Review Article, J Infect Dis Immune Ther Vol: 9 Issue: 1
Review on Q Fever and Its Public Health Importance
Walde Abdias and Haregawi Tesfaye*
Department of Veterinary Medicine, College of Agriculture and Veterinary Medicine, Jimma University, Jimma, Ethiopia
*Corresponding Author: Haregawi Tesfaye
Department of Veterinary Medicine, College of Agriculture and Veterinary Medicine, Jimma University, Jimma, Ethiopia
E-mail: haregawitesfaye@ymail.com
Received date: 09 October, 2023, Manuscript No. JIDITH-23-116144;
Editor assigned date: 11 October, 2023, PreQC No. JIDITH-23-116144 (PQ);
Reviewed date: 25 October, 2023, QC No. JIDITH-23-116144;
Revised date: 17 January, 2025, Manuscript No. JIDITH-23-116144 (R);
Published date: 24 January, 2025, DOI: 10.4172/2329-9541.1000355
Citation: Abdisa W, Tesfaye H (2025) Review on Q Fever and Its Public Health Importance. J Infect Dis Immune Ther 9:1.
Abstract
Q fever is recognized as a global zoonotic disease. The objective of this review paper is to provide an overview of Q Fevers and its public health importance, as well as transmission, control, and preventive measures and the current status in Ethiopia. It affects a wide range of mammals, birds, arthropods, and even humans. The main reservoirs of Coxiella burnetii are cattle, sheep, and goats. Also, ticks are one of the reservoirs. Coxiella burnetii is found in high numbers in amniotic fluid, placenta, and fetal membranes, as well as in the milk, urine, and faeces of infected animals. The organism can persist in spore-like form for more than 40 months. The airborne transmission of Coxiella burnetii associated with its highly resistant environment and the ability to easily produce huge quantities of Coxiella burnetii after the birth of aborted ewes or goats have led to classifying Coxiella burnetii as a Category B biological terrorism agent. Coxiellosis produces both acute and chronic forms of clinical manifestations in humans and also premature fetal death in pregnant women. Sheep and goats may exhibit abortion, stillbirth, premature delivery, and the delivery of weak offspring, while cattle and camels may develop infertility, metritis, and mastitis. Four categories of diagnostic tests are available: isolation, serologic assays, PCR, and stained methods. The recommended treatment for ruminants is to administer oxytetracycline during the last month of gestation. Also, doxycycline is the best medication for humans. In general, Q fever is a global health problem, an OIE-notifiable disease, and an infectious disease that is considered to have economic and public health importance. The control and prevention methods are antibiotic treatment and vaccination. Also, using insecticides to control ticks and following appropriate hygiene practices to avoid the spread of infectious diseases should also be addressed in this review.
Keywords: Biological terrorism; Coxiella burnetii; Doxycycline; OIE; PCR; Q fever
Introduction
Q fever is recognized as a global zoonotic disease that has been declared as potential bioterrorism category B select agent by the Centre for Disease Control and prevention (CDC) [1]. Q fever in animals has been detected worldwide, whilst the only country with an apparent zero prevalence is New Zealand [2]. It is a notable disease in the United States and the European Union, where it has increased the number of cases reported [3]. In the African context Q fever was first reported in 1947, but since then the quantity and quality of epidemiological research on this pathogen has been limited. Ethiopia was ranked highest in Africa in the health burden of zoonotic diseases. This reported a high seroprevalence of C. burnetii, (31.6% in cattle, 90.0% in camels and 54.2% in goats). A 6.4% prevalence of C. burnetii in Ethiopia was also report from different Ixodid ticks species by quantitative real time polymerase chain reaction targeting two different genes followed by Multi-spacer Sequence Typing (MST). Q fever is caused by the small, obligately intracellular, pleomorphic gram-negative bacterium C. burnetii that is characterized by high tenacity and virulence. C. burnetii can potentially survive for years in the environment, being highly resistant to chemical and physical stresses, including disinfectants, desiccation, UV light, sonication and osmotic stress [4]. The disease is classified as an emerging zoonotic infectious disease according to WHO, FAO, OIE and EFSA/ECDC. Domestic ruminants such as cattle, sheep, and goats are the main reservoirs of the disease. This bacterium is secreted in birth products (such as placenta), urine, milk, and faeces. The major route of transmission of Coxiella burnetii to human is through inhalation of contaminated aerosols and dust particles, and less commonly by handling and ingestion of infected meat and milk. And also, those who are in close contact with the animals, such as farmers, abattoir workers and veterinarians are at highest risk [5].
Human to human transmission was described and might happen through contaminated blood transfusion, sexual contact, and exposure to contaminated birth products of women. Mainly, this disease is reported in humans having close contact with infected animals and their products [6]. It can manifest as an acute or chronic disease. Acute infections are mostly asymptomatic (60%) or manifests as a flu-like and often self-limiting disease. Symptoms include but are not limited to flu-like symptoms, endocarditis, hepatitis, pneumonia, abortion and premature fetal death in pregnant women and neuropathies, meningitis, encephalitis, osteomyelitis. Differentiation of acute from chronic Q fever solely on clinical manifestation may be misleading. Currently, acute and chronic forms are differentiated on the basis of different antibodies present in the sera of the patient. This demonstrates that presence of IgG to phase I indicate the chronic form while detection of IgG to phase II antigen demonstrates acute form. Q fever is frequently asymptomatic, in sheep and goats it causes abortion, stillbirth, premature delivery, and delivery of weak offspring and in cattle and camel may develop infertility, metritis, and mastitis. Q fever is diagnosed in the laboratory using serological test by detection of antibodies, Polymerase Chain Reaction (PCR) amplification of specific targets have also been employed for more rapid diagnosis for molecular test. Doxycycline is the first choice of drug treatment for all adults and children in both acute and chronic case those has severe illness. For pregnant patient we use drug like Trimethoprim and Sulfamethoxazole. Cotrimoxazole and rifampin can be used in case of allergy to tetracycline’s or contraindication. Although vaccination is recommended for people in high-risk occupations, its usage is not advised to other groups due to the side effects. The economic and public health impacts of Q fever remain a major concern in developing countries because Q fever causes significant loss of animal productivity and is a zoonotic risk to humans. To protect humans from Q fever, the OIE recommends preventive measures, such as protocols for diagnostic testing and vaccinations in small ruminants and cattle.
Therefore, the objective of this seminar paper is:
- To review Q fever’s and its public health importance.
- To review transmission, control and preventive measures of Q fever.
Literature Review
History and morphology
Firstly, in the 1930’s, the causative agent of Q fever was described simultaneously in two near concurrent incidences in two different continents; Queensland, Australia and in Montana, USA [7]. After the august 1935 incident of undiagnosed febrile illness among abattoir workers in Brisbane, Queensland; Edward Derrick was assigned to investigate the cause of this epidemic [8]. In 1935, Davis and Cox, after Edward Holbrook Derrick had isolated in Queensland as the causative agent of Query fever, subsequently found the same organism as that found by Derrick. Soon after then, in 1939, the organism was named as Coxiella burnetii in honor of Cox and Burnet, who had identified the organism as a new Rickettsial. It was later identified as Coxiella burnetii infection. It also known by several synonyms such as Abattoir fever, Australian Q fever, Balkan influenza, Coxiellosis, Ninemile fever, and Pneumorickettsiosis [8]
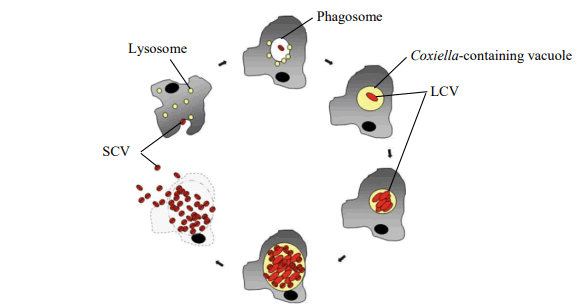
The bacteria Coxiella burnetii has two morphologically distinct cell variants an intracellular, metabolically active Large Cell Variant (LCV) and a spore-like Small Cell Variant (SCV) [9]. These two forms are morphologically and functionally distinct. The LCV is larger, elongated less electron-dense bacteria and metabolically active and replicating by a large amount [10]. While the SCV presents a compact rod shaped with a very dense central region, and it is considered as the metabolically dormant and less replicating. SCV is resistant to environmental stress and can survive longer in harsh environments. Coxiella burnetii is not able to replicate outside host cells naturally. Despite the long generation time of up to 12 hours replication follows a typical bacterial growth curve with a lag phase, exponential growth (log phase) and stationary phase. Interestingly, Coxiella burnetii displays a biphasic developmental cycle with a replicative (LCV) and a spore-like dormant (SCV) cell form Minnick et al. During growth, these cell forms show characteristic appearance, gene expression, regulatory and structural components (Figure 1).
Figure 1: Proposed biphasic developmental cycle of Coxiella burnetii.
Etiology and taxonomy
Q fever is a gram-negative, strictly intracellular, pleomorphic bacterium ranging in size from 0.2 μm to 0.5 μm in width and 0.4 μm to 1.0 μm in length. It belongs to domain bacteria, phylum proteobacteria, class gamma-proteo-bacteria, order Legionellales, family Coxiellaceae, genus Coxiella and species Coxiella burnetii [11]. Coxiella burnetii display two antigenic phases based on changes that occur in the organism during in vitro culture, such as phase I and phase II. They are vulnerable to the membrane's Lipopolysaccharide (LPS). Compared to other gram-negative bacteria, phase-I Coxiella burnetii antigens are more infectious and belong to the smooth phase. Whereas phase-II antigen corresponds to the less virulent granular (Rough) phase. Since Coxiella burnetii was once believed to be the only species in the genus Coxiella, numerous new species, including Coxiella cheraxi found in crayfish and a novel Coxiella-like organism present in birds and ticks. Coxiella cheraxi has the highest genetic homology with Coxiella burnetii and Coxiella massiliensis found in reptiles. Candidatus Coxiella avium is another novel pleomorphic Coxiella-like organism isolated from birds. It multiplies within the acidic vacuole of host macrophage cells, leading to systemic infection and mortality. Similarly, Coxiella-like Endosymbionts (CLE) is also present in ticks [12]. Genomic gather contains reference C. burnetii strain that were separated from contaminated human and creatures. Strain in genomic bunch 1, 2 and 3 have been disconnected from tick, human blood (intense Q fever), from drain of diligently contaminated dairy cattle, and prematurely ended fetal tissue. Strains in genomic gather 4 and 5 have been separated from the heart of people with incessant Q fever and prematurely ended tissue of creatures. Strains in genomic bunch 6 have been separated as it were from rodents; these strains are of obscure destructive for people and creatures.
Pathogenesis
Coxiella burnetii is a small; obligate intracellular, pleomorphic gram-negative bacterium. Because of its high tenacity, Coxiella burnetii can be infectious in raw milk for 90-273 days at 4-6°C as well as in raw milk products like butter and soft cheese for 42 days at 20°C. In dust and wool, it can be infectious over 7-24 months depending on the surrounding temperature. Coxiella burnetii evokes a zoonotic and mainly airborne disease called Q fever. Furthermore, the organism can survive for more than 6 months in 10% salt solution. The pathogenesis of Coxiella burnetii infection in humans and animals is not clearly understood. But, it is believed that bacterial LPS play an important role in the pathogenesis of Q fever in both humans and animals. The organism probably follows the oropharyngeal route as its port of entry into the lungs and intestines of both humans and animals. It is highly infectious, and a very low dose is sufficient to initiate infection. Primary multiplication takes place in the regional lymph nodes after the initial entry, and a transient bacteremia develops which persists for five to seven days, as shown in sheep. The SCVs are shed by infected animals. After infection the organism attaches to the cell membrane of phagocytic cells. After phagocytosis, the phagosome containing the SCV fuses with the lysosome. The SCVs are metabolically activated in the acidic phagolysosome and can undergo vegetative growth to form LCVs. The LCVs and the activated SCVs can be divided by binary fission and they can also undergo saprogenic differentiation. The spores that are produced can undergo further development to become metabolically inactive SCVs, and both spores and SCVs can be released from the infected host cell by either cell lysis or exocytosis. The entire developmental cycle of metabolically active Coxiella burnetii takes place in acidic phagolysosome; Coxiella burnetii is resistant to microbicidal activities in the host macrophages. The acidic environment also protects Coxiella burnetii from the effects of antibiotics, as the efficacy of antibiotics is decreased in the acidic PH. The SCV and spore forms are more difficult to denature than LCVs, possibly due to differences in cell wall composition and thickness as well as water content. The replicating Large Cell Variant (LCV) of Coxiella burnetii and the non-replicating, infectious Small Cell Variant (SCV) alternate during the organism's biphasic life cycle. The SCV is extremely resistant to environmental factors and has a unique spore-like shape with highly condensed chromatin [13].
Epidemiology
Global distribution: Q fever is a zoonotic disease which is endemic worldwide except in New Zealand and Antarctica [14]. Its outbreaks are reported for many countries worldwide such as Egypt, Germany, the Netherlands, Switzerland or Australia [15,16]. It affects a wide range of mammals, birds and arthropods. The largest Q fever outbreak ever recorded occurred in 2007 in the Netherlands, with more than 4000 acute human cases. A study done in Southern Taiwan demonstrated the overall seroprevalence of Q fever as 26.3% in humans engaging in veterinary and animal-related work and exposure to goats was significantly associated with seropositivity. The seroprevalence studies are available from nearly all Northern, Western, Central, Eastern and Southern African countries. Nevertheless, only few surveys were conducted with random sampling or correlated prevalence in human and animal populations [17]. Q fever seropositivity among integrated human and animal studies was 13%, 23%, 33% and 16% in Egypt. Animal serological studies found that 13.9% of cattle, 12.4% of goats, and 9.4% of sheep were Coxiella burnetii seropositive in West Africa. In Kenya, 10.5% of cattle in the outbreak were seropositive. In Ethiopia, Gumi et al., reported seroprevalence of Q fever as 90%, 32 % and 54 % in camels, cattle and goats respectively.
Reservoirs of Q fever: The main reservoirs of Coxiella burnetii are cattle, sheep, and goats. Although, an increasing number of animals have been reported to shed the bacterium, including domestic mammals, humans, marine mammals, reptiles, ticks, and birds. Little is known about the infection in wildlife, in spite of the significant role as reservoir of some wild species, such as rabbits, red deer and small mammals. In addition to all these sources of transmission, ticks are able to spread the pathogen and to be a source of infection. It has been described that one gram of tick faeces contains more than one billion Coxiella, and that less than ten organisms are capable of causing Q fever. The bacterium has been isolated from more than 40 hard tick species, and it was demonstrated the different affinity of Mediterranean ticks for Coxiella burnetii in Dermacentor marginatus Sulzer, Rhiphicephalus sanguineus Latreille, Rhiphicephalus pusillus Gil Collado and Hyalomma lusitanicum Koch. In consequence, ticks have been suggested to play an important role in the maintenance of Coxiella burnetii in nature, as a bridge between wild and domestic animal hosts. It has only been experimentally confirmed traits related to vector competence in seven tick species (Dermacentor andersoni Stiles, Haemaphysalis bispinosa Neumann, Haemaphysalis humerosa Warburton and Nuttall, Hyalomma aegyptium L, Hyalomma asiaticum Schulze and Schlottke, Ixodes holocyclus Neumann and R. sanguineus Latreille).
Risk factors
Agent factor: Coxiella burnetii can persist for long in the environment, resist to physical and chemical stresses, and easily dispersed due to a pseudo-sporulation process. The severity of the infection depends on the strains of the infecting bacteria. Phase I type bacteria are more virulent than the phase II type. Phase I bacteria corresponds to the smooth phase (smooth) of gram negative bacteria and are more highly infectious and to phase II, to the granular phase (rough) which has a lower virulence. Based on the Restriction Fragment Length Polymorphism (RFLP), strains of Coxiella burnetii are grouped into six (I-VI) genomic groups. Acute infection in humans is caused by Coxiella burnetii genomic type I-III, whereas type IV and V are responsible for chronic infection. The virulence of type VI is unknown. Coxiella burnetii is resistant to acids (up to PH 4.5), temperature (62°C for 30 min), UV light and pressure (up to 300,000 kPa).
Host factor: Prevalence is higher in dairy cows than in beef cattle. Increasing animal density increases the infection load in the environment, and this is a potential risk factor of Coxiella burnetii infection. Several studies in cattle show that seroprevalence increases with an increasing herd size. Flock size is reported to have a similar effect in sheep. A relationship of Coxiella burnetii infection with age and sex was also found in animals, particularly in cattle. Several studies have shown that the prevalence of Coxiella burnetii infection increases with age or with the number of parity in cattle and sheep. Age and gender are the two risk factors which are shown to influence the occurrence of Q fever in humans. People aged 30-60 years are the most vulnerable group, and the clinical disease is mostly prevalent in men. People with a previous history of valvulopathy, an immunosuppressive disease like AIDS and pregnant women are the most susceptible. People in certain occupations like veterinarians, animal farm workers, abattoir workers and laboratory personnel are at a higher risk of being infected or seropositive than others and studies show a comparatively higher prevalence in these groups.
Season, environment and management factors: Seasonal variation is observed in the occurrence of human Q fever. However, this variation is varies according to geographical region. But most cases of Q fever have been reported in the spring or early summer. Human Q fever has been shown to have a relationship with rainfall rather than season. A high prevalence of Q fever was observed among people living in close proximity to infected animals or in areas with a high livestock density. Several management factors such as housing systems, isolation of a newly introduced animal may also contribute to the seroprevalence of Coxiella burnetii infection in animals. Q fever is considered an occupational disease in farmers, abattoir workers, and veterinarians, although community outbreaks around farms with infected ruminants, especially during the kidding season have also been reported.
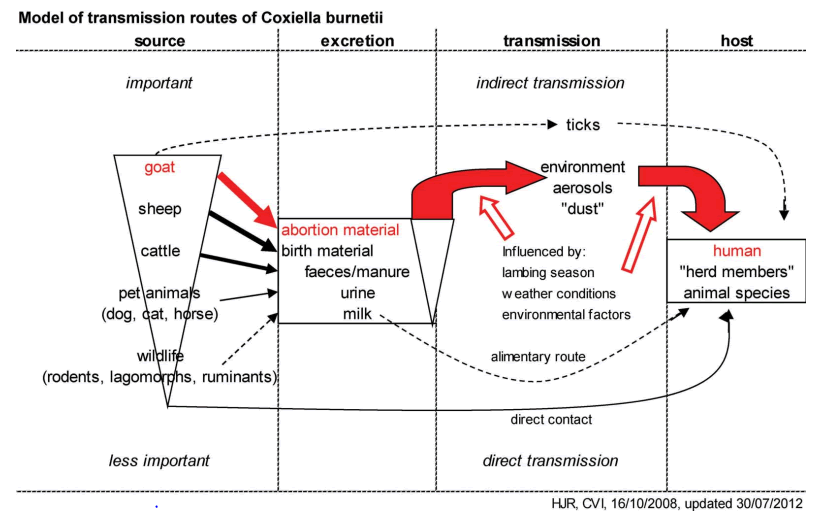
Transmission and source of infection: The zoonotic potential of C. burnetii originates from contact between humans and infected animals, such as wild or domestic mammals and ticks, which can shed the pathogen. It is found in high numbers in amniotic fluid, placenta and fetal membranes as well as in milk, urine and faeces of infected animals. Consumption of raw/unpasteurized milk and tick bites have also been claimed as possible routes of transmission, but they are probably far less frequent than the airborne one.
The main routes of introduction of C. burnetii on a farm are the aerosolized spore-like forms transported by the wind. It is likely that Coxiella burnetii contaminated manure plays a role on the maintenance of infection in animal populations. Ticks may act as reservoirs of C. burnetii in nature, as they transmit the agent transstadially and transovarially to their progeny. C. burnetii transmission by tick bite to animals has been proposed, but this is not the most important route of infection for livestock. A strong correlation has been reported between seropositivity and tick’s infestation in animals. Maintenance of C. burnetii infection in animal populations may be also affected by other factors such as manure management (capture, storage, treatment and utilization), farm characteristics (herd/flock size, animal and herd/flock density) and farm environmental conditions (temperature and relative humidity) [18].
Most commonly in humans, Q fever causes a flu-like illness, which can progress to atypical pneumonia and life threating acute respiratory distress syndrome. Humans are at highest risk of inhaling C. burnetii through distant and close contact with animals, especially small ruminants. The transmission of infection to human beings occurs through direct and indirect routes. Ruminants are considered as the main reservoir for human infections. The direct routes of transmission of infection from infected animals to humans are contact with unattended birth products and body fluids. Consumption of raw milk and contact with placentae of livestock has been shown to be high-risk human behaviors for the acquisition of Q fever. Close and distant contact between humans and small ruminants may cause single infections, as well as large outbreaks, in the human population. Animal owners, their families and employees or veterinarians have frequent close contact with small ruminants and contaminated materials, and thus are at a high risk of being infected with C. burnetii. The most common indirect source of infection are aerosols from infected farm animals because C. burnetii can remain in the environment over long periods of time and is transported by winds over long. As Q fever is an airborne disease, the pathogen can be spread over longer distances by wind and may pose a risk for human infection. Therefore, the greatest risk of infection is within a radius of 2-4 km from the source of the pathogen. Moreover, in gale force winds, C. burnetii may reach distances up to 18 km. Infected small ruminants can excrete the pathogen at high concentrations in abortion and birth materials, as well as at lower doses in milk, feces, urine and semen. As a result, contamination of the environment can occur, which means that C. burnetii can be detected in dust, manure, pastures or wool, and it can also be spread by wind. Moreover, laboratory staff may become infected via the inhalation of contaminated aerosols in workplaces. As mentioned above there different methods of transmission of the disease. However, the primary mode of human infection involves the aerosol route, and the domesticated animals most often implicated in human disease are sheep, cattle and goats (Figure 2).
Figure 2: Transmission model for Q fever adapted from Roest et al.
Clinical sign
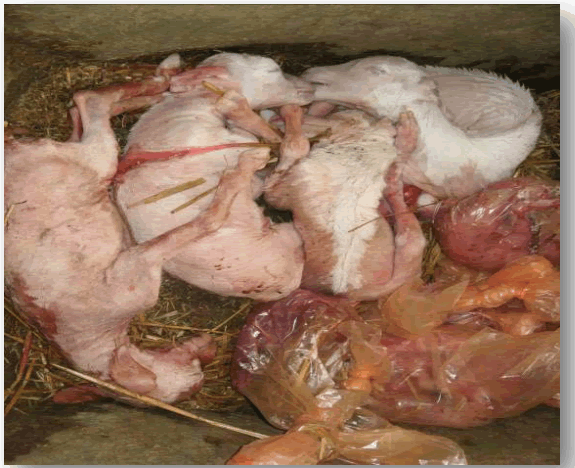
Infection in animals, Q fever is frequently asymptomatic. Sheep and goats may exhibit abortion, stillbirth, premature delivery, and delivery of weak offspring while cattle and camel may develop infertility, metritis, and mastitis. The organism is found in the blood, lungs, liver and spleen during acute experimental infection, whereas chronically infected animals persistently shed bacteria in their faeces and urine. Infection in most domestic animals remains unrecognized. Coxiellosis is considered a cause of abortion and reproductive disorders in domestic animals. Abortion rate is comparatively higher in ewes and goats than in cows. Abortion is usually observed in late pregnancy in both ewes and cattle (Figure 3).
Figure 3: Abortion during late gestation due to Coxiella burnetii infection in a small ruminant. Sources.
Coxiellosis produce both acute and chronic forms of clinical manifestations in humans. However, 60% infection remains asymptomatic with a few patients developing severe illness. The acute symptoms caused by infection with Coxiella burnetii usually develop within 2-3 weeks of exposure. Clinical signs of acute Q fever are nonspecific and vary among patients. A self-limiting febrile condition is the most frequent manifestation in clinical cases, which is accompanied by severe headaches, myalgia, arthralgia and a cough, general malaise, chills/sweats, non-productive cough, nausea, vomiting, diarrhea, abdominal pain and chest pain, however, it is important to note that the combination, duration and severity of symptoms vary greatly from person-to-person. Children with Q fever generally have a milder acute illness than adults. Although most persons with acute Q fever infection recover, others may experience serious illness with complications that include pneumonia, granulomatous hepatitis, and rarely myocarditis or central nervous system. A typical pneumonia is another common symptom of acute Q fever. Pneumonia is mild in most cases being characterized by a dry cough, fever, and minimal respiratory distress. Patients may also develop hepatitis with hepatomegaly, but without jaundice, subclinical hepatitis and granulomatous hepatitis with a prolonged fever. Pregnant women who are infected may be at risk for pre-term delivery, miscarriage, stillbirth or low infant birth weight. A prolonged fever, which may reach 39°C to 40°C, usually stays for 2 to 4 days and then gradually decreases to a normal level through the following 5-14 days. Chronic Q fever may develop from an acute infection. Possible predisposing factors are preexisting vascular grafts, cardiac valvulopathy, immunosuppression, and aneurysms.
Manifestations of chronic disease are most commonly endocarditis (culture-negative) in patients with underlying heart valve disease, or with prosthetic valves, vascular aneurysms or vascular grafts. Chronic hepatitis is another common feature, as is chronic infection during pregnancy, chronic fatigue syndrome and fever of unknown origin. More rare manifestations are osteomyelitis, pericarditis, meningitis, Guillain–Barre syndrome, osteoarticular infections with tenosynovitis and vertebral infections skin rash and chronic itch.
Diagnosis: Q fever diagnosis is only possible through laboratory testing. Serological tests can detect antibodies against phase I and phase II antigens of Coxiella burnetii, and distinguish acute from chronic disease. Coxiella burnetii has two different antigenic phases: Phase I and phase II. Such an antigenic difference is important in the diagnosis. In acute cases of Q fever, the titer of antibody against phase II is usually higher than phase I antibody. Acute disease is mostly diagnosed via an increase in the antibody titer within three to four weeks of the onset of the disease. In comparison, in chronic cases, the titer of antibody is higher against phase I compared to phase II.
This increase in the titer of antibodies against phases I and II may persist within months to years after the first infection of this disease. The clinical signs of Q fever are nonspecific both in human and animal because of this laboratory evidence of infection is needed for diagnosis. Four categories of diagnostic tests are available: Isolation of the organism, which must be conducted in a biosafety-level 3 laboratory using tissue-culture; laboratory animals, or embryonated eggs; serologic tests. A variety of indirect methods (serologic assays) have been used to detect Coxiella burnetii antibodies in animal serum samples, including Complement Fixation Test (CFT), Enzyme Linked Immunosorbent Assay (ELISA), Micro Agglutination test (MA), Indirect Immunofluorescence Assay (IFA) and Indirect Fluorescent Antibody Test (IFAT). The CFT is weakly sensitive and the antigen used in this test frequently fails to detect antibodies in sheep or goats. The ELISA is more sensitive than the CFT and is able to test a higher number of animals and flocks. PCR can be used to detect Coxiella burnetii DNA in a wide range of samples, including placenta tissues, faeces, vaginal mucus and milk. High level of specifity and sensitivity were acquired by PCR method applied with the primers consist INS of repetitive transposon like element [19]. Routine diagnosis of Q fever in animals is usually established by examination of fixed impressions or smears prepared from the placenta stained by the Stamp, Gimenez or Machiavello methods, associated with serological tests. It is stains poorly with pigmented Gram stain, but gimenez staining is traditionally used to stain the Coxiella burnetii pathogen from pathological materials and crop. In many countries, diagnosis of Q fever in domestic ruminants still relies mainly on Modified Ziehl Neelsen (MZN) stained smears of placental material from aborted fetuses, supplemented by Immunohistochemistry (IHC) where appropriate, although Polymerase Chain Reaction (PCR) is increasingly being used for disease confirmation in developed countries.
Differential diagnosis: There is some disease that we appreciate the same sign with Q fever such as Salmonellosis, Brucellosis, Leptospirosis, Campylobacteriosis, Listeriosis, Elective Abortion, Influenza, and Rickettsial Infection. At initial stages, i.e., before pulmonary symptoms are present, influenza may be suspected. Listeriosis is called circling disease, affected animal circle in one direction only and show swallowing, fever, blindness and head pressings. There is necrosis of placenta which leads to abortion and the fetus may be macerated or delivered weak and moribund, paralysis and death follow in 2 to 3 weeks later. Listerial abortion occurs in late gestation. Brucella is life longer infection it causes in female animal abortion around seventh month of pregnancy and retention of placenta and metritis are common and in male it causes orchitis, epididymitis, synovitis and sterility. Salmonellosis has sign like fever, dehydration and foul smelling diarrhea and cause abortion in the last two months of gestation. Leptospirosis is show sign like excessive salivation, muscular rigidity, conjunctivitis, hemoglobinuria, pallor of mucosa and jaundice. Leptospiral abortion occurs with or without placental degeneration and encephalitis, Abortion usually occurs 3-4 weeks later. Most affected animals are found dead, apparently from septicemia.
Treatments: Treatment is indicated for all infections, even for those that are subclinical. For domestic small ruminants’ oral therapeutic dose may be given for 24 weeks. However, most patients were treated with combination doxycycline-hydroxychloroquine. Two injections of oxytetracycline (20 mg per kg body weight) in the last trimester of pregnancy are usually recommended for animals, although this may not completely suppress abortions or stop bacterial shedding during parturitions. For human treatment of acute Q fever cases, a standard course of antibiotics belonging to the drug group’s tetracyclines (doxycycline, glycylcycline), macrolides (erythromycin, clarithromycin, roxithromycin) and quinolones (ciprofloxacin, ofloxacin, trovafloxacin) is recommended. Doxycycline is the most effective treatment for Q fever. Treatment is most effective if given within the first 3 days of symptoms, shortens the illness, and reduces the risk for severe complications. Other antibiotic regimens that can be used if doxycycline is contraindicated because of allergies include moxifloxacin, clarithromycin, trimethoprim/sulfamethoxazole, and rifampin. Long-term antibiotic therapy and cardiac surgery are recommended in order to treat chronic Q fever infection depending on the particular condition of each patient. New generation antibiotics are being applied in clinical trials. In some paediatric cases, gammainterferon is the treatment of choice. The recommended administration for human chronic Q fever is Doxycycline (100 mg per day) and Hydroxychloroquine (600 mg) for >18 months for adult, Trimethoprim and Sulfamethoxazole for >18 months for children. Treatment of Q fever in pregnancy is difficult as first line antibiotics (doxycycline, hydroxychloroquine and luoroquinolones) are contraindicated and Cotrimoxazole remains the only effective antibiotic. Administration of Cotrimoxazole probably prevents abortion but not the development of chronic infections or placental colonization. Trimoxazole treatment of pregnant women diagnosed with acute Q fever with once daily throughout pregnancy significantly decreases the risk of adverse consequences for the fetus. In adults 100 mg of doxycycline in every 12 hours and 200 mg of hydroxychloroquine in every 8 hours is indicated for Chronic Q fever. Standard duration of treatment is 18 months.
Control and prevention
The best methods available for control and prevention of Coxiellosis are antibiotic treatment and vaccination. In the case of acute Q fever in humans, doxycycline is the antibiotic of choice. For chronic Q fever, long-term treatment with doxycycline and hydrochloroquine is recommended. Although thorough evaluations of these therapies are lacking, they are considered to be effective. Control options can be divided into four main groups: 1) Measures to identify infected farms; 2) Measures to reduce excretion of Coxiella burnetii; 3) Measures to reduce the dispersion of Coxiella burnetii and 4) Measures to reduce human exposure.
A prerequisite for this is the availability of adequate diagnostic tests and awareness of the presence of the disease by general practitioners and veterinarians. Notification criteria may vary per-country, but notification of abortions is always one of the criteria. Measures to reduce the excretion of Coxiella burnetii are important for the control of human Q fever as well as controlling Q fever on the farm level. Vaccination with a phase 1 vaccine is effective in reducing abortions as well as the excretion of Coxiella burnetii. Modeling studies on the effectiveness of control measures suggest that vaccination is the most effective long-term intervention to prevent excretion of Coxiella burnetii on goat farms. The phase I vaccine Coxevac has been effective in decreasing abortion rates and bacterial load in vaginal mucus, feces, and milk in goats. Vaccination is also effective in preventing shedding of Coxiella burnetii in infected dairy cattle herds.
At human level, prevention of exposure to animals or wearing gloves, boots, and masks during manipulation of animals. Pasteurization at 145°F (63°C) for at least 30 minutes or 161°F (72°C) for 15 seconds sterilization is sufficient to destroy Coxiella burnetii, as well as other pathogens that can be present in raw milk. Vaccination may also be considered in livestock handlers, processors of animal products, veterinarians and laboratory workers likely to handle infected specimens. Coxiella burnetii is able to survive for long periods in the environment and in wild animals. The only way to really prevent the disease in ruminants is to vaccinate uninfected flocks, with an efficient vaccine. Three types of vaccine have been proposed for providing human protection against Q fever: The attenuated live vaccine (produced and trialled in Russia but subsequently abandoned because of concern about its safety); chloroform methanol residue extracted vaccine or other extracted vaccines (trialled in animals but not humans); and the whole cell formalin inactivated vaccine, which is considered acceptably safe for humans.
Coxiella burnetii can be reduced in the farm environment by regular cleaning and disinfection of animal facilities, with particular care of parturition areas, using 10% sodium hypochlorite. In the UK, Health Protection Agency guidelines mention the use of 2% formaldehyde, 1% lysol, 5% hydrogen peroxide, 70% ethanol, or 5% chloroform for decontamination of surfaces. Appropriate tick control strategies and good hygiene practice can decrease environmental contamination. Infected fetal fluids and membranes, aborted fetuses and contaminated bedding should be incinerated or buried. In addition, manure must be treated with lime or calcium cyanide 0.4% before spreading on fields; this must be done in the absence of wind to avoid spreading of the microorganism faraway. In feed addition of tetracycline or injectable oxytetracycline prepartum has not been shown to prevent Coxiella burnetii shedding in feces, milk and vaginal secretions. Preventive vaccination, manure management including covering and compositing of manure or treating manure with lime, better livestock farm and wool shearing practices, use of isolated calving pens, restrictions on free animal movement, and proper disposal and burial of aborted materials are important measures to prevent the spread of Coxiella burnetii infection. Hygienic practices, especially calving pen cleanliness, is considered an important measure in preventing this infection. Similarly, disinfection of calving pens, naval cord disinfection, and proper disposal of aborted fetuses and fetal membranes, and provision of new bedding at the time of calving are important measures to reduce the risk of disease transmission. Birth products including fetal membranes and dead fetuses should immediately be disposed to avoid their ingestion by stray dogs, wild carnivores and even domesticated animals, which may also spread the infection in the environment.
Discussion
Status of Q fever in Ethiopia
According to a few studies conducted in Ethiopia, 6.5% of abattoir workers in Addis Ababa had Coxiella burnetii on their bodies. Additionally, sheep and goats killed at the Addis Abeba abattoir and its per-urban areas were found to have antibodies against Coxiella burnetii. A seroprevalence of 31.6%, 90%, and 54.2% of Coxiella burnetii was recorded in cattle, camels and goats respectively in South Eastern Ethiopian pastoral zones of the Somali and Oromia regional states. Ticks were tested for Coxiella burnetii in Ethiopia by quantitative real time polymerase chain reaction targeting two different genes followed by Multispacer Sequence Typing (MST). An overall prevalence of 6.4% of Coxiella burnetii was recorded. Coxiella burnetii was detected in 28.6% of Amblyomma gemma, 25% of Rhipicephalus pulchellus, 7.1% of Hyalomma marginatum rufipes, 3.2% of Amblyomma variegatum, 3.1% of Amblyomma cohaerens, 1.6% of Rhihipicephalus praetextatus, and 0.6% of Rhipicephalus (Boophilus) decoloratus. Significantly higher overall frequencies of Coxiella burnetii DNA were observed in Amblyomma gemma and Rhihipicephalus pulchellus than in other tick species. It also seroprevalence of 6.17 and 11.79 Coxiella burnetii was reported in dairy farms and slaughterhouse respectively in Jimma town, South Western Ethiopia. Abortion is one of the most important reproductive health problems of dairy cows in Ethiopia in terms of economic impact. Both infectious and non-infectious agents may cause abortion in cattle. Q fever is one of infectious disease which causes abortion in Ethiopia.
Public health importance
Human population is at high risk of acquiring emerging infectious diseases particularly those of zoonotic nature. Among zoonotic diseases, Q fever is of great significance with special reference to human public health. The causative agent of Q fever is Coxiella burnetii which is a gram-negative bacterium. It is highly infectious for risky groups including veterinarians, laboratory workers, farmers and abattoir workers. Surveys have shown that significant numbers of livestock handlers have antibodies indicating exposure to the organism. Less than half of people infected become ill, and most infections are mild. But affected persons can develop a high fever with headache, muscle pains, sore throat nausea and vomiting, chest and stomach pains. The fever can last for one or two weeks, and lead to pneumonia or affect the liver. People with suppressed immune systems and those with pre-existing heart valve problems are at risk of this complication, which is often fatal. There is also a post Q fever syndrome of chronic fatigue. Q fever is the second most commonly reported laboratory infection with several recorded outbreaks involving 15 or more persons. Human infection occurs due to inhalation of dust contaminated by infected animal fluids, consumption of unpasteurized dairy products and contact with milk, urine, faeces, vaginal mucus or semen of infected animals. In humans, initial exposure to Coxiella burnetii may result in asymptomatic or mild infection but also in acute or chronic disease. The clinical diagnosis can be very difficult. The reasons for this high clinical polymorphism are largely unknown, even if risk factors of severity (Example: Pregnancy, immunosuppression, preexisting cardiac valvulopathy, vascular grafts, and aneurysms) have been described. Although rarely fatal, the disease may lead to substantial morbidity and can be highly debilitating, even under treatment.
Conclusion
In general, Q fever is a global health problem, an OIE-notifiable disease, and an infectious disease that is considered to have economic and public health importance. The only country not affected by this bacterium is New Zealand. It is the second most typical laboratory infection to be reported. Farm animals like cattle, goats, and sheep are the most frequently discovered causes of illnesses in humans. Wild animals and pets have both been shown to be potential sources of disease outbreaks. For both humans and animals, infected dust from faces, urine, milk, and ticks is the main cause of infection. Because Coxiella burnetii is so resistant to environmental factors and chemicals, it can endure in the environment for months. Through direct contact with animal reproductive products and aerosol transmission, the causal agent is conveyed to humans and other animals. It is advised to get vaccinated against Q fever and use tetracycline-group medications. It can be controlled by using insecticides for ticks, and following appropriate hygiene practices to avoid the spread of infectious diseases is covered in this seminar.
Recommendations
Therefore, based on the above conclusion the following recommendations are forwarded:
- Inform the public about potential illness sources and only consume pasteurized milk and milk products.
- It is advised to immunize people who are occupationally exposed, including veterinary professionals, livestock handlers, and abattoir workers.
- The government ought to vaccinate any animals discovered in an endemic area.
- Inform the public for using insecticide to control ticks.
References
- Abnave P, Muracciole X, Ghigo E (2017) Coxiella burnetii lipopolysaccharide: What do we know?. Int J Mol Sci 18: 2509.
[Crossref] [Google scholar] [PubMed]
- Agerholm JS (2013) Coxiella burnetii associated reproductive disorders in domestic animals-a critical review. Acta Vet Scand 55: 1-11.
[Crossref] [Google scholar] [PubMed]
- Ahmed A, Ijaz M, Ayyub RM, Ghaffar A, Ghauri HN, et al. (2020) Balantidium coli in domestic animals: An emerging protozoan pathogen of zoonotic significance. Acta Tropica 203: 105298.
[Crossref] [Google scholar] [PubMed]
- Anderson A, Bijlmer H, Fournier PE, Graves S, Hartzell J, et al. (2013) Diagnosis and management of Q fever-United States, 2013: Recommendations from CDC and the Q fever working group. MMWR Recomm Rep 62: 1-30. [Crossref]
[Google scholar] [PubMed]
- Angelakis E, Raoult D (2010) Q fever. Vet Microbiol 140: 297-309.
- Angelakis E, Million M, D’amato F, Rouli L, Richet H, et al. (2013) Q fever and pregnancy: disease, prevention, and strain specificity. Eur J Clin Microbiol Infect Dis 32: 361-368.
[Crossref] [Google scholar] [PubMed]
- Basanisi MG, La Bella G, Nobili G, Raele DA, Cafiero MA, et al. (2022) Detection of Coxiella burnetii DNA in sheep and goat milk and dairy products by droplet digital PCR in south Italy. Int J Food Microbiol 366:109583.
[Crossref] [Google scholar] [PubMed]
- Bond KA, Vincent G, Wilks CR, Franklin L, Sutton B, et al. (2016) One Health approach to controlling a Q fever outbreak on an Australian goat farm. Epidemiol Infect 144: 1129-1141.
[Crossref] [Google scholar] [PubMed]
- Bontje DM, Backer JA, Hogerwerf L, Roest HI, Van Roermund HJ (2016) Analysis of Q fever in Dutch dairy goat herds and assessment of control measures by means of a transmission model. Prev Vet Med 123: 71-89.
[Crossref] [Google scholar] [PubMed]
- Bosnjak E, Hvass AM, Villumsen S, Nielsen H (2010) Emerging evidence for Q fever in humans in Denmark: role of contact with dairy cattle. Clin Microbiol Infect 16: 1285-1288.
[Crossref] [Google scholar] [PubMed]
- Bruschke C (2010) Actual veterinary situation and developments in the Netherlands. One health in relation to Q fever in humans and animals. Ministry of Economic Affairs, Agriculture and Innovation, Breda, the Netherlands.
- Carcopino X, Raoult D, Bretelle F, Boubli L, Stein A (2009) Q Fever during pregnancy: A cause of poor fetal and maternal outcome. Ann N Y Acad Sci 1166: 79-89.
[Crossref] [Google scholar] [PubMed]
- Carcopino X, Raoult D, Bretelle F, Boubli L, Stein A (2007) Managing Q fever during pregnancy: The benefits of long-term cotrimoxazole therapy. Clin Infect Dis 45: 548-555.
[Crossref] [Google Scholar] [PubMed]
- Centers for Disease Control and Prevention (2017) Q Fever. Atlanta, GA: U.S. Department of Health and Human Services.
- Ceylan E, Berktas M, Keles I, Agaoglu Z (2009) Seroprevalence of Q fever in cattle and sheep in the east of Turkey. Asian J Anim Vet Adv 4.
- CFSPH (2017) Centre for food security and public health, Q fever: Query fever, Coxiellosis, Abattoir fever, Iowa state university, collage of veterinary medicine.
- Chang CC, Lin PS, Hou MY, Lin CC, Hung MN, et al. (2010) Identification of risk factors of Coxiella burnetii (Q fever) infection in veterinaryâ?ÂÂÂassociated populations in southern Taiwan. Zoonoses Public Health 57: e95-e101.
[Crossref] [Google Scholar] [PubMed]
- Chiu CK, Durrheim DN (2007) A review of the efficacy of human Q fever vaccine registered in Australia. N S W Public Health Bull 18: 133-136.
[Crossref] [Google Scholar] [PubMed]
- Clark NJ, Soares Magalhaes RJ (2018) Airborne geographical dispersal of Q fever from livestock holdings to human communities: A systematic review and critical appraisal of evidence. BMC Infect Dis 18: 1-9.
[Crossref] [Google Scholar] [PubMed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi