Review Article, J Infect Dis Immune Ther Vol: 1 Issue: 1
Role of Acanthamoeba in Granulomatous Encephalitis: A Review
Shalini Dewan Duggal1, Sharon Rainy Rongpharpi1, Ashish Kumar Duggal2*, Avinash Kumar1 and Indu Biswal3
1Department of Microbiology, Dr Baba Saheb Ambedkar Hospital, Rohini, Delhi, India
2DM Neurology, Department of Neurology, Gobind Ballabh Pant Hospital, Delhi, India
3Faculty Senior Resident, Department of Microbiology,VMMC and Safdarjung Hospital, Delhi, India
*Corresponding Author : Dr Ashish Kumar Duggal
DM Neurology, Assistant Professor, Department of Neurology, Gobind Ballabh Pant Hospital, Delhi, India
Tel: 009810523332
E-mail: ashishduggal2005@rediffmail.com
Received: November 30, 2017 Accepted: December 13, 2017 Published: December 20, 2017
Citation: Duggal SD, Rongpharpi SR, Duggal AK, Kumar A, Biswal I (2017) Role of Acanthamoeba in Granulomatous Encephalitis: A Review. J Infect Dis Immune Ther 1:1.
Abstract
Amoebic encephalitis is an infrequently encountered, fatal infection of the central nervous system (CNS) seen mostly in immunocompromised individuals and rarely in immunocompetent. The mechanisms associated with the pathogenesis of GAE remains undefined, however various hypothesis have been suggested. Diagnosis is based on neuroimaging, microscopy, cerebrospinal fluid (CSF) culture, serology, and histopathology and recently by Molecular methods. Combination drug therapy along with immunotherapy has been tried in most cases despite poor outcome. Vaccine against Acanthamoeba infections is still in infancy. Research should be focused to convert these ‘Trojan horses’ of infections into ‘Caravans’ of disease prevention.
Keywords: Amoebic encephalitis; Serology; Immunotherapy
Introduction
Acanthamoeba are free-living amoebae which have gained significant interest over the years due to their ability to produce serious human infections in immunocompromised patients and contact lens wearers, their role in a variety of ecosystems, ability to act as a host/reservoir for microbial pathogens, and as a model organism for motility studies [1]. These are mitochondria-bearing aerobic and amphizoic protozoa [2]. Acanthamoeba spp. are amphizoic as they may exist as free-living or as saprobes or parasites in different animals.
History
In 1930, Castellani discovered Acanthamoeba as a contaminant in the culture of a yeast Cryptococcus pararoseus and placed it in the genus Acanthamoeba [3]. These were ignored as contaminants for the next three decades till in 1958, Culbertson demonstrated its pathogenic potential by demonstrating cytopathic effects in monkey kidney cell cultures [4]. The first proven case of granulomatous amoebic encephalitis (GAE) in humans was observed by Jager & Stamm in 1972 [5] while the first case of Acanthamoeba keratitis was reported in 1974 [6].
Classification
Acanthamoeba has been classified under Super Group Amoebozoa of the new schema of the Eukaryotes. It belongs to the Centramoebida clade of Class Discosea of the subphylum Lobosa. Lobosa consists of non flagellate lobose amoebae; those with tube-shaped pseudopodia are grouped in class Tubulinea while those with flattened cells and different means of locomotion are grouped in class Discosea [7]. More than 24 species of Acanthamoeba have been identified based on morphology. Acanthamoeba species most commonly associated with human infections are A. castellanii, A. culbertsoni, A. hatchetti, A. healyi, A. astroonyxix, A. divionensis and A. polyphaga. These species were initially categorised into three groups based on their morphology and cyst size. Later identification was done on the basis of sequencing of the amoebae 18S rRNA genes and 17 different genotypes (T1–T17) of Acanthamoeba were established. Each genotype exhibits 5% or more sequence divergence between different genotypes. Majority of human infections have been associated with the T4 genotype due to their greater virulence and/or properties that enhance their transmissibility as well as their decreased susceptibility to chemotherapeutic agents [8]. The genotypes commonly associated with encephalitis include T1, T4, T10 and T12 [2].
Structure
The genus Acanthamoeba comprises of several species of freeliving amebae. The term ‘acanth’ is a greek word which means “spikes” to indicate the presence of spine-like structures (also known as acanthopodia) on the surface of the amoeba. It contains one or more prominent contractile vacuoles to expel water for osmotic regulation. Other types of vacuoles in the cytoplasm include lysosomes, digestive vacuoles and a large number of glycogencontaining vacuoles. Mitochondria provide them the energy required for locomotion, feeding, reproduction and other metabolic and cellular activities. The plasma membrane consists of proteins (33%), phospholipids (25%), sterols (13%), and lipophosphonoglycan (29%). The major phospholipids in Acanthamoeba are phosphatidylcholine (45%), phosphatidylethanolamine (33%), phosphatidylserine (10%), phosphoinositide (6%), and diphosphatidylglycerol (4%). The main fatty acids chains in Acanthamoeba are oleic acids (40-50%), and longer polyunsaturated fatty acids (20-30%) [1].
Ecology and Habitat
Acanthamoeba has the ability to tolerate a wide range of temperature, osmolarity and pH conditions, which allows them to survive in distilled water, tissue culture, mammalian body fluid. It can also survive at 37OC and at higher body temperatures. Acanthamoeba can be isolated from diverse habitats like soil, sewage, air-conditioning units, hot tubs and fresh water ponds; in domestic areas like kitchen and bathroom drains and faucets, home humidifiers, ventilating and air conditioning units, cooling towers, aquarium. They have also been isolated from hospital settings [9,10] where they may contaminate contact lens solution, hospital hydrotherapy or physiotherapy pools, dialysis machines, dental units, dental irrigation systems, eye wash stations and gastrointestinal washings. Thus they can be causes of Hospital associated infections especially in the immunocompromised.
Human exposure to Acanthamoeba
Because of the wide spread distribution of Acanthamoeba, human contact with the organism is inevitable and frequent. In a study, majority of healthy individuals were shown to possess anti- Acanthamoeba antibodies in titers varying from 1:20 to 1:80 [11]. They are responsible for opportunistic and non-opportunistic infections in humans and other animals. They have also been isolated from swabs obtained from nasopharyngeal mucosa of patients with respiratory complaints as well as healthy individuals, ear discharge, pulmonary secretions, mandibular autografts, and stool samples. They have also been isolated in 2-24% in human paranasal sinuses [10].
Epidemiology
Amoebic encephalitis is an infrequently encountered serious infection of the central nervous system (CNS) seen mostly in immunocompromised individuals. It is caused by free-living amebic organisms and is broadly divided into granulomatous amebic encephalitis (GAE) and primary amebic meningoencephalitis (PAM) [12]. GAE is a subacute to chronic infection caused by Acanthamoeba spp. and also by Balamuthia mandrillaris organisms, unlike primary amoebic encephalitis caused by Naegleria fowleri which is acute in onset, rapidly progressive, resulting in fatality within 48-72 h. The true incidence of Acanthamoeba GAE is difficult to ascertain due to limited diagnostic expertise, lack of adequate resources for diagnosis, lack of proper monitoring, lack of proper healthcare systems and low autopsy rates [13]. The incubation period of this disease is not known and is postulated to be more than 10 days. The disease occurs in the 2nd and 4th decades and 10 times more in men [14]. More than 400 cases have been reported in world literature with only two to three percent survival. There are reports from India where acanthamoebae were either demonstrated in direct CSF or on autopsy but there are very few reports where these have been successfully cultured from CSF [15]. There are also reports of cases of GAE from northern [14] and southern India [16]. Acanthamoeba encephalitis has been reported from a 63-year-old female in India where acanthamoebae were demonstrated and cultured from the CSF [17,18]. Based on the total number of GAE deaths and the total deaths in HIV/ AIDS patients, the approximate rate was calculated as 1.57 GAE deaths per 10 000 HIV/AIDS deaths in the USA [2]. Acanthamoeba infections in persons with lupus erythematosus who were being treated with corticosteroids have been reported [19]. Corticosteroids, besides reducing inflammation, impair the immune response, facilitating infection and disease caused by Acanthamoeba [20].
Risk factors
Risk factors for acquisition of infection include acquired immune deficiency syndrome (AIDS), steroids, Immunosuppressive therapy or prolonged and excessive use of antibiotics, organ/ tissue transplantation, diabetes mellitus, malignancy, haematological disorders or malignancies, Chronic alcoholism, liver cirrhosis, lupus, malnutrition, pregnancy, surgical trauma, burns, wounds, and radiation therapy [21].
Life Cycle
It has two stages during its life cycle: a vegetative or trophozoıte stage (8-40 mm) and a dormant cyst stage (8– 29 mm). Trophozoıtes are characterized by the presence of a single nucleus and a fine acanthopodia projecting outward from the surface of the body. The reproduction is by binary fission. The acanthopodia allows parasite to adhere to surfaces and capture it’s prey by cellular movements. Trophozoıte feed on bacteria, algae, yeast or small organic particles. The infectious and invasive form cannot survive for a long time on an adverse environmental condition and differentiate into uninucleate cyst with a double-walled structure (endo- and ectocyst). During encystment, excess matter including RNA, proteins, triacylglycerides and glycogen is expelled. The trophozoite condenses itself into a rounded structure, a. precyst which further matures into a double-walled cyst [2]. Factors affecting encystations are adverse environmental stimuli like differences in pH, osmotic pressure, temperature conditions, and nutritional requirements. It is protected from desiccation, starvation and a variety of chemical and physician agents. A study found that conditions triggering encystment in the Neff strain of Acanthamoeba, included osmolarity, starvation and increase in pH. Several surface binding monoclonal antibodies and MgCl2 have also been shown to induce encystment [22]. Acanthamoeba can resist the effect of chemotherapeutic drugs by differentiating into cysts, further adding to its pathogenic potential [1]. Cysts have been known to survive in vitro for greater or equal to 20 years. This resistant form possesses pores known as ostioles, which are used to monitor environmental changes. With return of optimal growth conditions, the cysts germinate to give rise to trophic forms leaving behind the outer shell [8]. Acanthamoeba trophozoites can both grow and divide producing more vegetative cells, or, alternatively they may cease dividing and encyst, forming a cellulose-containing cyst wall around a resting stage cell, until conditions allowing excystment and replication return [22,23]. Acanthamoeba causes intense necrosis but it is not known whether this necrotic phase is caused directly by trophozoites or by release of cytokines and other enzymes.
Pathogenesis of Acanthamoeba Encephalitis
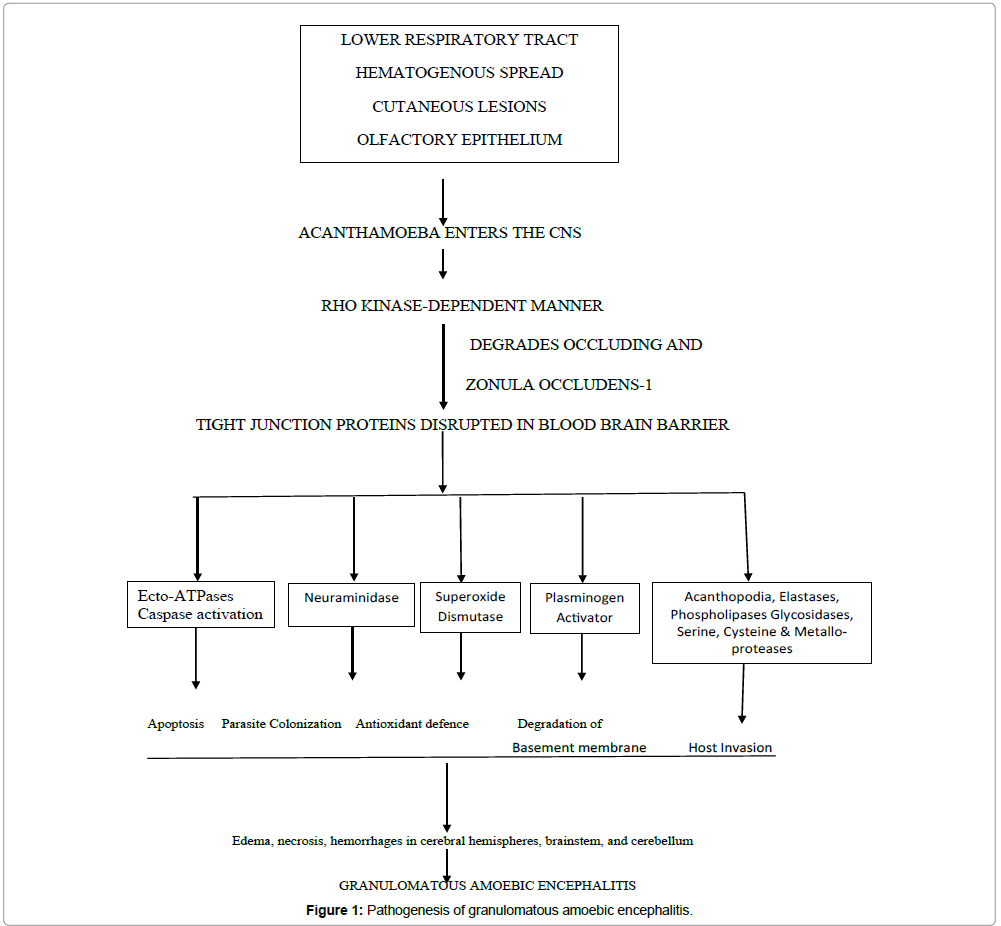
The mechanisms associated with pathogenesis of GAE remain undefined. However, the pathological complications involving the CNS most likely include induction of the pro-inflammatory responses, invasion of the blood-brain barrier and the connective tissue and neuronal damage leading to the brain dysfunction [24-28]. The routes of entry include lower respiratory tract leading to amoebae invasion of the intravascular space, followed by the haematogenous spread. Amebae may enter the body through breaks in the skin, resulting in hematogenous dissemination to the brain and other organs. In addition, olfactory neuroepithelium provides another route of entry into the CNS [29]. In a series of experiments, intranasal instillation of acanthamoebae was done in laboratory mice. In one group, the immune response was suppressed by administration of corticosteroids or tetracycline. In control animals, only acanthamoebae were administered. The control group showed 10% mortality as compared to 50-60% mortality observed in corticosteroid/ tetracycline treated mice in whom acanthamoebae were isolated from various organs of the body [30]. Following CNS invasion, amoebae penetrate the brain tissue to produce disease. Amoebae entry into the CNS most likely occurs at the sites of the blood-brain barrier. Major virulence factors are adhesion, phagocytosis, plasminogen activation, acanthaporin proteins, enzymes like Ecto-ATPases, euraminidases, superoxide dismutases, elastases, phospholipases, proteases and glucosidases [31]. The mannose basic protein (MBP), a 130 kDa protein the parasite expressed on the surface of Acanthamoeba has been implicated in initiating Acanthamoeba keratitis by adhering to the host cell [32]. This protein may also have a role in GAE by acting as an adhesin against human brain microvascular endothelial cells [33]. Only the trophozoites express MBP whereas cysts lack MBP, therefore cannot bind to the host cells. Following adhesion to the mannose binding receptor on surface of brain microvascular endothelial cells, the Rho-associated intracellular signalling cascade is activated leading myosin light chain phosphorylation which disturb the function of tight junctions and cause increased permeability of blood brain barrier. Acanthamoeba also releases serine proteases which induce degradation of the tight junction proteins ZO-1 and occludin, type I, III, IV collagen, elastin, fibronectin (main component of extracellular matrix), IgG, IgA, Hb leading to increased permeability of the basement membrane [34]. Other factors that may contribute to Acanthamoeba pathogenesis include ecto-ATPases [35] and caspase-3 activation [35]. The neuraminidase activities of Acanthamoeba could be relevant in the colonization of the parasite, causing alterations of glycolipids associated with meningoencephalitis (Figure 1). Two superoxide dismutases have also been identified in Acanthamoeba; an iron superoxide dismutase (~50 kDa) and a copper-zinc superoxide dismutase (~38 kDa). The superoxide dismutase catalyzes the dismutation of superoxide into oxygen and hydrogen peroxide, thus helping in antioxidant defence. Another study has suggested that Acanthamoeba possesses plasminogen activator activity which catalyzes the cleavage of host plasminogen to form plasmin. Plasmin in turn activates host matrix metallo-proteinases leading to degradation of the basement membranes. Some hydrolytic enzymes like elastases, phospholipases, glycosidases and a variety of serine, cysteine and metalloproteases also cause disease by host cell and tissue invasion, migration, catabolism of host proteins, cytoadherence, and evasion of host responses [2,27,37]. Host complement pathways and the antibodies together with neutrophils and macrophages show potent amoeba lytic activities, thus suppressing infection [2]. Acanthamoeba induces cell cycle arrest by inhibiting pRb phosphorylations in human brain microvascular endothelial cells [2]. There is a multi-layered microtubule organising centre in Acanthamoeba helps in organising the acanthopodia. Acanthamoeba induces host cell phagocytosis by means of amebostome like specialized food cups and production of different proteases to digest extracellular matrix of the nervous tissue [38]. The plasma membrane of Acanthamoeba accumulates Ca2+ ions in abundance. Host cell death may also be caused by an increase in intracellular calcium when the Acanthamoeba injects it through its acanthopodia [39].
Other Acanthamoeba Infections in the Immunocompromised
Acanthamoeba infections remain undiagnosed or poorly diagnosed in immunocompromised patients due to more focus on diagnosis of fungal and bacterial diseases. In these patients, besides brain, the amoebae can disseminate to internal organs like liver, kidneys, trachea, or adrenals, pancreas, prostate, lymph nodes and bone marrow [2]. Acanthamoeba pneumonitis has also been reported where trophozoites and cysts were found in pulmonary alveoli [27]. Leukocytoclastic vasculitis, amebic osteomyelitis, and endophthalmitis due to Acanthamoeba have also been reported in AIDS patients [40].
Immunology-Host Immune Defences against Acanthamoeba
Amoebae are recognised by the neutrophils, macrophages, and probably lymphocytes. The B-cells are stimulated to produce immunoglobulins which further stimulate the neutrophils to release lysosomal enzymes and reactive oxygen intermediates, producing interleukins (IL-1α and β, and tumor necrosis factor), which together with macrophages bring about the destruction of amebic trophozoites [26,29]. The ability of microglia to exhibit antimicrobial properties is also mediated by the generation of free radicals, i.e. nitric oxide (NO), and production of pro-inflammatory and anti-inflammatory cytokines. This along with stimulation of helper and cytotoxic T-cells results in the formation of granulomas [10]. IFN-γ is one of the earliest cytokines to be involved and may play an important role in the activation of immune cells. IFN-γ, through the pro-inflammatory network, upregulates the release of specific cytokines, including TNF-α, IL-6, IL-1β and IL-1α, which may initiate the immune response to Acanthamoeba in the brain. Other studies showed that microglial cells also secrete IL-1β, IL-1α and TNF-α in response to the parasite. Microglia primed with IFN-γ and TNF-α also exhibit amoebicidal effects [40,41]. The alternative complement pathway is activated ultimately to cause rupture of the cell.
Clinical Features
Free-living amebae can cause three types of disease in humans: amoebic keratitis, primary amoebic meningoencephalitis (PAM), and granulomatous amoebic encephalitis (GAE). Primary Amoebic Meningoencephalitis is caused by Naegleria fowleri, while granulomatous amoebic encephalitis is caused by Acanthamoeba species and Balamuthia mandrillaris. GAE caused by Acanthamoeba spp. usually occurs in chronically ill, debilitated individuals, in immunosuppressed patients including those who have HIV/ AIDS, or in those who have received broad-spectrum antibiotics or chemotherapeutic medications [42-45]. Use of TNF- antagonist agents may also be a predisposing factor for GAE [45]. Unlike PAM, cases of Acanthamoeba GAE can occur at any time of the year and bear no relation to fresh water exposure. Cysts or trophozites enter via the respiratory system or through the skin followed by hematogenous dissemination and subsequent invasion of the central nervous system (CNS). Incubation period is not known but is probably weeks to months. GAE due to Acanthamoeba species almost always has a subacute or chronic presentation and symptoms frequently resemble those of single or multiple space occupying CNS lesions. GAE may mimic bacterial leptomeningitis, tuberculous or viral meningitis. Onset is usually insidious with headache, nausea, irritability, dizziness and low grade fever. Neurological symptoms and signs will depend on area(s) of the brain involved. Altered mental state is predominant, they may also present with seizures, focal neurologic signs, diplopia, cranial nerve palsies, ataxia, confusion, and personality changes [2,13,26-29,38]. GAE is a progressive disease and death ensues within one to two months of symptom onset often from herniation of the brain due to increased intracranial pressure.
Diagnostic Methods
Neuroimaging
Brain image analyses can be done by using computed tomography (CT) or magnetic resonance imaging (MRI) scans. CT usually shows single or multiple enhancing lesions involving the cerebral cortex, basal ganglia, subcortical white matter, cerebellum, or pons with mild mass effect [46,47]. MR imaging may reveal multifocal lesions showing T2 hyperintensity and a heterogeneous or ring like pattern of enhancement, with a predilection for the diencephalon, thalamus, brain stem, and posterior fossa structures [48,49]. Intralesional hemorrhage is considered an important diagnostic clue by some authors and can be explained by the necrotizing angiitis described in severe cases of GAE [50].
Microscopy
CSF findings are non-specific. There is usually pleocytosis with lymphocytic predominance with elevated numbers of polymorphonuclear leukocytes. There may be increased protein concentration, normal or decreased glucose concentration and minimal cloudiness [27]. For direct microscopic observation of amoebae in the CSF, it 2-3 ml of CSF should be centrifuged at 250 g for 10 minutes to avoid deformation or rupture of amoebae [39]. They are frequently mistaken for monocytes, polymorphonuclear leukocytes or macrophages. Hence, they should be patiently looked for movement by pseudopodia but here again they may be mistaken for reactive macrophages. Diagnosis of Acanthamoeba should be suspected in an immunocompromised patient with absence of fungal and bacterial pathogens on the stained smear examination.
CSF culture
A few drops of the CSF and/or brain biopsy material should be inoculated on solid media for amoebic culture. Acanthamoeba feed on bacteria, yeasts, other protozoa and organic particles as a food source, so any of these can be used as substrates to promote growth of Acanthamoeba in the laboratory [2]. Non-nutrient agar should be used as solid media which contains minimal nutrients and thus inhibit the growth of unwanted organisms. Late log phase cultures of Gramnegative bacteria (Escherichia coli or Enterobacter aerogenes) are inoculated onto the culture media and incubated at 37ºC. Depending on the number of amoebae present in the specimen, trophozoites can be observed within a few hours (up to 12 h) to upto 7 days [51]. The agar culture plate can be examined under 10x of light microscope in which tracks formed by the organism and the dividing trophozoites can be seen while round double walled cysts with polyhedral inner cyst wall can be seen in the zone of clearing [52]. For subculture, a 1 cm × 1 cm agar block can be cut with a sterile blade from the zone of clearing and inoculated on another plate of non-nutrient agar with E. coli lawn cultures. Acanthamoeba spp. can also grow on Blood agar, Chocolate agar but the tracks and the clearing produced on these media can be easily overlooked without microscopic examination of the plates. It should be noted that if the patient is on antibiotics the CSF inoculated on the agar plate may inhibit the bacterial lawn and produce a zone of inhibition which can be easily mistaken to be due to these amoebae, however microscopic examination confirms their absence. Acanthamoeba can be grown ‘axenically’ in the absence of external live food organisms, though some bacteria may still be present as endosymbionts. Examples of axenic media include liquid PYG medium [proteose peptone 0.75% (w/v), yeast extract 0.75% (w/v) and glucose 1.5% (w/v)] or Nelson’s medium containing fetal calf serum or brain extract. Axenically cultivated amoebae cannot be utilized for virulence studies as loss of virulence has been reported in serial cultivations. For virulence studies, these must be passaged in the animal models like mice, monkeys, Locusta, Drosophila,etc. [39] They can also be cultured on mammalian cell lines like E6 monkey kidney cells, human embryonic lung cells. It produces characteristic cytopathic effects, therefore initially presumed to be a virus.
Serology
Diagnosis should not be solely based on the presence of Acanthamoeba-specific antibodies in the patient’s serum since healthy population may react to specific antigens in low titers as mentioned before. The demonstration of high levels of Acanthamoeba-specific antibodies in the patient’s serum may be useful in diagnosis of suspected GAE infection. Indirect immunofluorescence (IIF) assays are available in which serial dilutions of the patient’s serum are incubated with fixed amoebae-coated slides followed by incubation with fluorescein isothiocyanate (FITC)-labelled antihuman antibody and visualization under a fluorescence microscope. Complement fixation tests and Amoeba immobilization assay have also been tried. It should be noted however that serology may not be helpful in patients of GAE who are usually immunocompromised.
Histopathology
In GAE, the lesions are most numerous in the basal ganglia, midbrain, brainstem and cerebral hemiparesis, with characteristic lesions in the CNS parenchyma. On histopathological examination of frozen or paraffin-embedded sections of brain, affected areas show necrosis and hemorrhage, encephalomalacia, fibrinoid necrotizing panarteritis, thrombosis, perivascular centrifugal granulomas, polymorphonuclear and lymphoplasmacytic inflammatory infiltrate, foamy macrophages and multinucleated giant cells. Trophozoites and cysts are usually scattered in the vascular wall and in areas with and without inflammation [10]. The brain biopsy tissue can be stained with Giemsa-Wright, acridine orange or calcofluor white stain to facilitate identification of these amoebae. Immunohistochemistry can help in more specific identification where chromogen tagged specific anti-acanthamoeba antibodies are used to react with the tissue samples [2].
Molecular methods of diagnosis
Molecular techniques such as PCR and real-time PCR have also been used recently to identify Acanthamoeba in the CSF. Acanthamoeba DNA can be identified in the CSF where it can help in establishing the diagnosis of GAE even if amebae are not found [19]. A newer type of PCR, f-d-real-t PCR could detect and quantify ten different genotypes of Acanthamoeba ATCC strains [53]. To establish relatedness among isolates, typing methods like Isoenzyme electrophoresis or restriction fragment length polymorphism (RFLP) of mitochondrial or genomic DNA can be used. More recently the cytochrome oxidase gene, subunit-1 (COI gene) has been identified. It can determine the genus, genotype in the sample and utilizes a single primer for detection [54]. Though many PCR-based methods have been developed but microscopy and culture still remain the methods of choice.
Differential Diagnosis
The differential diagnosis of GAE includes infarcts from septic emboli, abscesses, toxoplasmosis, fungal granuloma, or neoplastic lesions. Solitary space-occupying lesions mimicking low-grade glioma or lymphoma have also been described in GAE [50,55]. Based on neuroimaging findings, amoebic meningoencephalitis can also be erroneously diagnosed as neurocysticercosis [56]. Organisms in tissue sections can be mistaken for yeast forms of Blastomyces dermatiditis, sporangia of Rhinosporidium seeberi, Cryptococcus neoformans, or Prototheca wickerhamii [27]. Thus, multiple sections or samples should be examined to demonstrate the amoebae.
Treatment
CNS infections caused by Acanthamoeba are difficult to treat because of several factors. The foremost reason is difficulty in diagnosing GAE. The symptoms are nonspecific which can be easily mistaken for other bacterial, viral diseases and in the absence of a reliable diagnostic test; diagnosis is most often made post-mortem. In almost all cases where successful treatment of infections due to Acanthamoeba species has been achieved, the disease was localized to the skin or sinuses, without CNS involvement. Most available antimicrobial agents are amebostatic rather than amebicidal, are too toxic, or do not penetrate the CNS in adequate concentrations. As a result optimal approach to treatment of systemic infection due to Acanthamoeba is still uncertain. Successful treatment is limited to case reports of a few patients, most of whom recovered with combination drug therapy in the setting of early diagnosis. In rare cases excision of a single brain lesion has helped in curing the disease [46]. Drug combination that have been tried include liposomal amphotericin B and Voriconazole, trimethoprim-sulfamethoxazole and rifampin, and meropenem, linezolid, moxifloxacin, and fluconazole [57- 59]. It has been suggested that alkylphosphocholine compounds, such as hexadecylphosphocholine, exhibit anti-Acanthamoeba properties and can cross the blood–brain barrier, thus can be used to treat [60]. Another drug, miltefosine, a hexadecylphosphocholine, has been successfully used to treat a GAE patient in Austria [61]. Both the encystment and the excystment processes require active macromolecule synthesis and can be blocked by cycloheximide (a protein synthesis inhibitor). In many cases, however, therapy could not be completed because of undesirable side-effects of the medications [2,7,26,29]. In addition it has been seen that drugs that have been effective in one patient, are ineffective in other cases of acanthamoebiasis. The outcome of an infection is influenced by how early drug treatment is initiated; the infective dose of amoebae, the virulence and antimicrobial sensitivity of the particular strain of amoebae and most importantly the immune status of the patient. As a result the prospect for successful treatment of GAE remains poor with nearly 90% case fatality rate [39]. In those who survive, sequelae like hearing loss and vision impairment may remain [2]. Apart from these, many natural products have been found to possess antiacanthamoebic properties. These include garlic extracts, Thymus, Origanum, coumarin rich extract from Pterocaulon balansae or Methanolic seed extracts of Peganum harmala, etc. Magainins, are defense peptides produced by certain animals and have antimicrobial activity [62].
Acanthamoeba as vehicles for other microbes
They have been described as “Trojan horses” as they can habour a diverse number of pathogens, including viruses, bacteria, yeasts and Protozoa. So they can act as a vector for these pathogens. It has been reported that approximately 20 to 24% of clinical and environmental isolates of Acanthamoeba harbor intracellular bacteria, these include Legionella spp., Mycobacterium avium, Listeria monocytogenes, Vibrio cholerae, Francisella tularensis, Burkholderia spp., Helicobacter pylori, Afipia felis, and Escherichia coli serotype O157 [63].
Vaccines
Various vaccines have been designed to protect against Acanthamoeba keratitis. The mannose-induced cytopathic protein (MIP-133) and Acanthamoeba plasminogen activator (aPA) have both been tried as oral vaccines and found effective in limiting keratitis. MIP-133 anti-IgA antibody elicited by immunization may inhibit the adhesion of Acanthamoeba trophozoites to the corneal epithelium further preventing the destruction of the corneal epithelial cells [64]. Oral immunization with recombinant MBP (Acanthamoeba castellanii mannose-binding protein) ameliorated Acanthamoeba keratitis in a hamster animal model [65]. These vaccines can be tried orally in animal models to ascertain their effectiveness in prevention of Acanthamoeba granulomatous encephalitis.
Research Prospects
Bacterium-amebae interactions may lead to the establishment of an endosymbiotic state or, alternatively, to destruction of either the bacterium or the ameba. Acanthamoeba also produces an alginate lyase enzyme which can act on the biofilms and destroy them [10]. However if the virulence markers are identified and if these organisms can be genetically modified to non- virulent ones, they can act as tools to remove biofilms from prosthetic devices, to deliver drugs on these sites, eat away the infectious microbes on these lines, salvage these devices where their removal may not be safe and prolong life. The avirulent acanthamoebae can be used as caravans to deliver attenuated vaccine strains, drugs and perhaps genetic material. These are mere hypotheses but may serve to guide the scientific community in future.
Conclusion
Acanthamoeba is a versatile organism with vast potential and adaptability. It can transform itself phenotypically according to the environmental conditions; it can feed on a variety of microbes with which it can establish parasitism, symbiosis or commensalism. It can infect immunocompetent or immunocompromised people and act as an opportunist wherever feasible. It is stubborn and resists treatment in majority of cases, leading to increased morbidity and mortality ratios. If however, it can be twisted to the use of mankind, the man would have his share of revenge.
References
- Siddiqui R, Khan NA (2012) Biology and pathogenesis of Acanthamoeba. Parasites & Vectors 5: 6.
- Khan NA (2006) Acanthamoeba: biology and increasing importance in human health. FEMS Microbiology Reviews 30: 564-595.
- Castellani A (1930) An amoeba found in culture of yeast: preliminary note. Am J Trop Med Hyg 33: 160.
- Culbertson CG, Smith JW, Minner JR (1958) Acanthamoeba: observations on animal pathogenicity. Science 127: 1506.
- Jager BV, Stamm WP (1972) Brain abscesses caused by free-living amoeba probably of the genus Hartmannella in a patient with Hodgkin's disease. Lancet 23: 1343-1345.
- Nagington J, Watson P G, Playfair TJ(1974) Amoebic infections of the eye. Lancet 304: 1537–1540.
- Smith TC, Flore-Donno AM, Chao E (2015) Multigene phylogeny resolves deep branching of Amoebozoa. Mol Phylogenet Evol 83: 293-304.
- Trabelsi H, Dendana F, Sellami A (2012) Review Pathogenic free-living amoebae: Epidemiology and clinical review. Pathol Biol 60: 399-405.
- Marshall MM, Naumovitz D, Ortega Y (1998) Water borne protozoan pathogens. Clin Microbiol Rev 11: 404.
- Visvesvara GS (2013) Infections with free-living amebae. Handb Clin Neurol 114: 153-168.
- Cursons RT, Brown TJ, Keys EA (1980) Immunity to pathogenic free-living amoebae: role of humoral antibody. Infect Immun 29: 410-417.
- Cursons RT, Brown TJ, Keys EA (1980) Immunity to pathogenic free-living amoebae: role of humoral antibody. Infect Immun 29: 410-417.
- Singh P, Kochhar R, Vashishta RK (2006) Amebic meningoencephalitis: Spectrum of imaging findings. AJNR Am J Neuroradiol 27: 1217-1221.
- Vyas S, Jain V, Goyal MK (2013) Granulomatous amoebic meningoencephalitis. Neurol India 61: 530-531.
- Visvesvara GS, Maguire JH (2006) Pathogenic and opportunistic free-living amebas: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol 2: 1114-1125.
- Hamide A, Sarkar E, Kumar N (2002) Acanthameba meningoencephalitis: a case report. Neurol India 50: 484-486.
- Lalitha MK, Anandi V, Srivastava A (1985) Isolation of Acanthamoeba culbertsoni from a patient with meningitis. J Clin Microbiol 21: 666-667.
- Hamide A, Sarkar E, Kumar N (2002) Acanthameba meningoencephalitis: a case report. Neurol India 50: 484-486.
- Kaushal V, Chhina DK, Kumar R (2008) Acanthamoeba encephalitis. Ind J Med Microbiol 26: 182-184.
- Petry F, Torzewski M, Bohl J (2006) Early diagnosis of Acanthamoeba infection during routine cytological examination of cerebrospinal fluid. J Clin Microbiol 44: 1903-1904.
- Bloch K, Schuster FL (2005) Inability to make a premortem diagnosis of Acanthamoeba species infection in a patient with fatal granulomatous amebic encephalitis. J Clin Microbiol. 43:3003–3006.
- Cogo PE, Scalgia M, Gatti S (2004) Fatal Naegleria fowleri meningoencephalitis. Emerging Infectious Disease 10: 1835–1837.
- Martinez AJ (1996) Free-Living Amebas: Naegleria, Acanthamoeba and Balamuthia (4th edition), Med Microbiol, Galveston, Texas.
- Cordingley JS, Trzyna WC (2008) Multiple Factors Affecting Growth and Encystment of Acanthamoeba castellanii in Axenic Culture ACTA Protozoologica 47: 307-316.
- Villemez CL, Carlo PL, Russell MA (1985) Differentiation of A. castellanii is induced by specific monoclonal antibodies. J Cell Biochem 29: 372-379.
- Martinez MS, Gonzalez-Mediero G, Santiago P (2000) Granulomatous amebic encephalitis in a patient with AIDS: isolation of Acanthamoeba sp. Group II from brain tissue and successful treatment with sulfadiazine and fluconazole. J Clin Microbiol 38: 3892–3895.
- Sissons J, Alsam S, Goldsworthy G (2006) Identification and properties of proteases from an Acanthamoeba isolate capable of producing granulomatous encephalitis. BMC Microbiol 6: 1-8.
- Martinez AJ, Visvesvara GS (1997) Free-living, amphizoic and opportunistic amebas. Brain Pathol 7: 583-598.
- Marciano-Cabral F, Cabral G (2003) Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev 16: 273-307.
- Schuster FL, Visvesvara GS (2004) Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int J Parasitol 34: 1001-1027.
- Lorenzo-Morales, Jacob, Naveed A. Khan (2015) An Update on Acanthamoeba Keratitis: Diagnosis, Pathogenesis and Treatment. Parasite 22: 10.
- Martinez AJ (1985) Free-living Amebas: Natural History, Prevention, Diagnosis, Pathology and treatment of disease. CRC Press 156.
- Mazur T, Jozviak M (1993) Extracerebral infections of Acanthamoeba spp. in mice. Wiad Parazytol 39: 357-366.
- Garate M, Cao Z, Bateman E (2004) Cloning and characterization of a novel mannose-binding protein of Acanthamoeba. J Biol Chem 279: 29849-29856.
- Alsam S, Kim KS, Stins M “Acanthamoeba interactions with human brain microvascular endothelial cells. Microb Pathog 35: 235-241.
- Khan NA, Siddiqui R (2009) Acanthamoeba affects the integrity of human brain microvascular endothelial cells and degrades the tight junction proteins. Int J Parasitol 39: 1611-1616.
- Losch FD (1875) Massenhafteentwickelung von amobenimdikdarm. J Anat Physiol 65: 196-211.
- Chusattayanond AD, Boonsilp S, Kasisit J (2010) Acanthamoeba isolate (T4) induced apoptotic death in neuroblastoma cells via the Bax-mediated pathway. Parasitol Int 59: 512-516.
- Sisson J, Alsam S, Jayasekera S (2004) Ecto-ATPases of clinical and non-clinical isolates of Acanthamoeba. Microb Pathog 37: 231-239.
- Visvesvara GS (2007) Pathogenic and opportunistic free living amebae. FEMS Immunol Med Microbiol 50: 2082-2091.
- N. A. Khan (2009) Acanthamoeba Biology and Pathogenesis. Caister Academic Press. Great Britain.
- Gonzalez MM, Gould E, Dickinson G (1986) Acquired immunodeficiency syndrome associated with Acanthamoeba infection and other opportunistic organisms. Arch Pathol Lab Med 110: 749-751.
- Carter WW, Gompf SG, Toney JF (2004) Disseminated Acanthamoeba sinusitis in a patient with AIDS: a possible role for early antiretroviral therapy. AIDS Read 14: 41-49.
- Heffler KF, Eckhardt TJ, Reboli AC (1996) Acanthamoeba endophthalmitis in acquired immunodeficiency syndrome. Am J Ophthalmol 122: 584-586.
- Khan NA (2008) Acanthamoeba and the blood–brain barrier: the breakthrough. J Med Microbiol 57: 1051-1057.
- Vernon SE, Acar BC, Pham SM (2005) Acanthamoeba infection in lung transplantation: report of a case and review of the literature. Transpl Infect Dis 7: 154-157.
- Ma P, Visvesvara GS, Martinez AJ (1990) Naegleria and Acanthamoeba infections: review. Rev Infect Dis 12: 490-510.
- Duarte AG, Sattar F, Granwehr B (2006) Disseminated acanthamoebiasis after lung transplantation. J Heart Lung Transplant 25: 237-240.
- Deetz TR, Sawyer MH, Billman G (2003) Successful treatment of Balamuthia amoebic encephalitis: presentation of 2 cases. Clin Infect Dis 37: 1304-1312.
- Fowler M, Carter RF (1965) Acute pyogenic meningitis probably due to Acanthamoeba: a preliminary report. Br Med J 2: 740-742.
- Sell JJ, Rupp FW, Orrison WW (1997) Granulomatous amebic encephalitis caused by Acanthamoeba. Neuroradiology 39: 434-436.
- Zagardo MT, Castellani RJ, Zoarski GH (1997) Granulomatous amebic encephalitis caused by leptomyxid amebae in an HIV-infected patient. American J Neuroradiology 18: 903-908.
- Khan NA, Paget TA (2002) Molecular tools for speciation and epidemiological studies of Acanthamoeba. Curr Microbiol 44: 444-449.
- Gupta N, Samantaray JC, Duggal S (2010) Acanthamoeba keratitis with Curvularia co-infection. Indian J Med Microbiol 28: 67-71.
- Pablo G, Sandrine D, Djida B (2009) New tool for the simultaneous detection of ten different genotypes of Acanthamoeba available from the American Type Culture Collection. Br J Ophthalmol 93: 1096-2022.
- Matson DO, Rouah E, Lee RT (1988) Acanthameba meningoencephalitis masquerading as neurocysticercosis. Pediatr Infect Dis J 7: 121-124.
- Crary MJ (2012) Genetic variability and its relationship to Acanthamoeba pathogenesis. Ohio State University.
- Kidney DD, Kim SH (1998) CNS infections with free-living amebas: neuroimaging findings. AJR Am J Roentgenol 17: 809-812.
- Walia R, Montoya JG, Visvesvera GS (2007) A case of successful treatment of cutaneous Acanthamoeba infection in a lung transplant recipient. Transpl Infect Dis 9: 51-54.
- Fung KT, Dhillon AP, McLaughlin JE (2008) Cure of Acanthamoeba cerebral abscess in a liver transplant patient. Liver Transpl 14: 308-312.
- Lackner P, Beer R, Broessner G (2010) Acute granulomatous acanthamoeba encephalitis in an immunocompetent patient. Neurocrit Care12: 91-94.
- Niyyati M, Dodengeh S, Lorenzo-Morales J (2016) A Review of the Current Research Trends in the Application of Medicinal Plants as a Source for Novel Therapeutic Agents Against Acanthamoeba Infections. Iran J Pharm Res 15: 893-900.
- Kotting J, Berger MR, Unger C (1992)Alkylphosphocholines: influence of structural variations on biodistribution of antineoplastically active concentrations. Cancer Chemother Pharmacol 30: 105-112.
- Aichelburg AC, Walochnik J, Assadian O (2008) Successful treatment of disseminated Acanthamoeba sp. infection with miltefosine. Emerg Infect Dis 14: 1743-1746.
- Greub G, Raoult D (2004) Microorganisms resistant to free-living amoebae. Clin Microbiol Rev 17: 413–433.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi