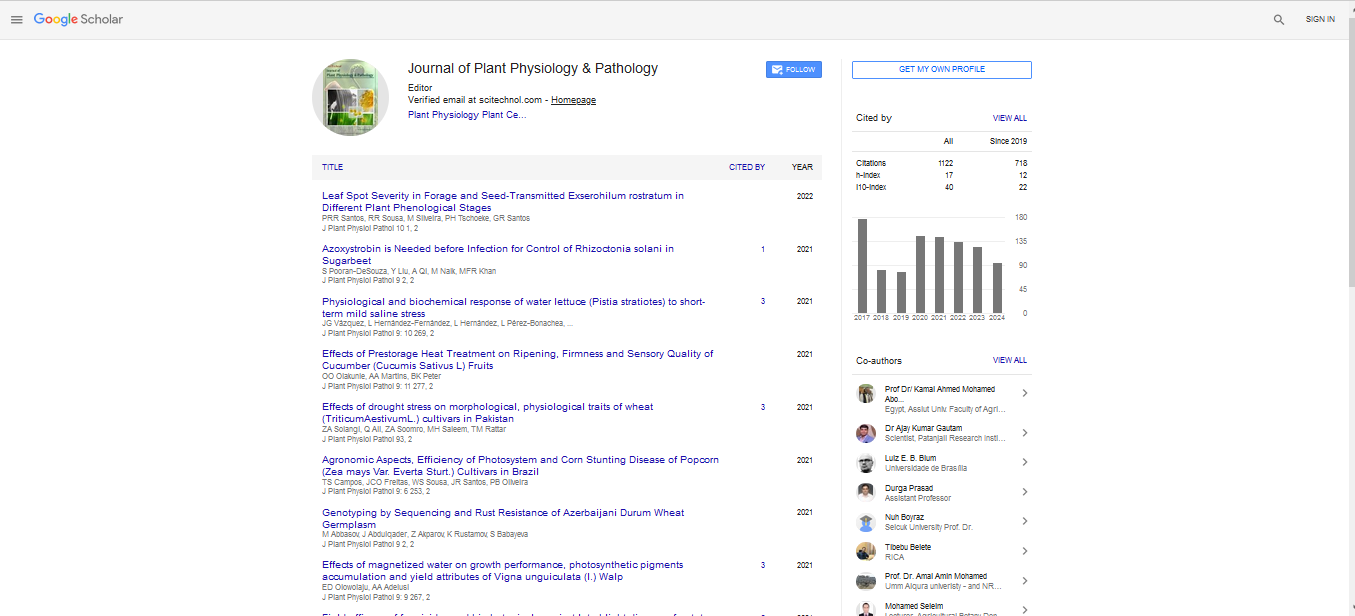
This study aimed to improve cowpea seed germination and seedling growth by using some antioxidant compounds naturally produced or up taken by plants. The effect of soaking cowpea (Vigna unguiculata) for 2 h in aerated solution of different antioxidants; ascorbic acid (AsA) and gallic acid (GA), each at 0.0, 50, 100, 150, 200, 250 and 300 ppm, sodium selenate (NaSeO4) and nanoselenium (SeNPs ≈ 33.4 nm) chemically prepared, each at 0.0, 6.25, 12.5, 25, 50 and 100 μM on seedling length and activities of some hydrolytic enzymes were tested. Seeds of treated and untreated cowpea were germinated at 25°C ± 0.5 under dark controlled conditions for 4 days. Results showed that AsA, GA, NaSeO4 and SeNPs at low concentrations significantly improved plumule and radicale lengths compared with their corresponding controls. Moreover, AsA and GA up to 150 ppm, NaSeO4 and SeNPs up to 25 μM significantly increased the activities of α-amylase, β-amylase and protease enzymes as well as the contents of total soluble sugars and total soluble proteins. The results indicate the successful use of ascorbic acid and gallic acid up to 150 ppm as well as sodium selenate and nanoselenium up to 25 μM in enhancing seedling growth and hydrolytic enzymatic activity in cowpea germinated seeds.Further promising results on the effect of these compounds on vegetative growth and yield can be concluded from the previous results on germination and seedling growth.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi