Research Article, Int J Cardiovasc Res Vol: 7 Issue: 5
Serum Adiponectin Levels and Severity of Coronary Artery Disease by Gensini Score
Rehab Ibraheem Yaseen1*,Walaa Farid1, and Alshaimaa Ali2
1Cardiology Department, Faculty of Medicine, Menoufia University, Egypt
2Sohag Cardiac Center, Sohag, Egypt
*Corresponding Author : Rehab Ibrahim Yaseen
Department of Cardiology, Menofia University, Abdel rahman Elsharkawy Street, Shebin El-Kom, Menoufia, Egypt
Tel: 01006235708
Fax: 20 (48) 222 6454
E-mail: dr_r.yaseen@hotmail.com
Received: August 01, 2018 Accepted: September 20, 2018 Published: September 26, 2018
Citation: Yaseen RI, Farid W, Ali A (2018) Serum Adiponectin Levels and Severity of Coronary Artery Disease by Gensini Score. Int J Cardiovasc Res 7:5. doi: 10.4172/2324-8602.1000361
Abstract
Background: Ischemic heart disease is the most common cause of death all over the world; its rate is expected to be accelerated in the next decade. Adiponectin is a protein hormone that modulates a number of metabolic processes, including glucose regulation and acid oxidation.
Objective: To correlate serum levels of adiponectin and CAD severity assessed by Gensini score.
Subjects and methods: Ninety subjects were recruited from catheterization unit (36 subjects with normal coronary arteries and 54 patients with CAD) and they were subdivided into four groups according to the number of affected vessel. Their modified Gensini score was calculated according to the severity of coronary lesions. Blood samples were withdrawn to measure adiponectin levels.
Results: Adiponectin levels were lower in patients with CAD than those with normal coronary arteries group by a highly significant statistically value (P<0.001). A significant negative correlation was found between adiponectin levels and modified Gensini score (r= -0.903; P<0.001). Adiponectin levels were significantly lower in patients with multi-vessels disease compared to patients with twovessel disease and single-vessel disease.
Conclusion: Adiponectin levels are lower in patients with CAD in comparison to group without CAD. The lower the levels the more severe CAD will be. So, measurement of adiponectin levels may be helpful in risk stratification of patients with CAD.
Keywords: Adiponectin; Coronary artery disease; Coronary angiography; Modified Gensini score
Introduction
Ischemic heart disease is the most common cause of death all over the world; its rate is expected to be accelerated in the next decade thus will lead to high financial cost [1]. It is considered that Adipose tissue is a storage depot of excess energy and it is known that it participates in mediating vascular complications. It has an endocrinal and paracrinal functions as it secrets numerous mediators called adipokines that share in different metabolic processes.
Leptin, TNF, and a plasminogen activator inhibitor-1 are adipokines that have atherogenic effects, while adipokines like adiponectin may have a protective role through its anti-inflammatory and anti- atherogenic effects [1]. It inhibits the class a macrophage scavenger receptor which leads to inhibition of foam cells formation.
Moreover, adiponectin inhibits LDL binding to biglycan; this ultimately decreases lipid accumluation in the subendothelial space, the cause of atherosclerotic plaque formation [2]. We aimed to study the correlation between adiponectin serum levels and CAD severity as assessed by coronary angiography using modified Gensini score [3].
Subjects and Methods
The study population included ninety subjects recruited from catheterization unit in Menoufia University Hospital and Sohag Cardiac Center who underwent elective coronary angiography in the period from January 2016 to December 2016; all patients were subjected to detailed history taking, full clinical examination and 12- lead electrocardiography.
Exclusion criteria were DM, infectious disease, thyroid dysfunction, and liver dysfunction based on medical history or laboratory tests, neoplastic disease, chronic renal failure (Cr>2.0 mg/dl) or on hemodialysis. The study was accepted by the ethics committee and written consent was taken from the patients.
Demographic, clinical data and routine biochemistry analyses in form of serum creatinine, lipid profile and serum adiponectin levels of all the subjects were performed. Serum samples for adiponectin were centrifuged and stored at -20 C in the laboratory then measured by ELISA method, using (Assay Max Human Adiponectin ELISA (Acrp30) kit, ASSAYPRO LLC Laboratory Medicine Inc., USA).
Selective coronary angiography by standard seldinger technique was performed for all subjects. Two cardiology interventionists who were blinded to study evaluated the coronary angiograms. The modified Gensini score was used to assess coronary lesions severity. Scoring was applied as follows: lesion in left main coronary was given score 5; lesions in proximal left anterior descending (LAD) artery and the left circumflex artery (LCX) were given score 2.5; while lesion in the mid-LAD artery was given score 1.5; lesions in the first diagonal branch (D1), the obtuse marginal branches and the right coronary artery were given 1 score; lastly lesions in the second diagonal (D2) and the LCX posterior-lateral branch were given score 0.5 [3]. Then the score was calculated as seen in Figure 1.
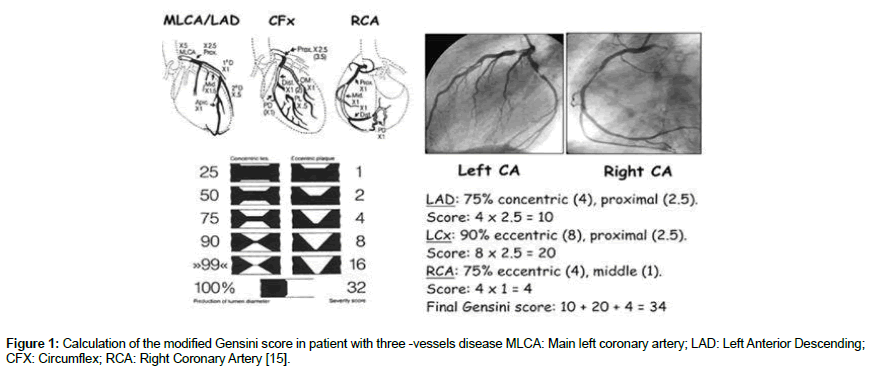
Figure 1: Calculation of the modified Gensini score in patient with three -vessels disease MLCA : Main left coronary artery; LAD : Left Anterior Descending; CFX: Circumflex; RCA : Right Coronary Artery [15].
Statistical Analyses
Results were statistically analyzed by SPSS version 20 (SPSS Inc., Chicago, IL, USA). Student’s t-test was used to indicate the significance between 2 means. Chi-Squared (χ2) test was used for comparison regarding qualitative variables. Pearson’s correlation was used to show strength and direction of association between two quantitative variables. P value was considered significant if <0.05.
Results
Our study included ninety subjects divided into group A=36 subjects with normal coronary arteries (21 males and 15 females) with mean age 55.55 ± 11.62 and group B=54 patients with CAD (41 male and 13 females) with mean age 56.90 ± 11. Group B then subdivided into: group 1: 17 patients (13 males and 4 females) with single-vessel disease, group 2: 12 patients (7 males and 5 females) with two-vessels disease, group 3: 19 patients (16 males and 3 females) with multivessels disease and group 4: 6 patients (5 males and 1 female) with left-main disease.
Regarding age and sex; no significant difference was found in the studied groups (P>0.05). In patients with CAD, the levels of serum adiponectin were found to be lower than those without CAD by a statistically highly significant value (P<0.001). Moreover, the patients with two vessels, multi-vessels disease and LM disease had lower adiponectin levels significantly compared to normal coronary arteries group (P<0.001) (Table 1).
Group A
(N=36)CAD patients
Group B
(N=54)Mean ± SDMean ± SDAge (Y)55.55 ± 11.6256.90 ± 11.23t=0.550.582Sex
Male
Female N0
21
15%
58.3
41.7No
41
13%
75.9
24.1χ2
3.110.077Hypertension1541.72750.0χ2
0.600.438Family history513.9916.7χ2
0.120.722Dyslipidemia 1027.81731.5χ2
0.140.707Serum Adiponectin levels
µg/ml 5.04 ± 1.553.65 ± 1.636.02<0.001*
Table 1: Comparison between the studied groups as regard to age, sex, and risk factors of CAD and serum adiponectin levels.
On the contrary the adiponectin levels in patients with singlevessel disease and normal coronary arteries group showed no significant difference (P>0.05).
When analyzing subgroups of patients with CAD, we found that, serum levels of adiponectin were statistically significant lower in patients with two-vessel disease (G2), multi-vessels disease (G3) and, LM disease (G4) as compared to single-vessel disease (G1).Also, it was statistically significant lower in patients with multi-vessels disease (G3) than patients with two-vessels disease (G2) (P<0.05).
As regard to modified Gensini score, it was highly statistically significant higher in patients with two-vessel disease (33.75 17.73), multi-vessels disease (67.28 ± 41.61) and left-main disease (80.16 ± 47.25) than patients with single- vessel disease (8.02 ± 6.0) (P<0.001). While it had no significant difference between patients with left-main disease and those with multi-vessels disease this may be attributed to small number of patients in left-main disease group (Table 2).
(N=36)CAD patients GB (N=54)TestP valueG1
(n=17)G2
(n=12)G3
(n=19)G4
(n=6)Mean
± SDMean ± SDMean
± SDMean
± SDMean
± SDSerum adiponectin Levels (µg/ml)5.04
± 1.554.57
± 1.643.75
± 1.303.03
± 0.842.84
± 1.28F=
19.06
P<0.001*A vs. 1=0.092
Avs. 2<0.001*
Avs. 3<0.001*
Avs. 4<0.001*
1 vs. 2=0.024*
1 vs. 3=0.001*
1 vs. 4<0.001*
2 vs. 3=0.039*
2 vs. 4=0.055
3 vs. 4=0.670Modified Gensini score-8.02 ± 6.033.75 ± 17.7367.28 ± 41.6180.16 ± 47.25Kruskal-Wallis
=35.12
P<0.001*1 vs. 2<0.001*
1 vs. 3<0.001*
1 vs. 4<0.001*
2vs. 3=0.011*
2 vs. 4=0.013*
3 vs. 4=0.445
Table 2: Comparison of serum adiponectin levels and modified Gensini score in the studied groups and subgroups.
Receive-operating characteristics (ROC) curve showed that cutoff value of serum adiponectin level for detection of CAD was<4.6 μg/ml with sensitivity 85%, specificity 69%, accuracy 79%, PPV 81% and NPV 76%. As regard cut-off values of serum adiponectin level for prediction of severity of CAD in two-vessels disease (G2), it was ≤ 4.4 μg/ml, for multi-vessels disease (G3), it was<3.9 μg/ml, and finally for left main disease (G4), it was<3.8 μg/ml (Figure 2 and Table 3).
Table 3: Sensitivity, specificity and accuracy of serum adiponectin levels in the studied patients and subgroups for detection of CAD and prediction of its severity.
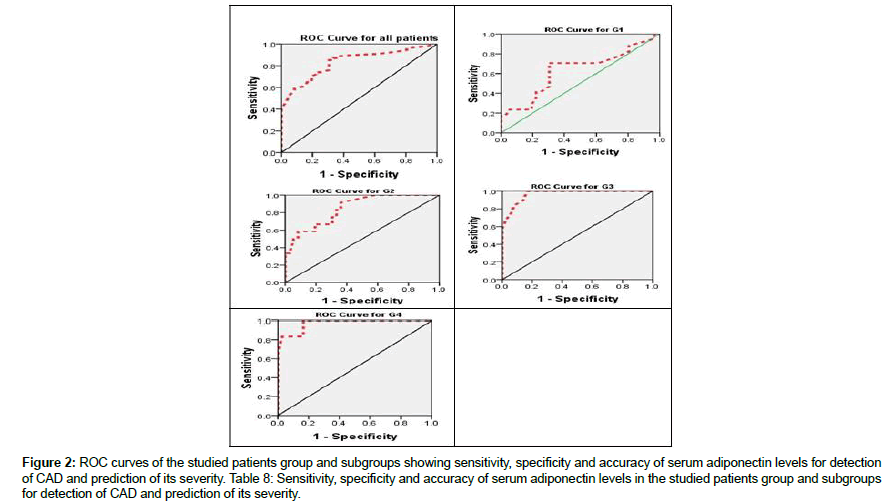
Figure 2: ROC curves of the studied patients group and subgroups showing sensitivity, specificity and accuracy of serum adiponectin levels for detection of CAD and prediction of its severity. Table 8: Sensitivity, specificity and accuracy of serum adiponectin levels in the studied patients group and subgroups for detection of CAD and prediction of its severity.
There was a significant negative linear correlation between modified Gensini score and serum levels of adiponectin in the studied patients group and subgroups (Figure 3 and Table 4).
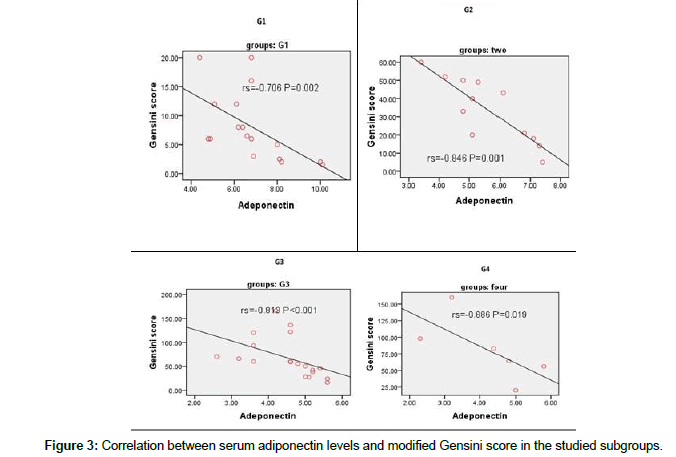
Figure 3: Correlation between serum adiponectin levels and modified Gensini score in the studied subgroups.
Patients
GBG1
(n=17)G2
(n=12)G3
(n=19)G4
(n=6)RP valuerP valuerP valuerP valuerP valueModified Gensini score-0.7060.002*-0.8460.001*-0.813<0.001*-0.8860.019*-0.903<0.001*
Table 4: Correlation between serum adiponectin levels and modified Gensini score in the studied patients group and subgroups.
Discussion
Coronary artery disease is a major cause of mortality and morbidity in developed countries. Although its rates of mortality decreased in the last four decades, it remains the cause of death in more than one-third of individuals over age of 35 [4-6].
Leptin, TNF, and a plasminogen activator inhibitor-1 are adipokines that have atherogenic effects, while adipokines like adiponectin may have a protective effect through its antiinflammatory and anti-atherogenic properties [1]. It inhibits the class A macrophage scavenger receptor which leads to inhibition of foam cells formation.
Moreover, adiponectin inhibits LDL binding to biglycan which is a vascular proteoglycan. This ultimately decreases lipid accumluation in the subendothelial space, the cause of atherosclerotic plaque formation [2]. Therefore, understanding the clinical importance of adiponectin may have a role in prevention of atherosclerosis [7].
Our study showed that, patients with angiographically diagnosed CAD had significantly lower levels of serum adiponectin than the normal coronary group (P<0.001). This agreed with Mohamed et al., who found that adiponectin levels were significantly lower in CAD group compared to the control group and revealed that this finding might be attributed to the acceleration of vascular inflammatory events caused by loss of protective effects of adiponectin on the vascular endothelium. Similarly, Selcuk et al. [8] found lower adiponectin levels in patients with significant CAD than those without CAD in a study performed in patients undergoing coronary angiography.
In addition, our study showed no significant difference in adiponectin levels in patients with single-vessel lesion compared to the normal coronary arteries group (P>0.05); whereas it was found to be significantly lower in patients with two-vessels, multi-vessels and left-main lesions when evaluated by usual method of scoring systems for severity of CAD. These results were similar to Gokosoy et al. [9] who found that, patient with single-vessel disease and control group had no significant difference in serum adiponectin levels, while patients’ subgroups with two-vessels and multi-vessels lesions had significantly lower levels compared to control group. As a result, low serum adiponectin levels may indicate multi-vessels (≥ 2 vessels) or left-main disease rather than single-vessel disease [10].
This disagreed with Mohamed et al., who found that adiponectin levels were significantly lower in patients with single-vessel lesion compared to the control group; this may be attributed to small numbers of patients of single-vessel disease in our study compared to their study. In another study done by Cavuflolu et al. [11] in which coronary angiography was performed for male patients with stable angina pectoris, unstable angina pectoris and NSTEMI, it was found that low serum levels of adiponectin had a predictive role in occurrence of acute MI and cardiac mortality. It has been suggested that CAD patients with complex lesions have lower levels of serum adiponectin that may indicate vulnerability of the plaque [12].
When we analyzed CAD patients subgroups, a significant negative correlation was found between adiponectin levels and modified Gensini scores, meaning that, there was lower levels with CAD progression, and it was highly statistically significant higher in patients with two-vessels disease, multi-vessels disease and left-main disease compared to single-vessel disease, and in multi-vessels disease and left-main disease compared to two-vessels disease. Similarly Shams et al. [13] found a significant linear negative correlation between adiponectin levels and severity of CAD based on usual method for evaluation in coronary angiogram. In our study, the mean Gensini score in the patients group was found to be 40.52 ± 41.02 and when we demonstrated the cut-off point of serum adiponectin level for detecting CAD, it was<4.6 μg/ml with sensitivity 85%, specificity 69%, accuracy 79%, PPV 81% and NPV 76%. As regard cut-off values of serum adiponectin level for prediction of severity of CAD in twovessel disease (G2), it was ≤ 4.4 μg/ml, for multi-vessels disease (G3), it was< 3.9 μg/ml, and finally for left main disease (G4), it was< 3.8 μg/ml.
Mohamed et al. showed that cut-off point of adiponectin level for detecting CAD was 7.21 pg/dl with sensitivity 100% and specificity 92%. Kumada et al. [14] revealed that, cut-off value was<4 μg/ml of adiponectin for detecting CAD prevalence in men.
The strength of our study was that, it approved the presence of a significant negative linear correlation between adiponectin levels and modified Gensini score as a predictor of severity of CAD not only in total patients but also in each subgroup of CAD patients categorized by usual method of scoring system for reporting coronary angiogram individually [15].
Limitations
Only total form of adiponectin was measured while High Molecular Weight (HMW) adiponectin alone may be a clinically useful marker of CAD than total form.
Conclusion
Serum adiponectin levels are lower in CAD patients in comparison to normal coronary arteries group. The lower the levels the more severe CAD will be. So, measurement of adiponectin levels may be helpful as a diagnostic tool in risk stratification of patients with CAD.
References
- Giannessi D, Maltinti, Del RYS (2007) Adiponectin circulating levels: Anew emerging biomarker of cardiovascular risk. Pharmacol Res 56: 459-467.
- Soydinc S, Davutoglu V, Sari I (2007) High serum levels of adiponectin improves coronary collateral development in patients with coronary artery disease. Tohoku J 211: 347-352.
- Gensini GG (1983) A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 51: 606.
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, et al. (2008) Heart disease and stroke statistics-update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee, Circulation 117: 25.
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethone M, De Simone G, et al. (2010) Executive summary: heart disease and stroke statistics-update: a report from the American Heart Association, Circulation 121: 948-954.
- Nichols M, Townsend N, Scarborough P, Rayner M (2014) Cardiovascular disease in Europe 2014: epidemiological update. Eur Heart J 35: 2950
- Abdelbar MG, Enany BE, Khalil AO (2013) The association between serum adiponectin levels and the severity of coronary artery disease. Med. J. Cairo Univ. 81: 215-219
- Selcuk MT, Selcuk H, Temizhan A, Orthan M, Saydam GS, et al. (2008) Impact of plasma adiponectin levels to the presence and severity of coronary artery disease in patients with metabolic syndrome. Coron. Artery Dis 19: 79-84.
- Goksoy H, Dursunoglu D, Ozturk M, Rota S (2009) The association between serum adiponectin levels and the severity of coronary artery lesions on the angiogram. Turk Kardiyol Dern Ars 37: 241-245.
- Liang KW, Sheu WH, Lee WL, Liu TJ, Ting CT (2008) Decreased circulating protective adiponectin level is associated with angiographic coronary disease progression in patients with angina pectoris. Int J Cardiol 129: 76-80.
- Cavuflolu E, Ruwende C, Chopra V, Yanamadala S, Eng C, et al. (2006) Adiponectin is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction in patients presenting with chest pain. Eur Heart J 27: 2300-2309.
- Otsuka F, Sugiyama S, Kojima S, Maryoshi H, Funahashi T, et al. (2006) Plasma adiponectin levels are associated with coronary lesion complexity in men with coronary artery disease. J Am Coll Cardiol 48: 1155-1162.
- Shams M, Rasekhi Kazerouni A, Ostovan MA, Omrani GR (2012) The Relationship between Serum Adiponectin Levels with the Presence and Severity of Coronary Artery Disease. Arch Iran Med 15: 611-616
- Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, et al​. (2003) Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol 23: 85-89.
- Montosri P, Ravagnani P, Galli S, Rotatori F, Veglia F, et al. (2006) Association between erectile dysfunction and coronary artery disease. Role of coronary clinical presentation and extent of coronary vessels involvement: the COBRA trial. Eur Heart J 27: 2632-2639.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 



