Review Article, Int J Cardiovasc Res Vol: 9 Issue: 4
Spot Stenting of Spontaneous Coronary Artery Dissection in a Patient Presenting with Anterior Wall ST Elevation Myocardial Infarction
Sajjad Ahmadi-Renani1, Abbas Soleimani2, Negar Moradian Shahrbabaky3 and Haleh ASHRAF4*
1Tehran Heart Center, Tehran University of Medical sciences, Tehran, Iran
2Sina Hospital, Tehran University of Medical sciences, Tehran, Iran
3Students’ Scientific Research Center (SSRC), Tehran University of Medical Sciences, Tehran, Iran
4Research Development Center, Sina Hospital, Tehran University of Medical Sciences, Tehran, Iran
*Corresponding Author: Dr. Haleh Ashraf
Research Development Center, Sina Hospital, Tehran University of Medical Sciences, Tehran, Iran
Tel: 00982166348551
E-mail: hashraf@sina.tums.ac.ir
Received: July 04, 2020 Accepted: July 24, 2020 Published: July 30, 2020
Citation: Ahmadi-Renani S, Soleimani A, Shahrbabaky NM, Ashraf H (2020) Spot Stenting of Spontaneous Coronary Artery Dissection in a Patient Presenting with Anterior Wall ST Elevation Myocardial Infarction . Int J Cardiovasc Res 9:4. doi: 10.37532/icrj.2020.9(4).408
Abstract
Background: Spontaneous Coronary Artery Dissection (SCAD) is a lesser recognized cause of myocardial infarction. Although some etiologies are suggested, the specific cause might not be identified in many cases. Many patients are the women in peri-partum or childbearing age with no significant atherosclerotic risk factors and who will undergo conservative or interventional treatment with close follow-up. Case Report: We report a case of SCAD patient who was diagnosed and treated in university hospital of Sina, Tehran, Iran. She had dissection in the middle and distal part of Left Anterior Descending artery (LAD) which was managed invasively with Percutaneous Coronary Intervention (PCI). Conclusion: Clinicians should have reasonable suspicion of SCAD, especially in young female patients with no apparent atherosclerotic risk factors who complain of Chest pain with signs of cardiac ischemia. Interventional cardiologists are to know SCAD diagnosis, management and follow up.
Keywords: Spontaneous coronary artery dissection; Acute coronary syndrome; Chest pain; Myocardial infarction.
Introduction
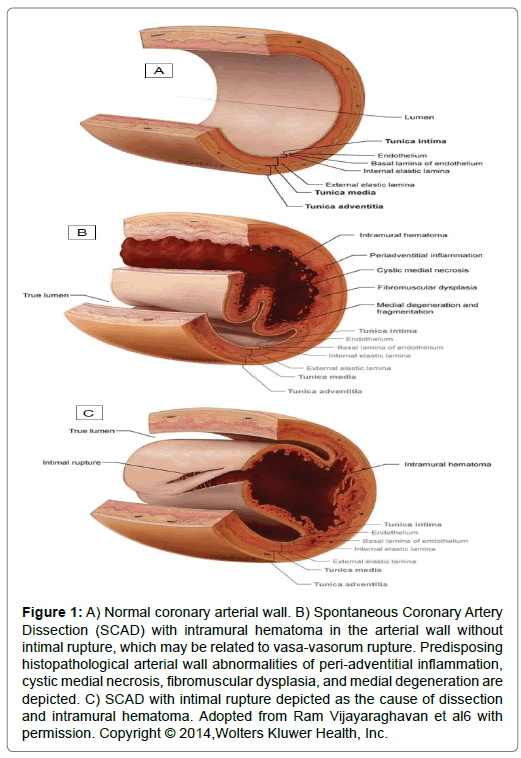
SCAD is a non-iatrogenic and non-atherosclerotic coronary artery disease that mostly present with Acute Coronary Syndrome (ACS) and might be a life threatening condition. It shouldn’t considered as a rare cause of ACS anymore because of its accounts for about 4% of ACS occurrence [1]. SCAD is more common in young peri-partum healthy women with no apparent risk factor for coronary artery disease [2]. In terms of the pathophysiology of SCAD, several potential disease conditions (connective tissue disease, pregnancy, coronary vasospasm and fibromuscular dysplasia) have been proposed. Although the pathogenesis of SCAD is not fully understood, some suggested mechanisms are hematoma formation due to spontaneous intimal tearing and tearing of vaso-vasorum of the media layer of coronary (Figure 1) [3,4]. Genetic factors and some connective tissue disorder such as Fibromuscular Dysplasia (FMD) are seem to be contribute in SCAD pathogenesis [5].
Figure 1: A) Normal coronary arterial wall. B) Spontaneous Coronary Artery Dissection (SCAD) with intramural hematoma in the arterial wall without intimal rupture, which may be related to vasa-vasorum rupture. Predisposing histopathological arterial wall abnormalities of peri-adventitial inflammation, cystic medial necrosis, fibromuscular dysplasia, and medial degeneration are depicted. C) SCAD with intimal rupture depicted as the cause of dissection and intramural hematoma. Adopted from Ram Vijayaraghavan et al6 with permission. Copyright © 2014,Wolters Kluwer Health, Inc.
SCAD known triggers are Valsalva-like activities which increase thoracoabdominal pressure (child delivery, coughing, vomiting, etc.) or catecholamine release due to physical (mostly isometric exercises) or emotional stress which cause increasing the coronary wall shear stress especially in patients with underlying arteriopathy. Other triggers include hormonal therapy, pregnancy and sympathomimetic drugs [6]. The treatment strategy of SCAD has not been clearly established. Conservative therapy is one of the options for management of SCAD. However, when ongoing ischemia is present, some type of alternative strategy to revascularize the ischemia-inducible dissected coronary artery needs to be formulated. We report a case of a middle age female, who presented with acute anterior wall myocardial infarction secondary to SCAD, Which could constitute a challenge in regards to treatment and also discuss the relevant literature.
Case description
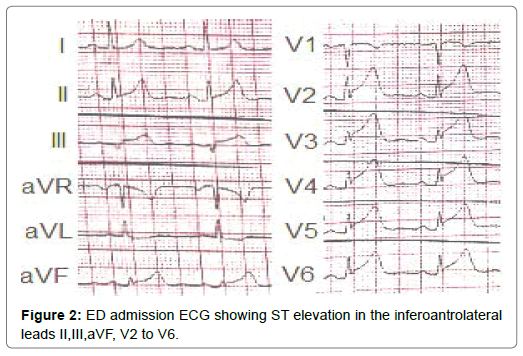
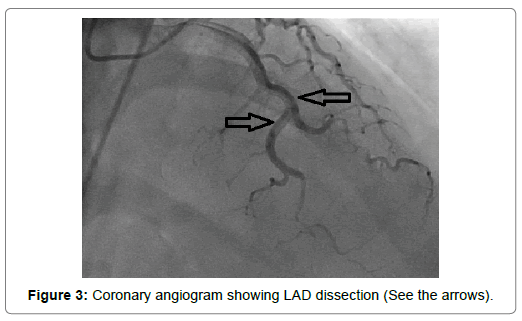
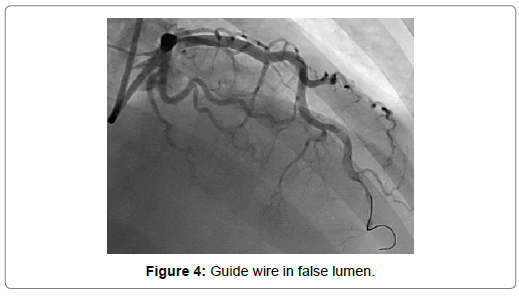
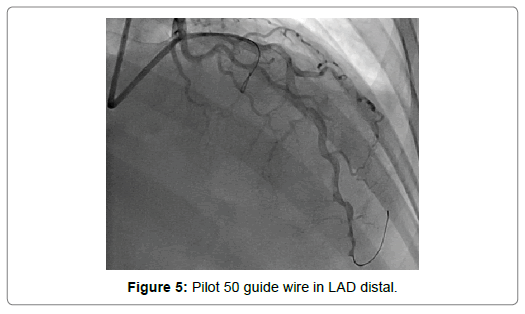
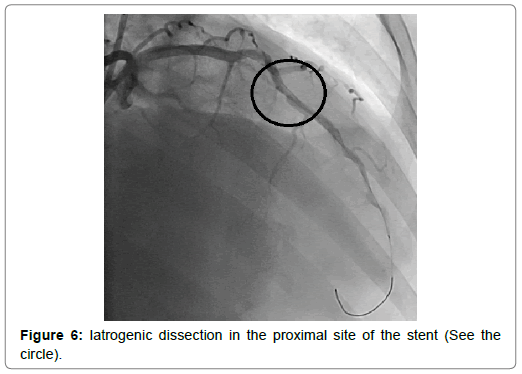
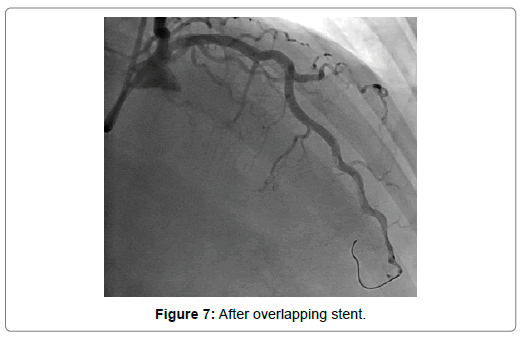
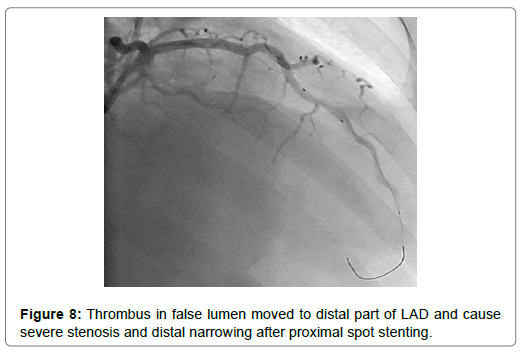
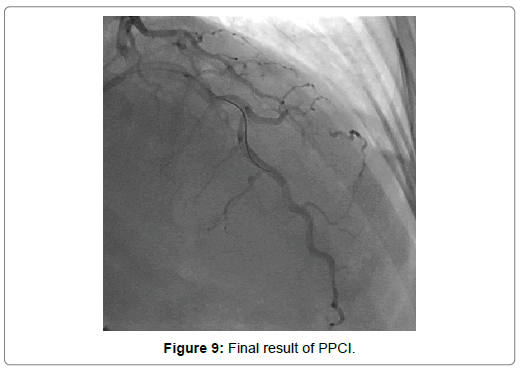
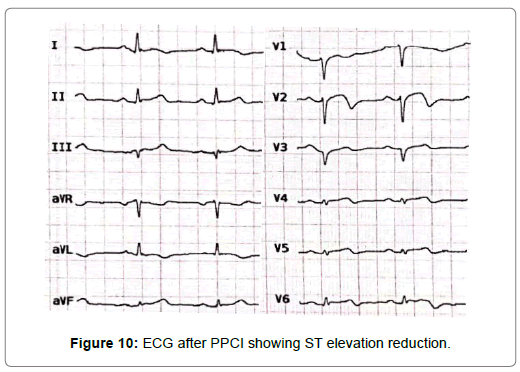
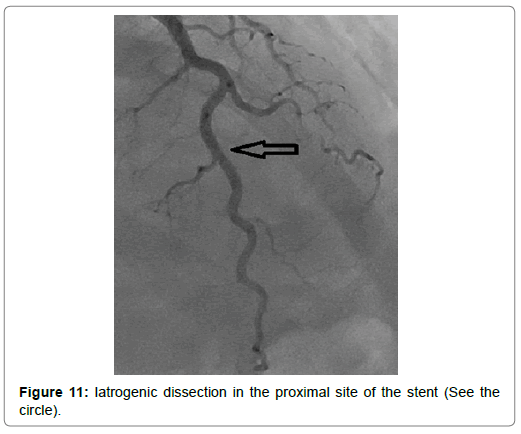
A 54-year-old woman with history of poor controlled Hypertension (HTN) and migraine, was brought to the Emergency Department (ED) by Emergency Medical Services (EMS) with complaint of chest pain from 5 hour ago. The patient described her chest pain burninglike, in left hemi-thorax which radiated to her jaw. The patient didn’t complain of diaphoresis, nausea, vomiting or dyspnea. There was no clear response to two pearls of Trinitroglycerin (TNG) consumption and chest pain didn’t resolved. She also mentioned some non-anginal chest pain in the past three months and positive Ischemic Heart Disease (IHD) in family history. At first examination the patient’s vital signs were unstable (Heart rate=71 p/m - O2saturation=92% - Respiratory rate=19 r/m - Blood Pressure=95/60 mmHg). Her first Electrocardiogram (ECG) (Figure 2). There is ST elevation in inferoanterolateral leads. The patient became a candidate for Primary Percutaneous Coronary Intervention (PPCI) with anterior wall ST-Elevation Myocardial Infarction (STEMI) diagnosis. In the angiography, the common pattern of coronary obstruction, which causes transmural myocardial infarction, was not seen. There was a sudden significant tapering in Left Anterior Descending Artery (LAD) mid part (Figure 3). It was speculated that it is a septal branch but due to the ECG pattern, LAD obstruction existed clearly (The LAD was wrap-around and also since LAD was cut at mid part, the ST vector became inferior axis. So in addition to ST elevation in anterolateral leads, there was ST elevation in inferior ones). Since BMW guidwire didn’t pass through the tapered part, it was guided to a vessel lumen other than the tapered one (Figure 4) that confirmed a SCAD type 2 extending to the distal part of coronary vessel (We didn’t have Intravascular Ultrasound (IVUS) or Optical Coherence Tomography (OCT) in cardiac catheterization lab). Afterwards, the wire was guided branch by branch until vessel tip. Due to the friction caused by tortuosity of the vessel, the BMW wire didn’t pass through after a while; so, we used hydrophilic Pilot 50 wire and went through the tapered area, reaching the distal part of LAD eventually (Figure 5). In order to progress in the right direction while trying to access the distal part, we predilated the vessel by balloon which the blood flow to the distal part was being observed with. Finally, having the distal part reached, the blood flow returned to LAD entirely. That changed SCAD type 2 to type 1. In this stage, the best strategy was in question: whether to choose a conservative or an invasive approach. We waited for 20 minutes, but the patient was complaining of chest pain which meant ongoing ischemia existed. So, we decided to do spot stenting in the entry site of the dissection in mid part of LAD. To prevent hematoma expansion into proximal part of the dissection, 6 mm of the stent was inserted proximally to the dissection entry. By then, an iatrogenic dissection had occurred in the proximal site of the stent (Figure 6) which made us plant another small stent overlapping the first one with lower pressure (Figure 7). Since the thrombus that had existed in false lumen moved to distal part of the stent, a severe stenosis in the distal part happened (Figure 8). Accordingly, we tried intra-stent dilation with balloon to spread the thrombus which resulted in alleviating the stenosis (Figure 9). Type 1 SCAD still existed after stenting, but the blood flow was improved and the chest pain disappeared. Hence, we finished the procedure and continued the patient’s treatment with heparin and Dual Antiplatelet Therapy (DAPT). The ECG which had been performed just after PPCI revealed a good reduction (>50%) in ST segment elevation (Figure 10). Echocardiography was performed the day after PPCI which reported Ejection Fraction (EF)=45% and regional wall motion abnormality. Apex and anteroseptal wall were akinetic, no significant valvular problem existed and she had normal Pulmonary Artery Pressure (PAP). The patient underwent Coronary Angiogram (CAG) the day after PPCI for second look. In the second CAG procedure it was determined that there was a stenosis in LAD which was caused by dissected flap but less filling defects (Figure 11). The patient was discharged a week after admission with no complication. In two weeks follow up, the patient had neither complaints of chest discomfort nor anginal pain nor exertional dyspnea. Two months later, patient examined again. She had no complaint and there was a greate betterment in EF (55-60%).
Discussion
Here, we report a case of a middle age female, who presented with acute anterior wall myocardial infarction which angiography revealed SCAD of LAD artery. Because ongoing ischemia was present, we choose invasive strategy using spot versus entire stenting. In the following, the relevant literature on diagnosis and treatment of SCAD will be reviewed.
Diagnosis
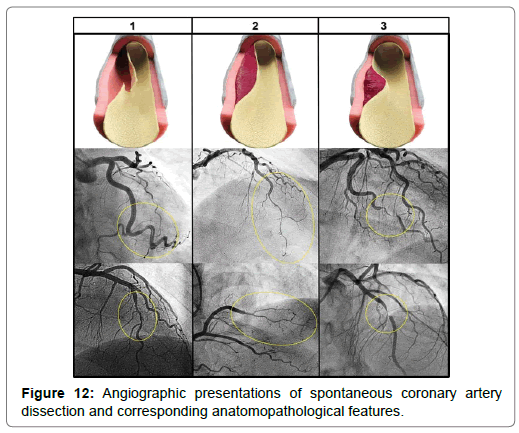
There are variable clinical presentations. A large cohort study which had been done on 196 SCAD patients, revealed that 96% of patients had chest discomfort. Other common presentations were: arm pain (49.5%), neck pain (22.1%), nausea/vomiting (23.4%), diaphoresis (20.9%), dyspnea (19.3%), and back pain (12.2%). Catastrophic presentations such as Ventricular Tachycardia (VT) and Ventricular Fibrillation (VF) were seen in 8.1% of patient and about 1% of patient initially presented with cardiac arrest. STsegment Elevation Myocardial Infarction (STEMI) happened in 24% of cases and others developed Non ST-segment Elevation Myocardial Infarction (NSTEMI) [7]. SCAD highly misdiagnosed because of its nonspecific presentation and absence of atherosclerotic risk factors. There are many reports of discharging young patients who were suffering from SCAD from ED without any diagnostic investigation [8]. Since SCAD has entirely different management from atherosclerotic form of ACS, the early accurate diagnosis of SCAD essential. In cases of patients with unusual features such as young women without any apparent risk factors for atherosclerosis who complain from chest pain, the physician should suspect nonatherosclerotic causes of ACS such as SCAD. When SCAD is suspected, the coronary angiography should be performed as soon as possible, especially in STEMI setting. Although CAG has some limitations for SCAD diagnosis, it is still the first line diagnostic tool for SCAD because of its availability and therapeutic potentials [9]. Since the traditional pathognomonic presentation of SCAD in CAG “multiple radiolucent lumens and extra luminal contrast staining” is detected only in a small proportion of SCAD cases, new classification of angiographic patterns of SCAD was created with the name of” Saw angiographic SCAD classification” [10].
• Type 1 or classic appearance: Multiple radiolucent lumens or arterial wall contrast staining
• Type 2: Diffuse stenosis could vary in severity and length(usually >20 mm)
• Type 3: focal or tubular stenosis(usually<20mm in length), that mimics atherosclerosis (Figure 12).
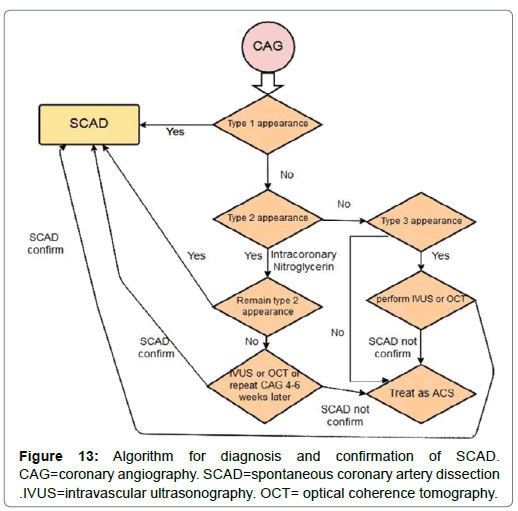
Type 2 is the most prevalent type, with 67.5% of cases. Type 1 occurs in about 29.1% cases. The most uncommon type is type 3, which occurs in just 3.4% of cases [11]. So if the physician who performs CAG relies only on pathognomonic pattern (multiple radiolucent lumens and extra luminal contrast staining), about 70% of cases would be missed. Hence, the physicians must be familiar to the different SCAD manifestation in angiography to reduce misdiagnosis. They must also be aware of Iatrogenic catheter-induced coronary artery dissection as a CAG complication. Interestingly the prevalence of coronary artery dissection due to Iatrogenic catheterinduced is more common(3.4%) in SCAD than that in standard diagnostic CAG(<0.2%) [12]. That means coronary dissection could be primary (spontaneous) or secondary (as a complication of CAG in SCAD or complication of CAG in atherosclerotic plaque rupture induce STEMI or even elective diagnostic CAG). It has been suggested that due to higher arterial wall fragility in SCAD patient, the risk of iatrogenic dissection is enhanced. There are some more imaging techniques, named intracoronary imaging such as Intravascular Ultrasonography (IVUS) and Optical Coherence Tomography (OCT) that could be used in suspected cases which angiography was not diagnostic. OCT has more resolution than IVUS and it is more sensitive to detect intimal tear and flap, but there is not any differences in Intramural Hematoma (IMH) detection. Fortunately, owing to cognition of various SCAD types in recent years, cases that need to undergo more expensive procedure have been diminished [13]. It is proved that novel techniques have the same level of risk for complications in comparison with angiography and these techniques must be performed just if the CAG has subtle results (mostly in type 3 of SCAD) [12]. In SCAD type 2, if CAG is not confirmatory and SCAD is highly suspicious (especially ACS in young healthy women without any traditional atherosclerosis risk factors and no evidence of atherosclerotic lesion in CAG), intracoronary nitroglycerin should be administered to rule out coronary spasm. If the lesion doesn’t improve, IVUS will be indicated. If the SCAD is not definitely confirmed, CAG could be re-done four weeks later. As SCAD would be resolved spontaneously, if the stenosis remains, we will conclude that it was an atherosclerotic lesion at first. In SCAD type 3, if the diagnosis is highly suspected, IVUS should be performed. The diagnosis approach is summarized in (Figure 13) [14].
Management
There are two main strategies of SCAD management: conservative and invasive. New guideline suggests early invasive intervention for revascularization of culprit lesion or conservative strategy individually based on the situation [15]
Conservative strategy
In conservative strategy, it is observed that about 70% to 97% of cases will spontaneously heal after 4 weeks but for some of them, the involved coronary artery remains dissected [16]. One study showed that if re-angiography was performed before 35th day, 88.5% of cases would show no dissection and if angiography was redone after 35th day, all cases would heal. So self-healing in SCAD is a matter of time [12]. Early complications of conservative management occur in about 5%-10% of patients who suffer from SCAD whom undergo conservative strategy. The most common complication is an extension of dissection in the first seven days [11]. These patients usually require emergent revascularization. Unfortunately, there is no known predictive factor for these patients so far [16].
Invasive strategy
Percutaneous coronary intervention: Several studies show that patients who underwent PCI for SCAD, are more prone to experiment complication [12,17-19] There are some known complications which routinely occur in SCAD patients’ PCI. It is possible that coronary guide wire enters the false lumen rather than true lumen and extends the dissection. This dissection may extend to the proximal part (retrograde dissection) or distal part (antegrade dissection) or even may cause spiral dissection to the distal artery. Extension of dissection may involve adjacent branches and cause sudden severe ischemia in cases of Left Main Coronary Artery (LMCA) involvement. If extension of dissection occurs, it is necessary to use longer coronary stent which increases the risk of stent thrombosis later. Balloon dilation also may cause extension of intimal dissection or IMH propagation to upstream or downstream which can worsen the coronary obstruction. Also, there is a potential risk of stent malposition due to IMH absorption that occurs in the nature course of disease which increases the risk of stent thrombosis [20,21]. There are some notes to improve PCI technique and reduce its complications, the operator should avoid deep coronary catheter engagement and non-coaxial positioning of catheter tip and also vigorous contrast injection. It is proven that radial access is associated with more complications rather than femoral access [12]. Post stent insertion, Dual Antiplatelet Therapy (DAPT) should be administrated [22].
Coronary Artery Bypass Grafting (CABG): CABG for SCAD patients was done for few cases so far and our knowledge about its outcome is limited to case reports. it’s a rescue technique for the patients who have left main coronary or proximal coronary involvement which is not suitable for PCI or who have failed from PCI or have been complicated with PCI. The patients’ follow-up shows that there is a huge risk of graft failure on the ground that SCAD resolves spontaneously and the competitive flow in the native coronary artery causes graft vein or artery occlusion [23].
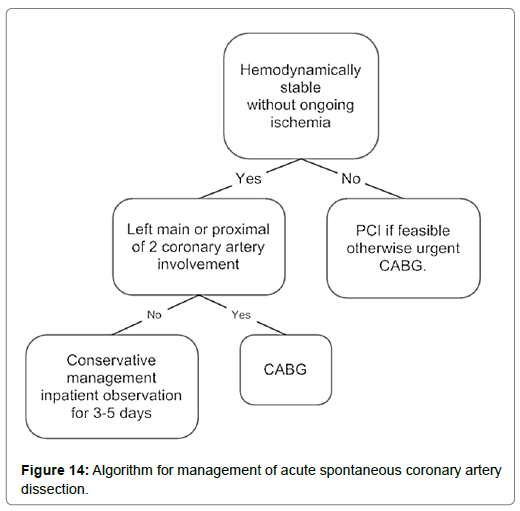
Accumulative data from multidisciplinary centers with different strategies for different patients and their outcomes were used to design an algorithm for management of patients who suffer from SCAD. Conservative therapy is the preferred approach in general for patients with stable condition and without ongoing ischemia (ongoing chest pain is not an indication for revascularization since it might be due to dissection itself and might not be related to ischemia). This strategy is not suitable for patients who suffer from ischemia in spite of conservative medication, who have left main coronary artery dissection, and are hemodynamically unstable patients. On the other hand, PCI is not appropriate for patients with involvement of distal part branches because of poor access; hence, those patients have to be managed conservatively (Figure 14) [16].
In case of Type 1 SCAD or Type 2B (type 2 with distal narrowing) which has transformed into type 1 after wire passing, the best option is medical treatment, especially if the dissection extends to distal part. But if patient remains symptomatic and ST elevation wouldn’t resolve, spot stenting from initial site of dissection occasionally by using IVUS for choosing the stent diameter and nominal pressure is the preferred option to improve distal perfusion and reduce infarcted area, because CABG is not a good option for these patients and full coverage of dissection area is nearly impossible by stent.
Medication
Anticoagulation and antiplatelet therapy
Although SCAD is of ACS category, its underlying pathophysiology of induced ischemia is entirely different from classic form of ACS which is caused by atherosclerosis that commonly is due to plaque rupture and its consequences. So although anticoagulation and antiplatelet therapy help to reduce the thrombosis burden, the real threat is the extension of the dissection or propagation of IMH. Therefore, it is rational to stop systemic anticoagulant if the patient had been managed medically (not invasively) in the absence of other conditions that require systemic anticoagulant, as soon as SCAD is proven [24]. The usage of glycoprotein IIb/IIIa inhibitors is similar to systemic anticoagulant.
There is not any certain accepted opinion for rule of antiplatelet therapy in SCAD and studies’ results are controversial but most experts recommend that if there is no contraindication for antiplatelet therapy, Acetylsalicylic Acid (aspirin or ASA) must be administrated for at least one year and if it’s possible, it’s better to use it lifelong. Dual antiplatelet therapy with ASA and Clopidogrel can be suggested for selected patients [25]. As discussed before, if PCI is performed, dual antiplatelet therapy should be prescribed at least for one year and ASA should be continued lifelong.
β-Adrenergic blockers
Using β-Adrenergic Blockers are also controversial among different studies. They are good options to control cardiac arrhythmias and hypertension but they may cause hypotension and vasospasm exacerbation [4].
Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers
It’s generally recommended for all types of ACS, including SCAD.9 Female patients should be aware of its teratogenic effect.
Statins
The nature of SCAD is not atherosclerotic, so statins don’t have any therapeutic effect and won’t reduce SCAD recurrence as studied in patients follow up. They can be prescribed for primary prevention of atherosclerosis if indicated [26].
Antianginal therapy
The most common usage of antianginal drugs is for eliminating post SCAD chest pain. It can be used in acute phase as well to reduce coronary spasm. The adverse effects are hypotension and headache [27].
Follow up
Cardiac rehabilitation
It is recommended to refer all SCAD patients to cardiac rehabilitation.27The program should focus on secondary prevention, atherosclerosis and cardiac diseases’ risk factors, and psychological and psychosocial issues. The main goal of cardiac rehabilitation is reducing emotional and physical stress that may play an important role in recurrence in 48.3% and 28.1%, respectively. Both of these two kinds of stressors are caused by catecholamine release which increases arterial wall shear stress [28]. There is no definite strategy for cardiac rehabilitation and it should be tailored to each patient [29]. There are two main reasons that physicians ignore cardiac rehabilitation in SCAD patients; the first reason is that some believe that activity may cause recurrent SCAD, and the second reason is that since SCAD is more prevalent in young individuals, some physician presume cardiac rehabilitation recommendation is unnecessary [30]. Most of the patients who start to participant in the rehabilitation program, do not complete it. The common excuses are job involvement, lack of time for attending the cardiac rehabilitation program, cost, transportation problems and concerns about danger of program for SCAD recurrence [31].
Physical activity restriction in acute phase and in future life
We should be aware that there is no distinct guideline for this matter and recommendations are mostly based on experts’ opinions. Most experts believe that heavy physical activities like lifting heavy objects and activities that need strain and Valsalva maneuver may lead to SCAD recurrence, but also extreme limitation causes sedentary life style which is a cardiac disease risk factor itself. General recommendations include keeping blood pressure during activities lower than 130/80 mmHg, maintaining 50% to 70% of reservoir heart rate of each person during activities and lifting weights for resistance training of 0.9 to 5.4 Kg by having a step up strategy which means starting from lower weight and gradually increasing it [31]
Psychosocial support
SCAD patients are more prone to have psychological distress in comparison with non-SCAD MI, especially in peri-partum individuals [32]. It’s proven that in all forms of MI, younger women are more likely at risk of post-MI chest pain syndrome and they are at higher risk of Posttraumatic Stress Disorder (PTSD) and reduced mental and physical activities after discharge from hospital. The guidelines recommend depression screening for these patients, but unfortunately most females and young patients do not receive such psychological services [33]. There is unique issue about psychological support in SCAD in comparison with classic form of MI (atherosclerotic induced), that is the prognosis and preventing factors are not well recognized. In the classic form of MI, psychological support usually focuses on encouraging patients to modify risk factors to prevent recurrent MI. But in SCAD, there is no obvious avoidable risk factor and patients don’t know how they can reduce the risk of recurrence. For example, we advise atherosclerotic MI patients to have regular physical activities for secondary prevention, but SCAD patients have fear of physical activities since they consider it as a trigger of recurrence [34].
After SCAD Chest pains syndrome
After MI chest pain syndrome is one of the most common challenging issues for patients and physicians. It causes economic and emotional burden for patients. It’s demonstrated that post MI chest pain syndrome is more common in SCAD rather than atherosclerotic MI patients. The symptoms are very variable including typical and atypical angina and also non-cardiac chest pain. The most concerning diagnosis for post SCAD chest pain syndrome is dissection extension or recurrent dissection. The key factor is an excellent history taking and physical examination. Clinicians must be aware of dissection extension, recurrent dissection and stent thrombosis possibility, if SCAD was managed by PCI. As chest wall discomfort and musculoskeletal pain are ruled out, the non-cardiac pain should be diagnosis of exclusion and these patients should be worked up for possible ACS as any other patients [35].
Post SCAD chest pains that occur in rest with emotional stress and induce no ischemia, respond well to nitrate agents. Long acting nitrate agents and calcium channel blockers can use periodically when the patients are symptomatic. The common adverse effects are hypotension and headache. Over time, the severity and the frequency of these non-ischemic chest pains reduce as a result of vessel healing or better self-controlling of anxiety [36].
Coronary reassessment after acute phase
After acute presentation of SCAD, it might be necessary to reassess the coronary artery. As we discussed before, there is potential risk of iatrogenic dissection during CAG in SCAD patients. So reassessment should be performed only if its benefits overweight its dangers. Some conditions need reassessment of coronary anatomy such as recurrent ischemic symptoms, abnormal cardiac function tests (for example positive Exercise Tolerance Testing (ETT)) and also critical anatomy (left main coronary or proximal or multi vessel dissection) which are needed to be re-evaluated considering residual dissection and their circumstances [37].
Screening for other conditions that associated with SCAD
As discussed previously, SCAD is not an isolated disease and it is associated with other diseases, especially FMD. Hence, in follow up, the clinicians must seek for signs and symptoms of the associated arteriopathies and connective tissue disorders [36].
It is recommended to perform a full vascular (Abdominal aorta, carotids, peripheral arteries including upper and lower extremities) physical examination with palpation and auscultation of artery in all patients who have experienced SCAD. Weak or asymmetric pulses and bruit are suggestive for stenosis or dissection while widened pulses are suggestive for aneurysmal artery [38]. A normal physical examination can’t rule out the underlying arteriopathies but the abnormal examination is highly suspicious. Brain and pelvic vascular imaging should be carried out in all patients who experienced SCAD, similar to patients who suffered from FMD. About 80% of FMD patients have renal artery involvement, and 74% of them have involvement of extra cranial artery, and it’s expected that this pattern also exists in SCAD patients.38 Imaging studies in SCAD patients showed presence of intracranial aneurysm in 14%-23% of patients. The final goal of these imaging studies is early detection of extra coronary abnormalities and performing interventions to avoid aneurysm rupture and its complications [39]. Patient should be aware of these diagnostic investigations’ consequences. The result of these investigations may be false positive which might lead to unnecessary interventions or might be false negative and future aneurysm rupture might happen. Even in case of true positive diagnosis, preventive intervention may cause iatrogenic dissection or aneurysm rupture.
Prognosis
A study designed to detect Major Adverse Cardiac Events (MACE) of SCAD in the first 2-3 years of incident. The MACE prevalence was 10%-30% and most prevalent event was re-MI because of coronary artery re-dissection, which occurred in 15% to 22% of patients [28]. In longer follow up, 5-7 years post incident, MACE was 15%-37% [24]. The only factor that observed as a real potential risk factor for SCAD recurrence is severe coronary tortuosity as future dissection usually occurs in tortuous segments but some studies suggest that uncontrolled hypertension is another risk factor for recurrence and using beta blockers reduce the risk of it by reducing coronary artery wall stress, especially in time of physical or emotional stress which the patients experience catecholamine surge [2].
Conclusion
SCAD is a non-atherosclerotic coronary artery disease and a rare cause of ACS which mostly occurs in young healthy women. It has a variable clinical presentation which makes it hard to diagnose, but since its management is entirely different from classic form of ACS, it is essential to be diagnosed truly. Despite the fact that there are some imaging modalities, the preferred one is coronary angiography. Having that said, some novel techniques named IVUS and OCT could be utilized in suspected cases which angiography was not diagnostic. Coronary dissection could be primary (spontaneous) or secondary (as a complication of CAG in SCAD or complication of CAG, anyway). There are two main strategies for SCAD management: conservative and invasive. Conservative strategy is preferred in stable patients with good outcomes. In unstable patients and who have critical anatomic involvement of coronary arteries, intervention is preferred. It is recommended to refer all SCAD patients to cardiac rehabilitation in follow up. The program should focus on prevention of SCAD recurrence, atherosclerosis risk factors modifying, psychological and psychosocial supports.
Acknowledgement
We are indebted to the Research Development Center of Sina Hospital for its support.
References
- Nishiguchi T, Tanaka A, Ozaki Y, Taruya A, Fukuda S, et al. (2016) Prevalence of spontaneous coronary artery dissection in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 5: 263-270.
- Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, et al. (2012) Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 126: 579-588.
- Vrints CJ (2010) Spontaneous coronary artery dissection. Heart. 96: 801-808.
- Saw J, Mancini GJ, Humphries KH (2016) Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol. 68: 297-312.
- Alfonso F, Bastante T, Rivero F, Cuesta J, Benedicto A, et al. (2014) Spontaneous coronary artery dissection. Circulation Journal. 78: 2099-2110.
- Vijayaraghavan R, Verma S, Gupta N, Saw J (2014) Pregnancy-related spontaneous coronary artery dissection. Circulation. 130: 1915-1920.
- Luong C, Starovoytov A, Heydari M, Sedlak T, Aymong E, et al. (2017) Clinical presentation of patients with spontaneous coronary artery dissection. Catheter Cardiovasc Interv. 89: 1149-1154.
- Saw J, Aymong E, Mancini GJ, Sedlak T, Starovoytov A, et al. (2014) Nonatherosclerotic coronary artery disease in young women. Can J Cardiol. 30: 814-819.
- Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, et al. (2012) 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non–ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 60: 645-681.
- Saw J. (2014) Coronary angiogram classification of spontaneous coronary artery dissection. Catheterization and Cardiovascular Interventions. 84: 1115-1122.
- Saw J, Aymong E, Sedlak T, Buller CE, Starovoytov A, et al. (2014) Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv. 7: 645-655.
- Prakash R, Starovoytov A, Heydari M, Mancini GJ, Saw J, et al. (2016) Catheter-induced iatrogenic coronary artery dissection in patients with spontaneous coronary artery dissection. JACC Cardiovasc Interv. 9: 1851-1853.
- Paulo M, Sandoval J, Lennie V, Dutary J, Medina M, et al. (2013) Combined use of OCT and IVUS in spontaneous coronary artery dissection. JACC: Cardiovascular Imaging. 6: 830-832.
- Yip A, Saw J (2015) Spontaneous coronary artery dissection-a review. Cardiovasc Diagn Ther. 5: 37.
- Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, et al. (2014) 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes: a report of the american college of cardiology/american heart association task force on practice guidelines. Circulation. 64: e139-228.
- Tweet MS, Eleid MF, Best PJ, Lennon RJ, Lerman A, et al. (2014) Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circulation: Cardiovascular Interventions. 7: 777-786.
- Rashid HN, Wong DT, Wijesekera H, Gutman SJ, Shanmugam VB, et al. (2016) Incidence and characterisation of spontaneous coronary artery dissection as a cause of acute coronary syndrome—a single-centre Australian experience. Int J cardiol. 202: 336-338.
- Gupta A, Wang Y, Spertus JA, Geda M, Lorenze N, et al. (2014) Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. J Am Coll Cardiol. 64: 337-345.
- Rogowski S, Maeder MT, Weilenmann D, Haager PK, Ammann P, et al. (2017) Spontaneous coronary artery dissection: angiographic follow‐up and long‐term clinical outcome in a predominantly medically treated population. Catheter Cardiovas Interv. 89: 59-68.
- Alfonso F, Bastante T, García-Guimaraes M, Pozo E, Cuesta J, et al. (2016) Spontaneous coronary artery dissection: new insights into diagnosis and treatment. Coronary artery disease. 27: 696-706.
- Lempereur M, Fung A, Saw J (2015) Stent mal-apposition with resorption of intramural hematoma with spontaneous coronary artery dissection. Cardiovas Diagn Ther. 5: 323-329.
- Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, et al. (2011) 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: executive summary: a report of the american college of cardiology foundation/american heart association task force on practice guidelines and the society for cardiovascular angiography and interventions. Circulation. 58: 2550-2583.
- Patel KP, Sotolongo RP, Myrick TW (2008) Off-pump coronary artery bypass grafting for spontaneous coronary dissection in a 26-year-old patient two weeks post-partum: a case report and review. J Extra Corpor Technol. 40: 127-129.
- Lettieri C, Zavalloni D, Rossini R, Morici N, Ettori F, et al. (2015) Management and long-term prognosis of spontaneous coronary artery dissection. Am J Cardiol. 116: 66-73.
- Tweet MS, Gulati R, Hayes SN (2015) What clinicians should know about spontaneous coronary artery dissection. Mayo Clin Proc 90: 1125-1130.
- Stone NJ, Robinson JG, Lichtenstein AH, Merz CN, Blum CB, et al. (2014) 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the american college of cardiology/american heart association task force on practice guidelines. circulation. 63: 2889-2934.
- Hayes SN (2014) Spontaneous coronary artery dissection (SCAD): new insights into this not-so-rare condition. Tex Heart Inst J. 41: 295-298.
- Saw J, Humphries K, Aymong E, Sedlak T, Prakash R, et al. (2017) Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol. 70: 1148-1158.
- Thomas RJ, King M, Lui K, Oldridge N, Piña IL, et al. (2010) AACVPR/ACCF/AHA 2010 Update: Performance Measures on Cardiac Rehabilitation for Referral to Cardiac Rehabilitation/Secondary Prevention Services. Circulation. 56: 1159-1167.
- Tweet MS, Gulati R, Hayes SN (2016) Spontaneous coronary artery dissection. Curr Cardiol Rep. 18: 60.
- Chou AY, Prakash R, Rajala J, Birnie T, Isserow S, et al. (2016) The first dedicated cardiac rehabilitation program for patients with spontaneous coronary artery dissection: description and initial results. Can J Cardiol. 32: 554-560.
- Liang JJ, Tweet MS, Hayes SE, Gulati R, Hayes SN, et al. (2014) Prevalence and predictors of depression and anxiety among survivors of myocardial infarction due to spontaneous coronary artery dissection. J Cardiopulm Rehabil Prev. 34: 138-142.
- Garavalia LS, Decker C, Reid KJ, Lichtman JH, Parashar S, et al. (2007) Does health status differ between men and women in early recovery after myocardial infarction?. J Womens Health. 16: 93-101.
- Saw J, Sedlak T, Ganesh SK, Isserow S, Mancini GJ, et al. (2015) Spontaneous coronary artery dissection (SCAD). Circulation. 131: e3-5.
- Mieres JH, Gulati M, Bairey Merz N, Berman DS, et al. (2014) Role of noninvasive testing in the clinical evaluation of women with suspected ischemic heart disease: a consensus statement from the American Heart Association. Circulation. 130: 350-379.
- Hayes SN, Kim ES, Saw J, Adlam D, Arslanian-Engoren C, et al. (2018) Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation. 137: e523-557.
- Motreff P, Souteyrand G, Dauphin C, Eschalier R, Cassagnes J, et al. (2010) Management of spontaneous coronary artery dissection: review of the literature and discussion based on a series of 12 young women with acute coronary syndrome. Cardiology. 115: 10-18.
- Olin JW, Froehlich J, Gu X, Bacharach JM, Eagle K, et al. (2012) The United States Registry for Fibromuscular Dysplasia: results in the first 447 patients. Circulation. 125: 3182-3190.
- Thompson BG, Brown Jr RD, Amin-Hanjani S, Broderick JP, Cockroft KM, et al. (2015) Guidelines for the management of patients with unruptured intracranial aneurysms: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 46: 2368-2400.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi