Research Article, J Plant Physiol Pathol Vol: 9 Issue: 8
Red Kidney Bean (Phaseolus vulgaris L.) Germination and Seedling Growth as affected by Selenium, Nano- Selenium and Sulfur
Nada Abou El-hamd and Eman Zakaria Ahmed*
Department of Botany and Microbiology, Faculty of Science, Helwan University, Cairo, Egypt
- *Corresponding Author:
- Eman Zakaria Ahmed
Department of Botany and Microbiology, Faculty of Science, Helwan University, Cairo, Egypt
Tel: + 201145103804
E-mail: em-_7891@yahoo.com
Received Date: July 31, 2021; Accepted Date: August 19, 2021; Published Date: August 26, 2021
Citation: El-hamd NA, Ahmed EZ (2021) Red Kidney Bean (Phaseolus vulgaris L.) Germination and Seedling Growth as affected by Selenium, Nano- Selenium and Sulfur. J Plant Physiol Pathol 9:8. 261.
Copyright: © All articles published in Dental Health: Current Research are the property of SciTechnol, and is protected by copyright laws. “Copyright © 2021, SciTechnol, All Rights Reserved.
Abstract
Selenium and sulfur similarity in chemical properties and transport pathways through plant pretend that they may share or compete in some metabolic activities during seed germination and plant growth. This work aimed to study the effect of soaking red kidney bean seeds (Phaseolus vulgaris L.) for 2h in aerated solution of Gum Arabic-Coated Selenium Nanoparticles (GA-SeNPs ≈ 48.22 nm), Sodium Selenate (Na2SeO4) and Sodium Sulphate (Na2SO4), each at 0.0, 0.5, 1, 5, 10, 25, 50 μM concentrations, on germination and seedling growth. The control and the treated seeds were germinated at 25°C ± 0.5 under dark controlled conditions for 4 days. GASeNPs, Na2SeO4 and Na2SO4 significantly improved germination percentage and seedling growth criteria of red kidney bean up to 10 μM, as compared with the controls. Red kidney bean responded in more or less similar trend to the different concentrations of the treatment solutions, with the magnitude of improvement was always greater for GA-SeNPs than for Na2SeO4 and Na2SO4. Moreover, both Na2SeO4 and Na2SO4 significantly decreased germination percent and seedling growth at 50 μM than the controls.
The results indicated the successful use of GA-SeNPs up to 50 μM, Na2SO4 and Na2SeO4 up to 5 μM, for enhancing the germination potential and subsequent seedling growth of red kidney bean under study.
Keywords: Red kidney bean seeds (Phaseolus vulgaris L.); Selenium nanoparticles; Sodium selenate; Sodium sulphate; Germination; Seedling growth
Introduction
Common beans are members of Fabaceae family, which are known for it’s economic and nutritional values. Red kidney bean (Phaseolus vulgaris L.) variety has low-fat, high protein contents and contains several bioactive compounds [1]. Red kidney bean is one of the most globally important legumes and considered as an essential component of human nutrition due to its high protein content (20%- 25%), complex sugars (50%-60%), vitamins especially vitamin E, minerals, poly‐unsaturated fatty acids and tangible amounts of folate and fibers (4%) [2-5]. It also contains a large number of phytochemical components including phenolic, flavonoids, vitexin and isovitexin [6]. It has the highest antioxidant activity compared with other types of legumes [7].
Selenium (Se) is essential for humans and animals, and its ingestion is associated with risk reduction of numerous diseases, such as cancer [8]. However, nearly 1 billion people in the world suffer from Se deficiency due to the consumption of foods with low Se concentration [9], especially as a result of the low and uneven availability of Se in most soils around world [10]. Considering the imbalance of Se contents in soils and those plants are primary sources for the entry of Se into the food chain, agronomic bio-fortification through the use of concentrated fertilizers is one of the primary alternatives to increase the bioavailability of Se in food [11]. The application of low concentrations of Se has been reported to have beneficial effects on crops. For example, Se application increased antioxidant activity and consequently increased yields [12, 13].
Recently, nanotechnology has been extensively employed in the field of plant sciences to explore its potential impacts in improving crop yields, selenium nanoparticles (SeNPs), have aroused worldwide attention due to their distinguished properties and excellent biological activities [14]. It is able to scavenge free radicals in vitro [15] and stimulate organogenesis [16]. Nano-Se has a higher efficiency in up regulating selenoenzymes and exhibits less toxicity compared with other Se compounds such as selenite [17], selenomethionine [18] and Se-methyl selenocysteine [19]. A new approach to fertilization of plants is the use of selenium nanomaterials [20].
Similarity between selenium and sulfur was proved in chemistry so, it is important to study the stimulatory or inhibitory action of both elements related to plant minerals nutrition and growth. Se and sulfur (S) are both group-16 “chalcogens” in the periodic table, meaning they have similar ionic radii, covalent radii, and chemical properties [21]. Indeed, selenate enters plant roots using sulphate transporters [22]. Considering S chemical similarity with Se, S forms tend to compete in such processes as absorption, translocation, and assimilation. For example, when Se is present as selenate, it enters the same pathway as sulphate, replacing it in the synthesis of such proteins as cystine and methionine [23, 22].
Therefore, the S compounds present in the rhizosphere may inhibit Se uptake by plants [24-26], thus influencing levels of these elements in plant tissues. Sulfur is also well known as an important macroelement, it plays a key role in the nutrition and production of agricultural crops. Sulphate taken up by the roots is the primary sulfur source for growth, but additionally plants are able to utilize absorbed sulfur gases by the shoot. Sulfur is a great indication for the structure of proteins and functioning of enzymes, and it plays an important role in the defense of plants against stresses and pests. Sulfur metabolites such as glutathione provide protection for plants against oxidative stress, heavy metals and xenobiotic. Humans and animals rely on plants for their reduced sulfur and plant sulfur nutrition has a decisive effect on food quality, e. g., availability of methionine [27].
The impact of selenium and sulfur on higher plants appears to depend on the species, age of plant, concentration and the experimental conditions. Se is a beneficial element for plants at low concentrations, but at high concentrations it is toxic. At low concentrations, Se show a promotive effect on germination percentage and physiological quality of seeds in several crops, such as barley, white mustard, oilseed rape [28], rice [29] and increases the antioxidant properties of higher plants, which reflect the reduction of reactive oxygen species [30]. In addition, Low concentration of Nano-Se and Se, improved tomato growth parameters and chlorophyll content under high and low temperature stress [31] and enhanced seedling growth and hydrolytic enzymatic activity in germinated cowpea seeds up to 25 μM [32]. On the contrary, high concentrations of Se reduced seed germination in bitter melon [33], Arabidopsis thaliana plants [34], inhibit germination and may lead to the death of the embryo in rice by inactivating hydrolytic carbohydrate enzymes [29]. Exposure to high Se (800 mg L-1); decreased seed germination and seedling growth, which reflect the increase of total sugars and sucrose concentration in both shoot and root of cowpea (Vigna unguiculata) [35]. Sulfur plays a role in building of proteins and chlorophyll [36,37], activation of Reactive Oxygen Species (ROS) scavenging enzymes and improves antioxidant defense under abiotic stresses [38]. Indirectly, S interacts with auxins, gibberellins, cytokinin, ethylene and salicylic acid, to counteract abiotic stresses [39]. On the other hand, its deficiency regulates the chlorophyll content of leaves, N content and photosynthetic enzymes [40] and suppresses cell sap osmotic pressure [41]. However, the mechanisms that mediate the effects of selenium and sulphur in plants remain unknown. Therefore, it is important to increase our knowledge about these mechanisms before implementing large-scale agricultural utilization of selenium and sulfur.
This work, aimed to study the effect of soaking red kidney bean (Phaseolus vulgaris L.) in different concentrations of Gum Arabic- Coated Selenium Nanoparticles (GA-SeNPs), Sodium selenate (Na2SeO4) and Sodium sulphate (Na2SO4) on the percent of seed germination and seedlings growth criteria in seedling.
Materials and Methods
Synthesis of selenium nanoparticles
Gum Arabic (GA) Coated Selenium Nanoparticles (GA-SeNPs ≈ 48.22 nm) was synthesized and various characterization techniques (UV-vis spectroscopy, Dynamic Light Scattering (DLS), Transmission Electron Microscopy (TEM) and Fourier Transform Infrared (FT-IR) spectroscopy) confirmed the formation of phytochemicals-capped SeNPs (under publication).
Plant materials and treatments
A pure lot of red kidney bean (Phaseolus vulgaris L.) seeds were provided by Sakha Horticulture Research Station, Horticulture Research Institute, Agricultural Research Center, Giza, Egypt.
Uniform seeds of red kidney bean were sterilized with 2.5% sodium hypochlorite solution for three minutes, and thoroughly rinsed with distilled water until complete removal of hypochlorite. Afterwards, seeds were equally divided into three batches and each batch was subdivided into groups (100 seeds each) to be soaked in the different working solutions. Soaking of seeds was carried out by putting a constant number of seeds of each batch for 2 h in glass containers, each containing a constant amount (100 ml) of the treatment solutions (Gum Arabic-Coated Selenium Nanoparticles (GA-SeNPs), sodium selenate and sodium sulphate) each at concentrations of (0.5, 1, 5, 10, 25, 50 μM). In addition, distilled water was used as a control. Soaking was carried out in a controlled cabinet (germinator). Afterwards, the seeds of the control and each treatment were washed thoroughly with distilled water, and then allowed to germinate in sterilized petri-dishes containing filter paper wetted with about 5 ml of distilled water (6 replicates, each contains 10 seeds) for 4 days. Germination was carried out in a controlled cabinet (incubator) at 25C° ± 0.5 under dark conditions.
Growth measurement
The germination percentage (%) was calculated at the 4th day, according to the following:

For measurements of different growth criteria (plumule and radicle length (cm), fresh and dry weights (g) per seedling), at least 12 randomly choice 4-day-old seedlings were taken from each treatment and the control. The collected samples were dried in an oven at 75°C until constant dry weight was obtained.
Statistical analysis
The data was expressed as mean of six replicates; each replicate consist of two seedlings. Statistical Analysis of the data was carried out using one-way Analysis of Variance (ANOVA) using least significant difference (LSD at 5% level) followed by Duncan’s Multiple Comparison Test.
Results
Germination percentage
Data presented in Table 1, show the germination percentage of the control (H2O) and the differently treated red kidney bean (Phaseolus vulgaris L.).
The germination percentage was generally significantly enhanced, as compared to control, in response to soaking for 2 hours in different concentrations of GA-SeNPs, Na2SeO4 and Na2SO4 at low concentrations (up to 10 μM), and then progressively decreased at higher concentrations (but still higher than control at 25 μM GASeNPs).
The best results were obtained in response to soaking seeds in solutions of GA-SeNPs at 1 μM and Na2SO4 at 5 μM, where, germination percent was improved by 21.36 and 17.99%, respectively relative to control.
However, at high concentration (50 μM) GA-SeNPs, Na2SeO4 and Na2SO4 negatively affected the germinative power in the order of 8.98, 15.05 and 21.12%, respectively in comparison to the control treatment (Table 1).
Growth criteria of seedling
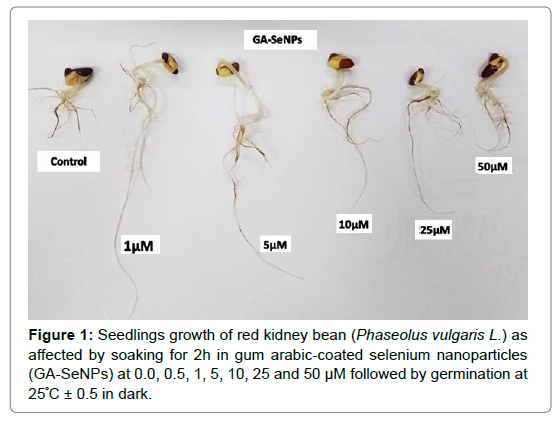
Table 1 shows the main growth criteria of 4-day-old seedlings resulting from pre-sowing seed soaking for 2 hours in different concentrations of GA-SeNPs, Na2SeO4 and Na2SO4. In case of the three applied treatments (nano-selenium, sodium selenate and sodium sulphate), the growth criteria (plumule and radicle lengths, fresh and dry weights) of seedlings were mostly significantly increased, relative to corresponding controls, from 0.5 to 10 μM concentrations. In this respect, best performance was induced by 1 and 5 μM GA-SeNPs, followed by 5 μM of Na2SO4 and Na2SeO4, where nano-Se at 1 μM induced the highest significant increase in plumule length (71.43%), radicle length (154.42%), fresh weight (34.11%) and dry weights (23.08%) of seedling in comparison to the control. Furthermore, nano-Se was more efficient than sodium sulphate and sodium selenate at comparable concentrations in increasing growth criteria of red kidney bean (Table 1 and Figure 1).
| Treatment µM | Germination percent | Plumule length | Radicle length | Fresh weight | Dry weight |
|---|---|---|---|---|---|
| % | Cm | Cm | g | g | |
| Control | 82.4h | 3.50ef | 2.83h | 1.29bcd | 0.39ab |
| Na2SeO4 0.5 | 85.0g | 3.63ef | 2.90h | 1.34bcd | 0.41ab |
| Na2SeO4 1 | 90.0f | 3.90de | 3.20g | 1.36bcd | 0.46a |
| Na2SeO4 5 | 90.0f | 5.23b | 4.60e | 1.48b | 0.47ab |
| Na2SeO4 10 | 90.0f | 4.63c | 4.43e | 1.38bc | 0.41ab |
| Na2SeO4 25 | 82.4h | 3.40ef | 2.57i | 1.29bcd | 0.37ab |
| Na2SeO4 50 | 70.0j | 3.00fg | 2.43i | 1.19cde | 0.35ab |
| GA-SeNPs 0.5 | 92.00d | 4.50c | 5.10d | 1.48b | 0.41ab |
| GA-SeNPs 1 | 100.00a | 6.00a | 7.20a | 1.73a | 0.48a |
| GA-SeNPs 5 | 95.00c | 6.00a | 5.80b | 1.71a | 0.46ab |
| GA-SeNPs 10 | 90.56de | 5.00bc | 5.60c | 1.42b | 0.41ab |
| GA-SeNPs 25 | 85.00g | 3.60ef | 4.50e | 1.37bcd | 0.40ab |
| GA-SeNPs 50 | 75.00i | 3.00fg | 3.70f | 1.29bcd | 0.37b |
| Na2SO4 0.5 | 90.0f | 3.67e | 3.20g | 1.42b | 0.43ab |
| Na2SO4 1 | 95.0c | 4.40cd | 3.37g | 1.45b | 0.46ab |
| Na2SO4 5 | 97.2b | 5.40b | 5.10d | 1.74a | 0.47a |
| Na2SO4 10 | 90.6de | 3.60ef | 2.87h | 1.32bcd | 0.40ab |
| Na2SO4 25 | 75.0i | 2.80g | 2.23j | 1.18e | 0.36ab |
| Na2SO4 50 | 65.0k | 2.77g | 1.67k | 1.08e | 0.34ab |
| LSD at 0.05 | 2.0 | 0.60 | 0.17 | 0.17 | 0.19 |
On the other hand, the growth criteria of red kidney bean seedling started to decrease at 25 μM of either Na2SeO4 or Na2SO4, while a moderate decrease was observed in plumule length (14.29 and 20.86%), radicle length (14.13 and 40.99%), fresh weight (7.75 and 16.28%) and dry weights (10.26 and 12.82%) of seedling in comparison to the control at 50 μM concentration of Se and S, respectively (Table 1).
Discussion
In the present study, the germination percentage and the main growth criteria (plumule and radicle lengths and fresh and dry weights) of 4-day-old red kidney bean seedlings resulting from soaking for 2 hours in different concentrations of gum arabic-coated selenium nanoparticles (GA-SeNPs), sodium selenate and sodium sulphate were mostly significantly increased, relative to controls, from 0.5 to up to 10 μM concentrations. Gum arabic-coated selenium nanoparticles stimulatory effects were more efficient than sodium sulphate and Na2SeO4 at comparable concentrations. The best performance was induced by GA-SeNPs at 1 and 5 μM, followed by 5 μM of Na2SO4 and Na2SeO4, where nano-Se at 1 μM induced the highest significant increase in plumule length (71.43%), radicle length (154.42%), fresh weight (34.11%) and dry weight (23.08%) of seedling relative to control (Figure 1 and Table 1). These conclusions were in alliance with those obtained with three common bean (Phaseolus vulgaris L.) cultivars, where rise in the concentration of AgNO3 or AgNPs from 20 to 80 ppm significantly improved germination percentage, germination rate and seedling growth criteria [42], SeNPs at low concentration (2-10 mg L-1) showed a positive effect on root growth and germination index of Dorema aucheri plant [43] and cowpea, in response to seed pre-soaking with Na2SeO4 and nano Se at 6.25-25 μM [32]. In accordance, Se at low concentrations act as an antioxidant and stimulated growth, whereas higher concentrations act as a pro-oxidant and reduced ryegrass yields [44]. Se has a role in mitochondrial membrane functions [45]; hence low concentration of Se improves respiration activity in young pea (Pisum sativum L.) [46]. The enhanced percentage of germination might be attributed to an increased permeability of the seed testa, thus facilitating the admission of water and di-oxygen into the cells, which would then accelerate germination and concomitant metabolic processes [47]. In addition, the enhanced seedling growth by seed soaking in Se, nano- Se and S, particularly at lower concentrations may be due to de novo synthesis of certain germination-promoting substances, promotion plant cell division, elevation effect of some hydrolytic enzymes (α-amylase, β-amylase and protease) that resulted in efficient utilization of seed reserves, stimulation of antioxidant activity, and increased abilities for absorbing and utilizing water as has been concluded by [48] in cauliflower, [49] in rice, [32] in cowpea and [29] who found that seeds priming with Se enhanced de novo synthesis of germination-promoting substances, membrane re-organization, activity of hydrolytic enzymes and reduced leakage of metabolites, through increased GSH-Px activity [12, 50], as Se is present in the GSHPx enzyme active site which participates in the reduction of toxic hydrogen peroxide and lipid peroxides [51]. Se at low levels (0.1- 0.75 ppm) can stimulate the shoots growth in 10-day-old mungbean seedlings by up-regulation carbohydrate metabolism enzymes, thus providing energy substrates for enhanced growth, where, the activity of starch hydrolysing enzymes (α-and β-amylases) and sucrose hydrolyzing enzyme (invertase) were stimulated significantly associated with elevation of activities of sucrose synthesising enzymes (sucrose synthase and sucrose phosphate synthase) [44]. Also, the maximum activities of α-amylase, β-amylase and protease enzymes in cowpea seedlings were obtained in response to soaking seeds in solutions of either Na2SeO4 or SeNPs at 6.25 μM [32]. Mahakham [52] proposed that different mechanisms underlying nanopriminginduced seed germination, including creation of nanopores for enhanced water uptake, stimulation the up-regulation of aquaporin genes, rebooting ROS/antioxidant systems in seeds, generation of hydroxyl radicals for cell wall loosening, and nanocatalyst for fastening starch hydrolysis compared to unprimed control and other priming treatments. With respect to sulfur, low concentrations of sodium sulphate and sodium chloride stimulated growth in Chenopodium rubrum L., but higher concentrations resulted in large decreases in dry weight and leaf area [53], foliar spray with 6 ppm of sulfur enhance the shoot and root biomass accumulation, physiological and biochemical attributes and alleviate the effect of heat stress in tomato plants [54]. Sulfur and its derivatives play vital roles in the activation of Reactive Oxygen Species (ROS) scavenging enzymes to improve antioxidant defense under abiotic stresses [38]. Indirectly, S interacts with auxins, gibberellins, cytokinin, ethylene and salicylic acid, to counteract abiotic stresses [39]. Plants need thiol-containing S biomolecules to develop a defensive mechanism against different abiotic stresses [55].
On the contrary, the percentage of germination and the growth criteria of 4-day-old red kidney bean seedlings started to decrease at 25 μM of either Na2SeO4 or Na2SO4, while a moderate decrease was observed in plumule length (14.29 and 20.86%), radicle length (14.13 and 40.99%), fresh weight (7.75 and 16.28%) and dry weight (10.26 and 12.82%) of seedling in comparison to the control at 50 μM concentration of Se and S, respectively (Table 1). In consistent, [34] noticed an inhibition in germination of Arapidopsis thaliana species sown in soil close to Se hyper accumulator species due to their apparent ability of concentrate Se. In cowpea, seed germination was inhibited at high concentrations of Se [29], plumule and radicle lengths was reduced in response to soaking in Se and nano-Se at 50-100 μM [32], root growth, (seedling length, the shoot and root fresh weight) started to decrease at 1 and 40 mg L-1 Se concentration, respectively, while a drastic decrease was observed in shoot (74 and 82%) and root (87 and 94%) growth in comparison to the control at 400 and 800 1 mg L-1 Se concentrations, respectively. The drastic decrease of photosynthetic pigments, increase of total sugars and sucrose levels in shoot and root are related to the lower seedling growth development in response to high Se exposure [35]. In Dorema aucheri plant, SeNPs at 30 mg L-1 decreased seed germination index by 20%, compared to the control [43], while application of ZnONPs (25, 50, and 100 mg L-1) had no obvious effects on the seed germination, however, it improved the early growth and related physio-biochemical attributes in rice [56].
In the present study, the observed decrease in red kidney bean seedling growth at an elevated concentration of either nano-Se, Se or S, may be attributed to elevated MDA levels and the activities of antioxidants in an attempt to cope with oxidative stress. Metabolic activities are expected to increase remarkably in kidney bean seeds following their soaking with nano-Se, Se or S that may lead to higher activity of ROS as secondary products of mitochondrial respiration. Additionally, [46] found higher respiratory activity associated with low doses of Se in young pea (Pisum sativum L.) plants. There is strong evidence that free radicals and peroxides are abundantly produced within seeds during germination [57] and are cooperatively tackled by enzymatic reactions [49]. The enhanced expression and activity of antioxidant enzymes has been proposed as part of seed strategy to cope with ROS produced during seed priming [29]. Se has been ascribed as a natural stimulant of antioxidant activity in plants [58]. Wang [59] found that low (non-toxic) Se concentrations upregulate proteins involved in ROS detoxification and resistance to pathogens. The expression of the same proteins was down-regulated by high (toxic) Se concentrations. In two fine rice cultivars (Super and Shaheen Basmati), the activity of α-amylase was significantly induced by priming with selenium (15-60 μmol L−1). Nevertheless, Se at 90 and 105 μmol L-1 had detrimental effect on α-amylase activity that was dropped even below than that of the control at 105 μmol L-1 [29]. Endogenous phytohormones that are known to balance cell proliferation are modulated by nanoparticles [60, 61]. At high concentrations, Selenium replaces sulfur in amino acids leading to malformed selenoproteins formed due to the misincorporation of selenocysteine/seleno-methionine in place of cysteine/methionine in protein chain which are nonfunctional proteins and enzymes and contributes to its toxicity [62]. Seleno-amino acids incorporated into protein may result in S-S bonds being replaced by the less stable Se-Se bonds, leading to changes in biological activity of the protein [63-65].
Conclusion
The present study indicates that soaking red kidney bean seeds in solutions of GA-SeNPs, Na2SeO4 and Na2SO4 at different concentrations (0.5-50 μM) improved germination percentage, and seedling growth criteria particularly at lower concentrations.
Generally, GA-SeNPs at 1 and 5 μM was the most effective treatments in enhancing germination and seedling growth parameters followed by Na2SO4 at 5 μM, then Na2SeO4 at the same concentration, compared to controls. A reverse situation was recorded at higher concentration 50 μM, where seedling growth criteria was the lowest in treatment with Na2SO4 followed by Na2SeO4, then GA-SeNPs. However, field studies are needed to determine the possible role of GA-SeNPs, Na2SeO4 and Na2SO4 in improving red kidney bean growth and yield under normal and different stresses conditions.
References
- Reynoso Camacho R, Ramos-Gomez M, Loarca-Pina G (2006) Bioactive components in common beans (Phaseolus vulgaris L.) Advances Agric Food Biotechnol 217-236.
- Reyes-Moreno C, Paredes-López O (1993) Hard-to-cook phenomenon in common beans-a review. Crit Rev Food Sci Nutr 33: 227-286
- Rehman Z AM, Salariya, Zafar SI (2001) Effect of processing on available carbohydrate content and starch digestibility of kidney beans (Phaseolus vulgaris L.). Food Chemistry 73: 351-355.
- Shi S, Xue J, Kakuda Y, Ilic S, Kim D (2007) Isolation and characterization of lectins from kidney beans (Phaseolus vulgaris). Process Biochemistry 42: 1436-1442.
- Hayat I, Ahmad A, Masud T, Ahmed A, Bashir S (2014) Nutritional and Health Perspectives of Beans (Phaseolus vulgaris L.). Food Sci Nutr 54: 580-592.
- Luo J, Cai W, Wu T, Xu B (2016) Phytochemical distribution in hull and cotyledon of adzuki bean (Vigna angularis L.) and mung bean (Vigna radiate L.) and their contribution to antioxidant, anti-inflammatory and anti-diabetic activities. Food Chemistry 201: 350-360.
- Kiyani-Sam M, Amjad L, Ranjbar M (2015) Comparative study of antioxidant activity, phenol and flavonoid content in Phaseolus vulgaris and Vigna sinensis in different stages of seed germination. Int J Biosci 6: 292-297.
- White PJ, Broadley MR (2009) Biofortification of crops with seven mineral elements often lacking in human diets iron, zinc, copper, calcium, magnesium, selenium and iodine, New Phytologist 182: 490-84.
- Rayman MP (2012) Selenium and human health. The Lancet, 379: 1256-1268.
- Kabata-Pendias A (2011) Trace elements in soils and plants. (4th edition), Taylor & Francis Group, Boca Raton London New York.
- Lyons G (2010) Selenium in cereals: Improving the efficiency of agronomic biofortification in the UK. Plant and Soil 332: 1-4.
- Djanaguiraman M, Devi DD, Shanker AK, Sheeba A, Bangarusamy U (2005) Selenium an antioxidative protectant in soybean during senescence. Plant and Soil 272: 77-86.
- Ramo SJ, Faquin V, Guilherme LRG, Castro EM, Ávila FW (2010) Selenium biofortification and antioxidant activity in lettuce plants fed with selenate and selenite. Plant, Soil and Environment 56: 584-588.
- Navarro-Alarcon M, Cabrera-Vique C (2008) Selenium in food and the human body: A review. Sci Total Environ 400: 115-141.
- Huang B, Zhang J, Hou J, Chen C (2003) Free radical scavenging efficiency of Nano-Se in vitro. Free Radical Biol Med 35: 805-813.
- Domokos-Szabolcsy E, Marton L, Sztrik A, Babka B, Prokisch J (2012) Accumulation of red elemental selenium nanoparticles and their biological effects in Nicotinia tabacum. Plant Growth Regul 68: 525-31.
- Zhang J, Wan H, Yan X, Zhang L (2005) Comparison of short-term toxicity between Nano-Se and selenite in mice. Life Sci 76: 1099-1109.
- Wang H, Zhang J, Yu H (2007) Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in mice. Biol Med 42: 1524-1533.
- Zhang J, Wang X, Xu T (2008) Elemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: comparison with Se-methylselenocysteine in mice. Toxicol Sci 101: 22-31.
- Bunglavan SJ, Garg AK, Dass RS, Shrivastava S (2014) Effect of supplementation of different levels of selenium as nanoparticles/sodium selenite on blood biochemical profile and humoral immunity in male Wistar rats. Veterinary World 7: 1075-1081.
- White PJ (2016) Selenium accumulation by plants. Ann Bot 227: 217-235.
- Gupta M, Gupta S (2017) An overview of selenium uptake, metabolism, and toxicity in plants. Frontiers in Plant Science 7: 1-14.
- Chang PT, Van Iersel MW, Randle WM, Sams CE (2008) Nutrient solution concentrations of Na2SeO4 affect the accumulation of sulfate and selenate in Brassica oleracea L. HortScience 43: 913-918.
- White PJ, Bowen HC, Parmaguru P, Fritz M, Spracklen WP (2004) Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. J Exp Bot 55: 1927-1937.
- Schiavon M, Pilon M, Malagoli M, Pilon-Smits EAH (2015) Exploring the importance of sulfate transporters and ATP sulphurylases for selenium hyperaccumulation-a comparison of Stanleya pinnata and Brassica juncea (Brassicaceae). Front Plant Sci 6: 1-13.
- Liu X, Yang Y, Deng X, Li M, Zhang W (2017) Effects of sulfur and sulfate on selenium uptake and quality of seeds in rapeseed (Brassica napus L.) treated with selenite and selenate. Environ Exp Bot 135: 13-20.
- Zhao FJ, Tausz M, De Kok LJ (2008) Role of Sulfur for Plant Production in Agricultural and Natural Ecosystems. in Sulfur Metabolism in Phototropic Organisms, Chapter 21 : 417-435.
- Molnárová M, Fargašová A (2009) Se (IV) phytotoxicity for monocotyledonae cereals (Hordeum vulgare L., Triticum aestivum L.) and dicotyledonae crops (Sinapis alba L., Brassica napus L.). Journal of Hazardous Materials 172: 854-861.
- Khaliq A, Aslam F, Matloob A, Hussain S, Geng M (2015) Seed Priming with Selenium: Consequences for Emergence, Seedling Growth, and Biochemical Attributes of Rice. Biol Trace Elem Res 166: 236-244.
- Silva VM, Boleta EHM, Lanza MGDB, Lavres J, Martins JT (2018) Physiological, biochemical, and ultrastructural characterization of selenium toxicity in cowpea plants. Environ Exp Bot 150: 172-182.
- Haghighi M, Abolghasemi R, Teixeira da Silva JA (2014) Low and high temperature stress affect the growth characteristics of tomato in hydroponic culture with Se and nano-Se amendment. Scientia Horticulturae 178: 231-240.
- Zeid IM, Gharib FA Ghazi SM, Ahmed SM (2019) Promotive effect of ascorbic acid, gallic acid, selenium and nano-selenium on seed germination, seedling growth and some hydrolytic enzymes activity of Cowpea (Vigna unguiculata) seedling. J Plant Physiol Pathol 7: 1.
- Hartikainen H, Xue T, Piironen V (2000) Selenium as an anti-oxidant and pro-oxidant in ryegrass. Plant and Soil 225: 193-200.
- El Mehdawi AF, Quinn CF, Pilon-Smits EAH (2011) Effects of selenium hyperaccumulation on plant-plant interactions: evidence for elemental allelopathy. New Phytologist 191: 120-131.
- Lapaz AM, Santos LFM, Yoshida CHP, Heinrichs R, Campos R (2019) Physiological and toxic effects of selenium on seed germination of cowpea seedlings. Bragantia 78: 1-11.
- Kertesz MA, Fellows E, Schmalenberger A (2007) Rhizobacteria and plant sulfur supply. Adv Appl Microbiol 62: 235-268
- Fox A, Kwapinski W, Griffiths BS, Schmalenberger A (2014) The role of sulfur and phosphorus mobilizing bacteria in biochar-induced growth promotion of Lolium perenne. FEMS Microbiol Ecol 90: 78-91
- Bashir H, Ibrahim MM, Bagheri R, Ahmad J, Arif IA, et al. (2015) Influence of sulfur and cadmium on antioxidants, phytochelatins and growth in Indian mustard. AoB Plants 7: 001.
- Mehar F, Masood A, Khan N (2013) Coordinate changes in assimilatory sulfate reduction are correlated to salt tolerance: Involvement of phytohormones. Annu Rev Res Biol 3: 267-295.
- Thomas SG, Bilsborrow PE, Hocking TJ, Bennett J (2000) Effect of sulphur deficiency on the growth and metabolism of suga rbeet (Beta vulgaris cv. Druid). J Sci Food Agric 80: 2057-2062.
- Kusaka M, Ohta M, Fujimura T (2005) Contribution of inorganic components to osmotic adjustment and leaf folding for drought tolerance in pearl millet. Physiol Plant 125: 474-489.
- Hegazi AZ, Gharib FA, Ghazi SM, Abd El Hafz AG, EL-Batal AI (2017) Bean (Phaseolus vulgaris L.) germination and seedling growth as affected by silver Nanoparticles. VIII International Scientific Agriculture Symposium, Jahorina 5-8, 205-213.
- Abedi E (2020) Effect of nanopriming for enhancing germination and seedling growth of Dorema aucheri L, Nano Mat 2020, 31st International Conference on Nanotechnology and Materials Engineering, Paris, France-12-13, 2020.
- Malik JA, Kumar S, Thakur P, Sharma S, Kaur R (2011) Promotion of growth in mungbean (Phaseolus aureus Roxb.) by selenium is associated with stimulation of carbohydrate metabolism. Biol Trace Elem Res 143: 530-539.
- Easwari K, Lalitha K (1994) Subcellular distribution of selenium during uptake and its influence on mitochondrial oxidations in germinating Vigna radiata L. Biol Trace Elem Res 48: 141-160.
- Smrkolj P, Germ M, Kreft I, Stibilj V (2006) Respiratory potential and Se compounds in pea (Pisum sativum L.) plants grown from Se-enriched seeds. J Exper Bot 57: 3595-3600.
- Zheng L, Hong F, Lu S, Liu C (2005) Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol Trace Element Res 104: 83-91.
- Fujikura Y, Karssen CM (1995) Molecular studies on osmo-primed seeds of cauliflower: A partial amino acid sequence of a vigor-related protein and osmopriming-enhanced expression of putative aspartic protease. Seed Sci Res 5: 177-181.
- Farooq M, Basra SMA, Wahid A, Khaliq A, Kobayashi N (2009) Rice seed invigoration: a review In: Lichtfouse E (ed) Organic Farming, Pest Control and Remediation of Soil Pollutants: sustainable agricultural reviews. Springer Science, Amsterdam, pp 137-175.
- Hawrylak-Nowak B, Matraszek R, Pogorzelec M (2015) The dual effects of two inorganic selenium forms on the growth, selected physiological parameters and macronutrients accumulation in cucumber plants. Acta Physiol Plant 37: 1-13.
- Frączek A, Pasternak K (2013) Selenium in medicine and treatment. Elem 18: 145-163.
- Mahakham W, Sarmah AK, Maensiri S, Theerakulpisut P (2017) Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Scientific reports 7: 1-21 pp. 8263.
- Warne P, Guy RD, Rollins L, Reid DM (1990) The effects of sodium sulphate and sodium chloride on growth, morphology, photosynthesis, and water use efficiency of Chenopodium rubrum. J Bot 68: 999-1006.
- Ali MM, Shafique MW, Gull S, Naveed WA, Javed T (2021) Alleviation of heat stress in tomato by exogenous application of Sulfur. Horticulturae 7: 21.
- Ihsan MZ, Daur I, Alghabari F, Alzamanan S, Rizwan S, et al. (2019) Heat stress and plant development: Role of sulphur metabolites and management strategies. Acta Agric Scand Sect. B-Soil Plant Sci69: 332-342.
- Li Y, Liang L, Li W, Ashraf U, Ma L (2021) ZnO nanoparticle-based seed priming modulates early growth and enhances physio-biochemical and metabolic profiles of fragrant rice against cadmium toxicity. Nanobiotechnol 19: 1-19.
- Bailly C, Benamar A, Corbineau F, Come D (2000) Antioxidant systems in sunflower (Helianthus annuus L.) seeds as affected by priming. Seed Sci Res 10: 35-42.
- Germ M, Stibilj V, Kreft I (2007) Metabolic importance of selenium for plants. Eur Plant Sci Biotechnol 1: 91-97.
- Wang YD, Wang X, Wong YS (2012) Proteomics analysis reveals multiple regulatory mechanisms in response to selenium in rice. J Proteomics 16: 1849-1866.
- Shu K, Liu XD, Xie Q, He ZH (2015) Two faces of one seed: hormonal regulation of dormancy and germination. Mol Plant 9: 34-45.
- Li C, Li J, Chong K, Harter K, Lee Y (2016) Toward a molecular understanding of plant hormone actions. Mol Plant 9: 1-3.
- Van-Hoewyk D (2013) A tale of two toxicities: malformed seleno proteins and oxidative stress both contribute to selenium stress in plants. Ann Bot 112: 965-972.
- Fu LH, Wang XF, Eyal Y, She YM, Donald LJ (2002) A selenoprotein in the plant kingdom: mass spectrometry confirms that an opal codon (UGA) encodes selenocysteine in Chlamydomonas reinhardtii glutathione peroxidase. Biol Chem 277: 25983-25991.
- Valkama EM, Kivimaenpaa H, Hartikainen, Wulff A (2003) The combined effects of enhanced UV-B radiation and selenium on growth, chlorophyll fluorescence ultrastructure in strawberry (Fragaria ananassa) and barley (Hordeum vulgare) treated in the field. Agric Forest Meteorol 120: 267-278.
- Chen CC, Sung JM (2001) Priming bitter gourd seeds with selenium solution enhances germinability and antioxidative responses under sub-optimal temperature. Physiologia Plantarum 111: 9-16.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi