Research Article, Int J Cardiovasc Res Vol: 9 Issue: 2
Remote Ischemic Preconditioning Provides Clinical Benefits by Decreasing Periprocedural Myocardial Injury in Indian Patients
Suresh Chandravanshi1, Smit Shrivastava2,3*, Jai Kumar Patel4 and Rimjhim Shrivastava5
1Department of Medicine, Pt. Jawahar Lal Nehru Memorial Medical College, Raipur, India.
2Department of Cardiology, Advanced Cardiac Institute, Pt. Jawahar Lal Nehru Memorial Medical College, Raipur, India
3Dr. Bhim Rao Ambedkar Memorial Hospital, Raipur, India.
4Department of Medicine, Pt. Jawahar Lal Nehru Memorial Medical College, Raipur, India.
5Department of Pediatrics, Cardiology Clinic, Avanti Vihar, Raipur, India.
*Corresponding Author: Dr. Smit Shrivastava
Associate Professor and Head, Department of Cardiology,
Advanced Cardiac Institute, Pt. Jawahar Lal Nehru Memorial Medical College
and Dr. Bhim Rao Ambedkar Memorial Hospital,
Avanti Vihar, Shankar Nagar, Raipur, Chhattisgarh, India
Tel: 09329613977
E-mail: dr.smit.shrivastava@gmail.com
Received: March 06, 2020 Accepted: April 30, 2020 Published: May 07, 2020
Citation: Chandravanshi S, Shrivastava S, Patel JK, Shrivastava R (2020) Remote Ischemic Preconditioning Provides Clinical Benefits by Decreasing Periprocedural Myocardial Injury in Indian Patients. Int J Cardiovasc Res 9:2.
Abstract
Background: Remote Ischemic Preconditioning (RIPC) in patients undergoing elective Percutaneous Coronary Intervention (PCI) may provide enhanced clinical benefits. However, published studies on PCI induced cardiac damage have yielded conflicting results. Aims: To determine whether RIPC reduces reperfusion injury and cardiac damage in Indian patients undergoing elective PCI. Materials and Methods: This prospective, randomized, control study was conducted among Acute Coronary Syndrome (ACS) patients undergoing PCI from July 2017 to October 2018 in Pt. Jawaharlal Nehru Memorial Medical College, Raipur and Dr. Bhimrao Ambedkar Memorial Hospital, Chhattisgarh. One group received RIPC (three 5-minute inflations and deflations of a standard blood-pressure cuff on the upper arm) at 1 hour before PCI. The other group which did not received RIPC served as control group. Cardiac biomarker release (Troponin-I and Creatine Kinase- MB), Electrocardiographic (ECG) and echocardiographic changes were measured in all patients before and after PCI at different time intervals. Results: A total of 52 patients were randomised and equally distributed into control and RIPC group (26 each). Post PCI, there was 77.29% reduction in mean Area Under Curve (AUC) for Troponin-I in RIPC group (8.84 ± 9.72) compared to control group (38.93 ± 79.11). Also, 64.82% reduction was found in mean AUC of CKMB in RIPC group (179.95 ± 120.7) compared to control group (511.65 ± 701.0). Conclusion: RIPC of the upper arm before primary PCI in patients with ACS could provide protection against cardiac damage and ischemia-reperfusion injury.
Keywords: Coronary heart disease; Myocardial reperfusion injury; Remote ischemic preconditioning; Cardiac biomarkers; Percutaneous coronary intervention; Troponin-I; Angioplasty.
Keywords
Coronary heart disease; Myocardial reperfusion injury; Remote ischemic preconditioning; Cardiac biomarkers,; Percutaneous coronary intervention; Troponin-I; Angioplasty.
Introduction
Coronary Heart Disease (CHD) is the very common problem globally, which causes morbidity and mortality despite the availability of optimal therapy [1]. In India and other South Asian countries, the prevalence of CHD and cardiovascular mortality has increased [2]. In India 52% of Cardio Vascular Disease (CVD) deaths occur before the age of 70 years while in western population, this number is 23% [3]. In patient with Coronary Artery Disease (CAD), myocardial revascularization remains the intervention of choice [4]. However, post revascularization the myocardial reperfusion is considered as a double edge sword. The myocardial reperfusion injury may negatively impact clinical outcomes of revascularization [5,6]. Depending on local practice and diagnostic criteria used Prasad A et al. [7] reported that 5% to 30% patient undergoing elective PCI has a Periprocedural Myocardial Infarction (PMI). In a meta- analysis performed by Nienhuis MB et al. [8], elevated Troponin-I was observed in 16% to 73% of patients undergoing PCI that is related to an increase in mortality which can be as high as 45%. Thus, novel cardio-protective modalities are warranted to enhance clinical consequences in CAD patients undergoing PCI.
RIPC, which includes a series of repetitive cycles of brief reperfusion alternating with brief occlusion immediately after reperfusion, showed encouraging outcomes in preclinical and clinical studies [9]. Iliodromitis et al. [10] reported an increase in troponin and C-reactive Protein (CRP) level in the RIPC group. However Prasad A et al. [7] and Wang X et al. [11] observed no significant difference in troponin level between RIPC and control group after 24 h. Ghaemian et al. [12], Ahmed et al. [13] and Luo et al. [14] found a reduction in median troponin rise and rate of Myocardial Infarction (MI). The Third Danish Study of Optimal Acute Treatment of Patients with ST-Elevation Myocardial Infarction-Ischemic Postconditioning (DANAMI-3-iPOST) trial has even shown non-beneficial effects of RIPC [15]. Thus, outcomes of RIPC in patient undergoing elective PCI have shown mixed results. Limited data on the effect of RIPC on cardiac damage has been reported from India.
Therefore, the present study was planned to determine the effect of RIPC on reperfusion injury and cardiac damage in Indian patients undergoing elective PCI. The cardiovascular effects of RIPC were expressed in terms of cardiac biomarker release (Troponin-I and CKMB), ECG and echocardiographic changes.
Methods
This was a prospective, randomized and controlled study. Participants were identified from the patient’s waiting list those who have opted for PCI scheduled in between July 2017 to October 2018. They were invited to participate in the trial before their Angioplasty/ Stenting in Pt. Jawaharlal Nehru Memorial Medical College and Dr. Bhimrao Ambedkar Memorial Hospital, Raipur, Chhattisgarh, India. The study was approved by the Institutional Ethics Committee of Pt. Jawaharlal Nehru Memorial Medical College and Hospital. Signed and written informed consents were obtained from all participants before the start of the study.
Patients were randomly assigned in a 1:1 ratio to control and RIPC groups using the random number table [16]. Eligible patients were adult (age ≥ 18 years), who were undergoing PCI and had baseline Troponin-I level and Creatinine kinase (CK) - MB level lesser than 0.08 ng/dl and 3.5 ng/dl, respectively. The primary outcomes of interest were the effect of RIPC on cardiac biomarker release (Troponin-I and CK-MB) in patients undergoing PCI. The secondary outcomes were ECG (ST segment elevation) and echocardiography (Left ventricular ejection fraction) changes following RIPC. The diagnostic criteria for myocardial injury associated with PCI were based on the fourth universal definition of myocardial infarction [17].
Baseline demographic and clinical characteristics
Patient’s sex, age, height, weight and Body Mass Index (BMI) were recorded prior to PCI. In addition, detailed history of disease, risk factors for coronary heart disease, drug treatment and physical examination were also captured
Procedural intervention
RIPC and control interventions: During the course of admission, patients were instructed to avoid any strenuous activity that could provoke angina before procedure. In RIPC group, blood pressure cuff was placed around their non-dominant upper arm and cuff was inflated to 200 mmHg pressure for five minutes, followed by five minutes of deflation, to allow reperfusion. This procedure was done for three times, 1hour before PCI. In control group blood pressure cuff was placed around the non-dominant arm similar to RIPC group, however cuff was inflated to 20 mmHg below a participant’s resting diastolic blood pressure for five minutes followed by five minutes of deflation [18,19].
Percutaneous coronary intervention: The intervention of PCI was performed via radial or femoral arterial approach with 6F or 7F guiding catheters. All patients received clopidogrel 600 mg at least 12 h before PCI and were anticoagulated with a heparin bolus (70 to 100 U/kg) after arterial sheath insertion to achieve an activated clotting time of 250 seconds. Glycoprotein IIb/IIIa antagonists were not administered. Omnipaque 350 (Iohexol) (GE Healthcare Pharmaceuticals) was used as the contrast agent in all cases. The PCI strategy and all post procedural medication were given at the discretion of the treating interventional cardiologist according to conventional practice.
Hydration procedure: Patients were hydrated with intravenous saline infusion (1 mL/kg/hr) 12 hours before and 12 hours after contrast. They were encouraged to drink lots of water after PCI (except those with left ventricular dysfunction).
Blood sampling and laboratory measurements: Venous blood samples were withdrawn at baseline, 6, 12 and 24 hours post PCI, to measure cardiac enzymes (CK-MB and Troponin-I). These cardiac enzymes were used to assess the status of ischemia-reperfusion injury after PCI at different time intervals based on the available diagnostic information of myocardial injury [17]. Cardiac enzymes were analyzed with an automated immunoassay (Roche analyzer). Other laboratory parameters like Haemoglobin (Hb), Total Leukocyte Count (TLC), Mean Corpuscular Volume (MCV), platelet counts, serum creatinine, Blood UUrea Nitrogen (BUN), and electrolytes (sodium, potassium) levels were also recorded before as well as 24-h after PCI in both groups. A prospective 25% rise in serum creatinine level of their baseline value at 24 hours was defined as a significant decrease in renal function.
Left Ventricular Ejection Fraction (LVEF) and ST segment elevations measurement: Left Ventricular Ejection Fraction (LVEF) levels and ST segment elevations were recorded before as well as 24-h after PCI in both groups. 2D echocardiography was done with Phillips EPIQ7C Echo machine while ECG was done with Philips Page writer TC 20.
Statistical Analysis
To calculate difference of mean with effect size of 0.8, α-error probability of 0.05 and power of 80% with allocation ratio of 1:1, a sample size of 52 patients (26 cases and 26 control) for study was derived by sample size software G*power (3.0.10). Analysis of variance or t-tests were used for comparing continuous variables and Fisher’s Exact test for discreet variable. P value<0.05 was considered as statistically significant. SPSS Software for WindowsTM version 17, IBMTM Corp NY and Microsoft ExcelTM 2007, Microsoft Inc USA were used to perform the statistical analysis.
Results
A total of 84 patients with ACS undergoing PCI were screened for study participation. Out of 84 patients, 18 patients were excluded because of higher baseline values for cardiac enzymes (CK‐MB and Troponin-I). Seven cases did not undergo PCI due to technical reasons. Another Seven patients were excluded from final analysis due to failure to follow up. Total 52 eligible patients were then randomly allotted into RIPC and control group.
Baseline demographic and clinical characteristics
There was no significant statistical difference between the two groups with respect to age, sex and risk factors for coronary artery disease. Baseline values for laboratory measurements and cardiac enzymes were found to be similar in both the groups (Table 1).
| Control group (n=26) | RIPC group (n=26) | p-value | |
|---|---|---|---|
| Age (Years) | 56.5 ± 8.7 | 55.1 ± 7.7 | 0.46 |
| Male | 19 | 22 | 0.31 |
| BMI | 23.5 ± 3.5 | 24.5 ± 3.8 | 0.37 |
| Diabetes | 06 | 13 | 0.44 |
| Hypertension | 12 | 13 | 0.78 |
| Smoking/Tobacco | 11 | 17 | 0.12 |
| Alcohol | 13 | 14 | 1.00 |
| Types of ACS | |||
| AWMI | 10 | 13 | 0.58 |
| ASMI | 4 | 2 | 0.67 |
| IWMI | 6 | 7 | 1.0 |
| NSTEMI | 1 | 1 | 1.0 |
| UA | 5 | 3 | 0.70 |
| Baseline laboratory values | |||
| Troponin-I (ng/mL) | 0.01 ± 0.02 | 0.01 ± 0.02 | 1.00 |
| CK-MB (U/L) | 1.23 ± 1.04 | 1.26 ± 0.86 | |
RIPC-Remote Ischemic Preconditioning; BMI-Body Mass Index; CK-MB-Creatinine Kinase MB; AWMI- Anterior Wall Myocardial Infarction; ASMI-Antero Septal Myocardial Infarction; IWMI-Inferior Wall Myocardial Infarction; NSTEMI-Non-ST Segment Elevation Myocardial Infarction; UA-Unstable angina
Table 1: Baseline parameters in control and RIPC groups.
Types of ACS
Anterior Wall Myocardial Infarction (AWMI) was found in 44.2% of our study population (10 patients from control group and 13 patients from RIPC group). Antero Septal Myocardial Infarction (ASMI) was found in 11.5% of our study population (4 patients from control group and 2 patients from RIPC group). Inferior wall myocardial infarction was found in 25.0% of our study population (6 patients from control group and 7 patients from RIPC group). Non ST Segment Elevation Myocardial Infarction (NSTEMI) was found in 3.8% of our study population (1 patient from control group and 1 patient from RIPC group). Unstable Angina (UA) was found in 15.4% of our study population (5 patients from control group and 3 patients from RIPC group).
Angiographic variables
No significant difference was observed in the number and distribution of affected blood vessels between the groups (Table 2).
| Control group (n=26) | RIPC group (n=26) | p-value | |
|---|---|---|---|
| Distribution of affected vessels | |||
| LAD | 14 | 16 | 0.67 |
| LCx | 03 | 01 | |
| RCA | 01 | 02 | |
| Multivessel | 08 | 07 | |
| Number of vessels involved | |||
| SVD | 18 | 19 | 1.00 |
| DVD | 07 | 06 | |
| TVD | 01 | 01 | |
RIPC-Remote Ischemic Preconditioning; LAD-left anterior descending artery; LCx-left circumflex artery; RCA-Right Coronary Artery; SVD-Single Vessel Disease; DVD- Double Vessel Disease; TVD-Triple Vessel Disease
Table 2: Distribution of affected vessels in control and RIPC groups.
Laboratory measures
There was no statistically significant difference between the two groups in biochemical values following PCI; though BUN was insignificantly higher in the RIPC group. The mean BUN concentration was 19.9 ± 10.2 mg/dL in the RIPC group compared to 25.1 ± 10.2 mg/dL in control group (p=0.07) (Table 3).
| Control group (n=26) | RIPC group (n=26) | p-value | |
|---|---|---|---|
| Hb (gm%) | 12.8 ± 1.4 | 12.9 ± 1.5 | 0.81 |
| TLC (103/µL) | 7.3 ± 1.5 | 8.1 ± 2.1 | 0.14 |
| MCV (fl) | 78.2 ± 17.9 | 79.1 ± 11.9 | 0.84 |
| Platelet counts (103/µL) | 263 ± 78.6 | 234 ± 78.7 | 0.19 |
| BUN (mg/dL) | 19.9 ± 10.2 | 25.1 ± 10.2 | 0.07 |
| Serum creatinine (mg/dL) | 1.1 ±1.1 | 1.0 ± 0.2 | 0.67 |
| Sodium (mmol/l) | 136.1 ± 3.5 | 137.3 ± 3.9 | 0.38 |
| Potassium (mmol/l) | 3.8 ± 0.3 | 3.7 ± 0.4 | 0.30 |
RIPC-Remote Ischemic Preconditioning; Hb-Haemoglobin; TLC-Total Leukocyte Count; MCV-Mean Corpuscular Volume; BUN- Blood Urea Nitrogen
Table 3:Laboratory measure in control and RIPC groups.
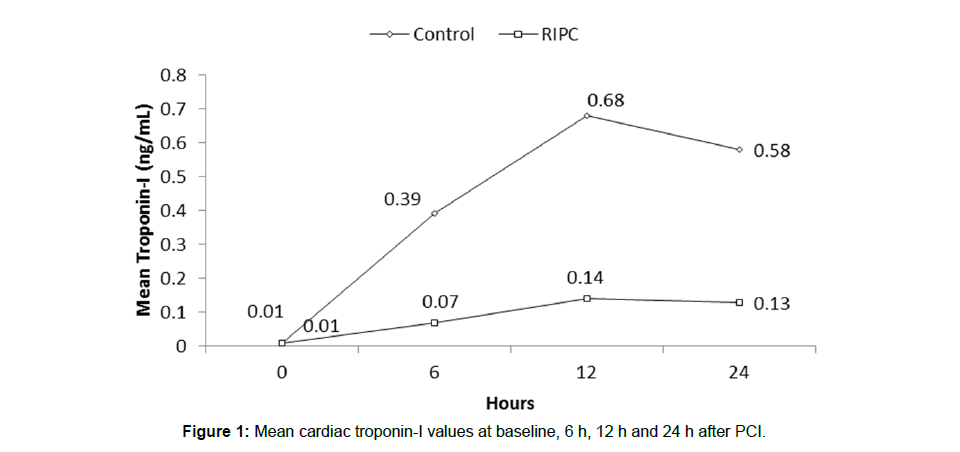
Troponin-I: Baseline troponin-I levels were 0.01ng/ml in both groups. RIPC reduced troponin I release over the 24-h after PCI (Figure 1). The total troponin-I released, expressed as the area under the curve over the 24 h after PCI, was reduced from a mean (SD) of 38.93 (79.11) in controls to 8.84 (9.72) with RIPC (p=0.05) a reduction of 77.29%.
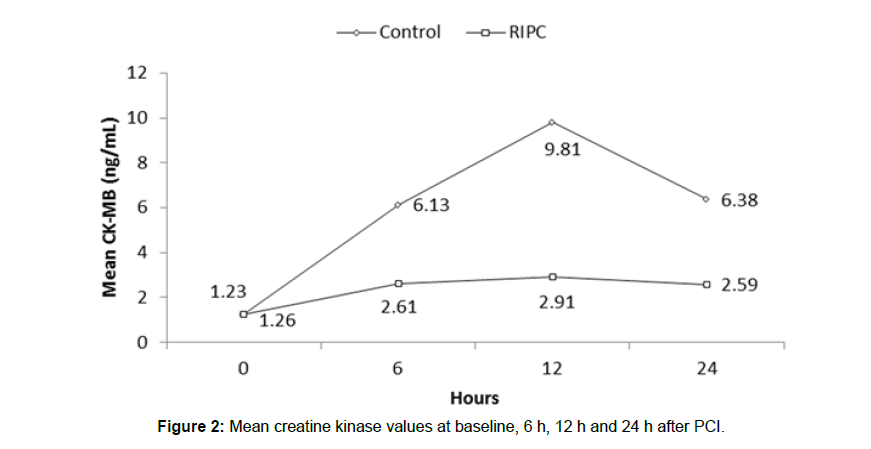
Creatine kinase-MB (CK-MB): Baseline CK-MB levels were 1.23 ng/ml in the control group and 1.26 ng/ml in the RIPC group. RIPC reduced CK-MB release over the 24-h after PCI (Figure 2). The total CK-MB released, expressed as the area under the curve over the 24 h after PCI, was reduced from a mean (SD) of 511.62 (179.95) in controls to 179.95 (120.70) with RIPC (p=0.02) a reduction of 64.82%.
ST segment elevation: Baseline ST segment elevations of ECG were 0.9 mm in RIPC group and 0.9 mm in control group. There was no significant resolution of ST segment elevation after PCI in both RIPC and control groups (0.7 ± 1.0 mm vs. 0.9 ± 1.1 mm, p=0.72).
Left ventricle ejection fraction (LVEF): LVEF values were increased in the RIPC group (48.8%) and the control group (49.4%) at 24-h after PCI compared to the pre-PCI (44.2% in control group and 43.8% in RIPC group). However, increase in LVEF values was not significant between the groups (p=0.55).
Discussion
This was a prospective, randomized, control study designed to test whether RIPC can reduce reperfusion injury and cardiac damage in patients undergoing PCI. Within this cohort, RIPC was safe.
PCI-related reperfusion injury and cardiac damage was reduced in RIPC group as evidenced by reduced troponin-I and CK-MB release. However, RIPC did not affect PCI-related outcomes such as ECG and 2D echocardiographic changes.
Myocardial injury, as indicated by the release of cardiac enzymes, occurs as a result of reperfusion injury encountered during PCI. Cardiac-specific biomarkers like troponin I and CK-MB are used routinely to quantify the myocardial injury and have been reported to be associated with worse short-term and long-term outcomes. Due to their high sensitivity and specificity, even minute amount of peri-procedural myocardial injury is conveniently detected and documented [20]. Elevation of troponin level after one year of PCI (greater than 5 times the baseline value which is diagnosed as type 4a MI in the latest guideline) is considered as a distinct predictor of a composite of death, MI, and revascularization [5]. Another observational study reported post-PCI troponin elevations as a predictor of increased long-term mortality and MI [21].
Ischemic reperfusion injury develops in about 30% of patients following elective PCI. RIPC has been shown to reduce the procedurerelated cardiac troponin I in the setting of primary PCI [22]. Thus, RIPC-induced cardioprotection during the early phase (1-2 hours before PCI) is considered ideally suited for patients undergoing PCI as it increases the tolerance of the myocardium to ischemia. A previous study has shown 3 cycles of 5-minute blood pressure cuff inflations as sufficient stimulus to confer cardioprotection [23]. It reduced ischemia and reperfusion injury by stimulating endogenous protection [24]. In this study RIPC, comprised of 3 cycles of 5-minute cuff inflation, was applied during the early window of protection at 1 hour before starting the PCI to avoid ischemia-reperfusion injury.
Liu and colleagues reported in a randomized study of 200 patients undergoing elective PCI that the median troponin-I and CK-MB levels at 24 h were lower in the L-RIPC group compared to control (0.01 vs. 0.03 ng/mL, 15 vs. 27 IU/L; p<0.05). This lower concentration of troponin-I and CK-MB was associated with myocardial protection in these patients. Hoole et al. [25] induced RIPC by applying the pressure of 200 mmHg around the upper arm for five minute using a blood pressure cuff followed by a five minutes reperfusion period for three times before the patient was operated for stenting. After 24 hours, median concentrations of Troponin-I (0.06 ng/mL) was found to be significantly reduced when compared with the control patients (0.16 ng/mL; p<0.04). In 2012, Ghaemian et al. [12] witnessed that two five minute lower limb ischemia before PCI reduced the absolute risk of post-procedure cardiomyocyte necrosis by 27.5% [12]. D’Ascenzo et al. [26] and Pei et al. [27] confirmed the decreased incidence of PMI by means of RIPC in the patients undergoing cardiac interventions in their meta-analysis study. Another meta-analysis investigating the effect of RIPC on Troponin-I levels did not find any effect of RIPC on the the concentration of Troponin-I at 12 h and 24 h after elective PCI [28].
The results of our study are consistent with previous studies and meta-analyses and suggest that RIPC is a safe, effective, and noninvasive strategy for providing cardio-protection when myocardial necrosis is expected. In this study, we compared RIPC group to control group and found reduction of Troponin-I and CK-MB level in RIPC group, thereby leading to reduction of PMI incidence, which is incompatible with meta-analysis findings by D’Ascenzo et al [26]. The mechanism behind RIPC induced cardio-protective mechanism is not yet clearly understood but it is mainly concentrated in molecular mechanism of signal transduction via NF-kappa B, protein kinase C, and mitochondrial ATP-sensitive potassium channel [29].
Acute Kidney Injury (AKI) is a serious post-operation complication in patients with cardiac and vascular interventions [30]. Patients with post-operative AKI have significantly higher morbidity and mortality. Our study did not find any effect of RIPC on the renal outcomes as evidenced by insignificant difference in BUN and creatinine level between RIPC and Control group. This is in agreement with previous meta-analysis showed that serum creatinine level was not reduced through RIPC [31]. However, Li B et al [32] in their meta-analysis concluded, that the contrast mediated AKI can be reduced after RIPC, in patients undergoing PCI. Similarly, in another meta-analysis done by Valappil SP et al. [33] RIPC significantly reduced Contrast Induced Nephropathy (CIN) incidence in patients undergoing cardiac or vascular interventions. These obvious inconsistencies may also be because of limitations in the low quantity of studies, a small pattern size, and different definitions of AKI.
Previous studies have demonstrated that ST-segment resolution measurements after PCI have prognostic value for predicting amount of myocardial damage [34,35]. It has also been found to be associated with lower left ventricular ejection fraction, larger final infarct size and higher mortality [36,37]. In the present study, RIPC insignificantly improved ST-segment resolution (0.9 mm to 0.8 mm) and LVEF (43.8% to 48.8%). The ECG measures correlated well with LVEF as determined by 2D echocardiography, reflecting protection against cardiac damage and reperfusion injury after PCI. The only limitation of the study was that the time from the first cuff inflation to the first balloon dilation was unforeseen and frequently delayed. It is possible that if the cuff-to-balloon time had been shorter, the cardio-protection would have been better. Also the effect of procedure and early outcomes of PCI may affect the results, which were not recorded
Conclusion
Our study confirmed that RIPC can reduce myocardial injury as indicated by a 77.29% reduction in Troponin-I and 64.82% reduction in CK-MB released over the 24 h periprocedural period. An optimal RIPC protocol shall be evaluated in large randomized clinical trials for assessing long-term clinical outcomes.
Acknowledgement
Authors acknowledge WorkSure, India for medical writing assistance.
References
- Prabhakaran D, Jeemon P, Roy A (2016) Cardiovascular diseases in India current epidemiology and future directions. Circulation 133: 1605-1620.
- Krishnan MN (2012) Coronary heart disease and risk factors in India- on the brink of an epidemic? Ind. Heart J 64: 364-367.
- Harikrishnan S, Leeder S, Huffman M, Jeemon P, Prabhakaran D (2014) A Race against time: The challenge of cardiovascular disease in developing economies(2nd ed), Centre for chronic disease control, New Delhi, India.
- Iqbal J, Serruys PW (2017) Optimal medical therapy is vital for patients with coronary artery disease and acute coronary syndromes regardless of revascularization strategy. Ann Transl Med 5: 140.
- Zeitouni M, Silvain J, Guedeney P, Kerneis M, Yan Y, et al. (2018) Periprocedural myocardial infarction and injury in elective coronary stenting. European Heart Journal 39: 1100-1109.
- Ejiri K, Miyoshi T, Kohno K, Nakahama M, Doi M, et al. (2018) Protective effect of remote ischemic preconditioning on myocardial damage after percutaneous coronary intervention in stable angina patients with complex coronary lesions. Circ J82:1788-1796.
- Prasad A, Herrmann J (2011) Myocardial infarction due to percutaneous coronary intervention. N Engl J Med 364: 453-464.
- Nienhuis MB, Ottervanger JP, Dambrink J-HE, Dikkeschei LD, Suryapranata H, et al. (2007) Troponin T elevation and prognosis after multivessel compared with single-vessel elective percutaneous coronary intervention. Netherlands Heart Journal 15: 178-183.
- Cao B, Wang FH, Zhang CC, Xia CM, Yang AX (2018) remote ischemic postconditioning of the upper arm results in protection from cardiac ischemia-reperfusion injury following primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. Med Sci Monit 24: 1017-1026.
- Iliodromitis EK, Kyrzopoulos S, Paraskevaidis IA, Kolocassides KG, Adamopoulos S, et al. (2006) Increased C reactive protein and cardiac enzyme levels after coronary stent implantation. Is there protection by remote ischaemic preconditioning?. Heart 92: 1821-1826.
- Wang X, Kong Na, Zhou C, Mungun D, Iyan Z, et al. (2017) Effect of remote ischemicpreconditioning on perioperative cardiac events in patients undergoing elective percutaneous coronary intervention: a meta-analysis of 16 randomized trials. Cardiol Res Pract 6907167.
- Ghaemian A, Nouraei SM, Abdollahian F, Naghshvar F, Dino A, et al, (2012) Remote ischemic preconditioning in percutaneous coronary revascularization: a double-blind randomized controlled clinical trial. Asian Cardiovasc Thorac Ann20: 548-554.
- Ahmed RM, Mohamed El-Haddad A, Ashraf M, Maithili S, Nabil F, et al. (2013) Effect of remote ischemic preconditioning on serum troponin T level following elective percutaneous coronary intervention. Catheter Cardiovasc Interv 82: 647-653.
- Luo SJ, Zhou YJ, Shi DM, Ge HL, Wang JL, et al. (2013) Remote ischemic preconditioning reduces myocardial injury in patients undergoing coronary stent implantation. Can J Cardiol 29: 1084-1089.
- Høfsten DE, Kelbæk H, Helqvist S, Kløvgaard L, RN, et al. (2015) The third DANish Study of optimal acute treatment of patients with ST-segment elevation myocardial infarction: ischemic postconditioning or deferred stent implantation versus conventional primary angioplasty and complete revascularization versus treatment of culprit lesion only: rationale and design of the DANAMI 3 trial program. Am Heart J 169: 613-621.
- Rand (2001) A million random digits with 100,000 normal deviates. Rand Corporation. [Assessed on 2020 April 28].
- Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, et al. (2018) Fourth universal definition of myocardial infarctio. J Am Coll Cardiol 72: 2231-2264.
- Kendra M, Allen C, Gidday JM, Lee JM, Hershey T, et al. (2017) Remote limb ischemic conditioning at two cuff inflation pressures yields learning enhancements in healthy adults. J N Mot Behav 49: 337-348.
- Young PJ, Dalley P, Garden A, Horrocks C, Flamme AL, et al. (2012) A pilot study investigating the effects of remote ischemic preconditioning in high-risk cardiac surgery using a randomized controlled double-blind protocol. Basic Res Cardiol 107: 256.
- Aydin S, Ugur K, Aydin S, Sahin I,Yardim M (2019) Biomarkers in acute myocardial infarction:current perspectives. Vascular Health and Risk Manag 15: 1-10.
- Ahn SH, Lee JS, Kim YH, Kim BJ, Kim YJ, et al. (2017) Prognostic significance of troponin elevation for long-term mortality after ischemic stroke. J Stroke 19: 312-322.
- Bøtker HE, Kharbanda R, Schmidt MR, Bøttcher M, Kaltoft AK, et al. (2010) Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. The Lancet 375: 727-734.
- Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, et al. (2002) Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation 106: 2881-2883.
- Zhou D, Ya J, Pan L, Wang Y, Ji X, et al. (2018) Remote ischemic conditioning: a promising therapeutic intervention for multi-organ protection. Aging (Albany NY) 10: 1825-1855.
- Hoole SP, Heck PM, Sharples L, Khan SN, Duehmke R, et al. (2009) Cardiac remote ischemic preconditioning in coronary stenting study. Circulation 119: 820-827.
- D’Ascenzo F, Moretti C, Omedè P, Cerrato E, Cavallero E, et al. (2014) Cardiac remote ischaemic preconditioning reduces periprocedural myocardial infarction for patients undergoing percutaneous coronary interventions: a meta-analysis of randomised clinicai 9: 1463-1471
- Pei H, Wu Y, Wei Y, Yang Y, Teng S, et al. (2014) Remote Ischemic Preconditioning Reduces Perioperative Cardiac and Renal Events in Patients Undergoing Elective Coronary Intervention: A Meta-Analysis of 11 Randomized Trials. PLOS ONE 9: e115500.
- Niu X, Zhang J, Chen De, Wan G, Zhang Y, et al. (2014) Remote ischaemic conditioning in percutaneous coronary intervention: a meta-analysis of randomised trials. Postep Kardiol Inter 10: 274-282.
- Carreira RS, Lee P, Gottlieb RA (2011) Mitochondrial therapeutics for cardioprotection. Curr Pharm Des 17: 2017-2035.
- Park JT (2017) Postoperative acute kidney injury. Korean J Anesthesiol 70: 258-266
- Brevoord D, Kranke P, Kuijpers M, Weber N, Hollmann M, et al. (2012) Remote ischemic conditioning to protect against ischemia-reperfusion injury: a systematic review and meta-analysis. Plos One 7: e42179.
- Li B, Lang X, Cao L,Wang Y, Lu Y, et al. (2016) Effect of remote ischemic preconditioning on postoperative acute kidney injury among patients undergoing cardiac and vascular interventions: a meta-analysis. J Nephrol 30: 19-33.
- Valappila SP, Kunjukrishnapillai S, Viswanathan S, Koshy AG, Gupta PN,et al. (2017) Remote ischaemic preconditioning for prevention of contrast induced nephropathy-Insights from an Indian study. Ind Heart J 70: 857-863.
- Ndrepepa G, Alger P, Kufner S, Mehilli J, Schömig A, et al. (2012) ST-segment resolution after primary percutaneous coronary intervention in patients with acute ST-segment elevation myocardial infarction. Cardiol J 19: 61-69.
- Fabris E, van’t Hof A, Hamm CW, Lapostolle F, Lassen JF, Goodman SG, et al. (2019) Clinical impact and predictors of complete ST segment resolution after primary percutaneous coronary intervention: A subanalysis of the ATLANTIC Trial. Eur Heart J Acute Care 8: 208-217.
- Galassi AR, Boukhris M, Toma A, Elhadj ZI, Laroussi L, et al. (2017) Percutaneous coronary intervention of chronic total occlusions in patients with low left ventricular ejection fraction. JACC: Cardiovas Interv 10: 2158-2170.
- Tajti P, Burke MN, Karmpaliotis D, Alaswad K, Jaffer FA, et al. (2018). Prevalence and outcomes of percutaneous coronary interventions for ostial chronic total occlusions: insights from a multicenter chronic total occlusion registry. Can J Cardiol 34: 1264-1274
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi