Research Article, J Polym Sci Appl Vol: 9 Issue: 1
Structural and Charge Transport Mechanism of Polyacetylene Composites
Muhammad Irfan1*, Waseem Abbas1, Umair Shehzad1, Ahsan Mazhar1, Mehrun Nisa2, Ambreen Kalsoom2, Alfa Riaz2, Sadia Malik2, Imran Saleem3, N. Bano4, M. Imran5 and Malik Tahir Mehmood6
1Department of Physics, Bahauddin Zakariya University, Multan, Pakistan
2Department of Physics, Govt Sadiq College Women University, Bahawalpur, Pakistan
3Department of Environmental Enginering, Myongji University, Seoul, South Korea
4Department of Physics and Astronomy, College of Science, King Saud University, Riyadh, Saudi Arabia
5Department of Physics, Govt College University of Lahore, Lahore, Pakistan
6Department of Physics, Islamia University of Bahawalpur, Bahawalpur, Pakistan
*Corresponding Author: Muhammad Irfan, Department of Physics, Bahauddin Zakariya University, Multan, Pakistan
E-mail: mirfanphysics@gmail.com
Received date: 09 July, 2024, Manuscript No. JPSA-24-141169;
Editor assigned date: 12 July, 2024, PreQC No. JPSA-24-141169 (PQ);
Reviewed date: 26 July, 2024, QC No. JPSA-24-141169;
Revised date: 17 January, 2025, Manuscript No. JPSA-24-141169 (R);
Published date: 24 January, 2025 DOI: 10.4172/Jpsa.1000155.
Citation: Irfan M, Abbas W, Shehzad U, Mazhar A, Nisa M, et al. (2025) Structural and Charge Transport Mechanism of Polyacetylene Composites. J Polym Sci Appl 9:1.
Abstract
Polyacetylene was synthesized by using Ziegler-Natta catalyst with chemical polymerization method and doped it with 10% iodine and 10% bromine to prepared the composites. The samples were characterized by XRD, SEM and temperature dependent DC electrical conductivity. The XRD pattern of doped polyacetylene displayed that the crystallinity was improved. The SEM morphology of doped polyacetylene demonstrated that granularity was increased. The calculated electrical conductivity shows the low electric conductivity of pure polyacetylene but when we doped polyacetylene with iodine and bromine the electrical conductivity was improved. This study explored that the improvement in the electrical conductivity which may confirm the doped polyacetylene behave as semiconductor and can by helpful for the potential application of devices and related fields.
Keywords: Polyacetylene, Ziegler-Natta catalyst, Crystallinity, DC conductivity
Introduction
Materials have been divided into three categories with respect to their electrical conductivity: Insulators, semiconductors and conductors. Conjugated polymers are in the category of semiconductors [1]. Conjugated polymers are also called synthetic metals. The conductivity of conjugated polymers is due to the movement of π-bonded electrons at backbone of polymer materials displays electronic effects such as small ionization potentials and greater electron affinities. Conjugated polymers provide impedance type gas sensors for low cost, highly sensitive at room temperature [2].
In conjugated polymers, the creation of holes and free electrons does not take place in the conduction bands as in crystalline semiconductors. The dissimilarity to inorganic semiconductors is in the shape of the polymer backbone that deformed when the polymer is oxidized or reduced by the dopants. The properties of conjugated polymers are optoelectronic, optical, electrical, mechanical properties and microwave absorbing properties [3-6]. The conjugated polymers are used in different areas like microelectronics, energy storage devices, rechargeable batteries, supercapacitor, photovoltaic devices, fuel cells, actuators, Polymeric Light Emitting Diodes (LED’s), corrosion protection technology, electro chromic device technology, bio medical applications, membrane technology and drug distribution of conducting polymers [7]. There are several conjugated polymers namely polyacetylene, polyaniline, polyphenylene, polypyrrole, polythiophene and their derivatives [8]. The polyacetylene is an organic polymer which is the combination of repeating small units and it will give the idea of polymerization of acetylene [9]. Polyacetylene is considered as the first synthesize simple organic conjugated polymer. The main advantage of use of the polyacetylene over the other organic conjugated polymer is air sensitive due to this reason that researchers have been focus on it [10]. The chemical formula of polyacetylene is H(HC=CH) nH. Where “n” represents the number of repeating units. The geometry of double bonds can be cis or trans form of polyacetylene [11]. When hydrogen atoms are linked to same side of carbon-carbon double bond in the structure of polyacetylene so this type of polyacetylene is known as cis polyacetylene [12-14]. Similarly, when hydrogen atoms are linked to the opposite sides of carboncarbon double bond in the structure of polyacetylene so this type of polyacetylene is known as trans-polyacetylene. The conjugated polymers can be doped to p-type or n-type and show a different class when mixed with impurities. Polyacetylene show p-type or n-type doping when it mixed with impurities [15-18]. The doping of polyacetylene with halogens such as 10% (0.25 g) AsF5, 10% (0.25 g) SbF5 presented p-type doping whereas mixed with alkalis provided ntype doping were studied in the literature review [18]. The electrical conductivity is also increased as dopants were mixed via enabling the transportation of charges through the finite polyenes [19,20]. The main aim of the present work is to examine the temperature dependent DC conductivity of low doped polyacetylene composites. For this purpose, we synthesized polyacetylene via chemical polymerization technique and doped it with iodine and bromine to study their charge transport mechanism which was not previously investigated.
Materials and Methods
In-situ chemical polymerization method was used to synthesize the polyacetylene. At first a solution of Ziegler-Natta catalyst was prepared by mixing 5 mol of tetra-butoxy-titanium and 10 mol of triethylaluminium into the 50 ml of toluene. The solution was stirred by using magnetic stirrer at room temperature for about 20 min. After that 30 ml solution was separated into the flask and cooled down by using dry ice. After that acetylene gas was introduced into the solution for the formation of polyacetylene film. The remaining catalyst solution was removed with syringes. Before toluene was used to wash the polyacetylene film until it became colorless. To avoid the cis/trans isomerization, the temperature of polymerization was kept maintained during the purification. At this time washed polyacetylene film and was dried under nitrogen at room temperature. The nitrogen was kept at -30°C temperature and this polymerization can be performed under different temperatures from -100°C to +180°C. In case when temperature less than 80°C, toluene is used as a solvent. The polyacetylene film was seen when there was a change in color take place. The variation in colors was occurs due to the different polymerization temperatures. When temperature was below 0°C then the red color film was observed. At room temperature, the color of the film was purple. At temperature greater than 100°C the film color was blue. When electron acceptors are mixed to the polyacetylene such as I2, Br2, AsF5 is termed as p-type doping whereas when electron donating species are mixed to the polyacetylene such as Li, K, Na is known as n-type doping. In this research the p-type doping of polyacetylene with iodine and bromine was performed. The kept the pressure during the doping process was almost one torr. The structural properties of pure and doped polyacetylene with bromine and iodine were confirmed by X-Ray Diffraction (XRD) technique by using ‘Panalytical X’pert PRO’ diffractometer with radiation source 1.54 Å CuKα operated at 40 kV and 30 mA at scan rate of 2°/min in the range of 2θ =10° to 70°. The surface morphologies were studied by ‘EVO 50 ZEISS’ Scanning Electron Microscope (SEM). The pellets of synthesized samples were connected to a Keithley-2400 electrometer and a current source electrometer for DC Conductivity and activation energy were measurements by using two probe technique.
Results and Discussion
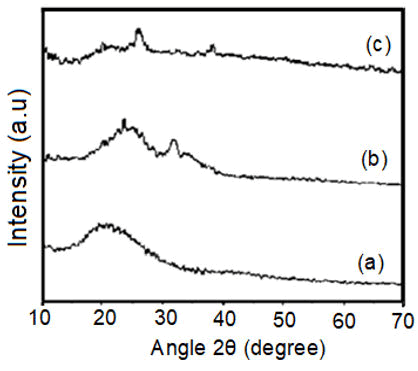
The obtained broad peak at the smaller angle 2θ =18.97° to 27.95°in XRD pattern of pure polyacetylene as shown in Figure 1 (a) revealed its amorphous structure. The broadness in the peak and the smaller angle indicated that higher inter-chain distance as well as more open structure and disorder was present in pristine polyacetylene. The dspacing values were calculated by using the Bragg’s Law.

Here, n=1, λ=constant (1.54Å), θ=peak angle, d=d-spacing
The XRD pattern represented in Figure 1 (b) of bromine doped polyacetylene revealed two sharp peaks at an angle 2θ=25.37° and 2θ=32.44° and their corresponding d-spacing values are 3.50 Å and 2.76 Å representing the improvement of the crystallinity of synthesized Polyacetylene-5% Bromine composite. The observed XRD pattern in Figure 1(c) for an iodine doped polyacetylene revealed three sharp peaks at angles of 2θ=19.88°, 26.04°, 38.38° and their corresponding d-spacing values are 4.46 Å, 3.41 Å, 2.34 Å. Therefore, these sharp peaks clearly indicate that the crystallinity of polyacetylene has been increased significantly by incorporated an iodine to form the Polyacetylene 5% iodine composite.
Figure 1: XRD of (a) Pure polyacetylene (b) Polyacetylene-10% Bromine composite (c) Polyacetylene 10% Iodine composite.
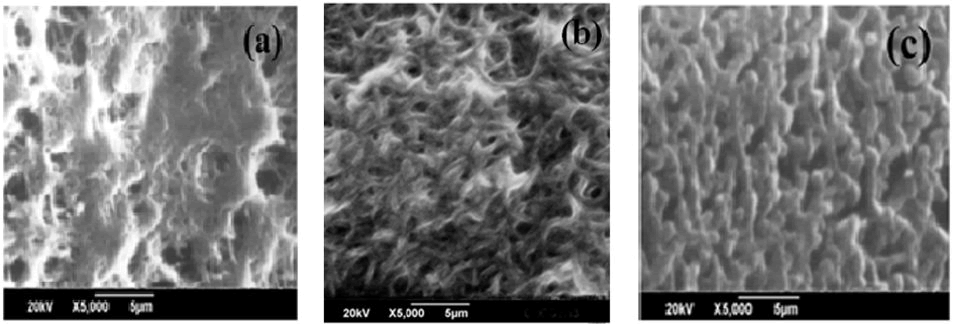
In order to investigate the surface morphology of the synthesized samples via utilizing the SEM analysis route. The SEM image of pure polyacetylene shows in Figure 2 (a) randomly oriented fibril network was observed. The SEM image of bromine doped polyacetylene composite as shown in Figure 2 (b) represents the fibrous morphology with increased in particle size of the fibril. The SEM photo of iodine doped polyacetylene composite is showing the more growth in particle size and the structure appears like fibrous nature and also shows the small and thick rod like fibrils as exhibited in Figure 2 (c).
Figure 2: SEM images of (a) Pure Polyacetylene (b) Polyacetylene-10% Bromine composite (c) Polyacetylene-10% Iodine composite.
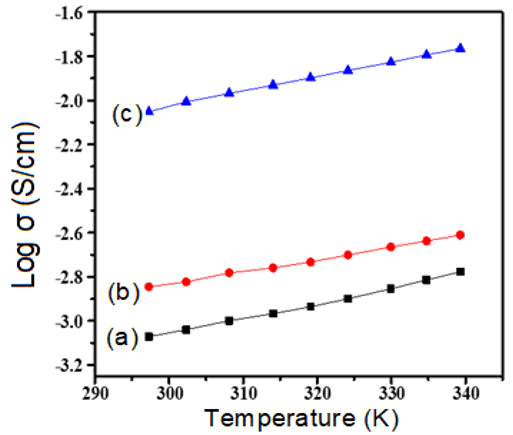
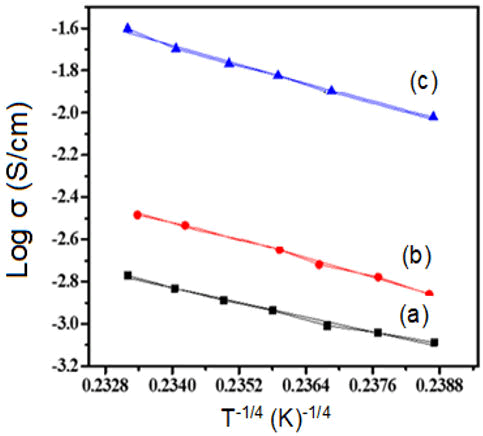
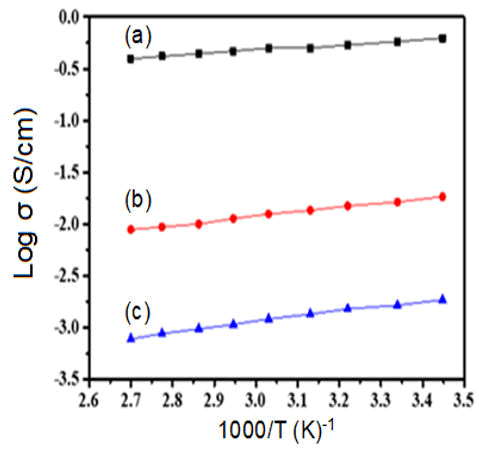
The nature as well as charge transport mechanism for polymer samples can be studied through the DC-conductivity characteristics. The electrical conductivity of doped polyacetylene decreased by dropping the temperature. The temperature range 293K and above the electrical conductivity of polyacetylene was increased due to the thermally activation process. At temperature greater than 200°C the fibrils are broken down aa well as reduce the crystallinity and therefore, the conductivity of iodine doped polyacetylene was observed to be reduce at higher temperature. When polyacetylene is exposed to the vapors of iodine its electrical conductivity is raised intensely similarly, when polyacetylene was doped to the bromine vapors then its electrical conductivity was increased as shown in Figures 3 and 4. The semiconducting behavior was observed for both composites’ bromine doped polyacetylene and iodine doped polyacetylene due to the delocalization of charge carriers. The conduction mechanism is due to the transfer of charges from conjugated polymer to the accepter due to the presence of iodine that generates the acceptor states within the energy band gap. The electrical conductivity was observed to be improved at room temperature in the range of 10-160 (ohm cm)-1 for composites. From graph Log σ vs. T-1/4 as shown in Figure 4, it was clear that the DC conductivity of bromine doped polyacetylene composite is less than the conductivity of iodine doped polyacetylene composite will follow the 3D-VRH model for charge transport mechanism. The activation energy is calculated using the Arrhenius exponential equation and from the slope of graph of Log σ vs. 1000/T as shown in Figure 5.
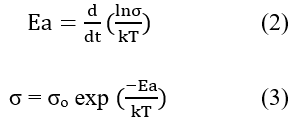
Where Ea=Activation energy, k=Boltzmann constant, T=Temperature
Figure 3: Graph of Log σ vs. Temperature (a) Pure Polyacetylene (b) Polyacetylene-10% Bromine composite (c) Polyacetylene 10% Iodine composite.
Figure 4: Graph of Log σ vs. T-1/4 (a) Pure Polyacetylene (b) Polyacetylene-10% Bromine composite (c) Polyacetylene 10% iodine composite.
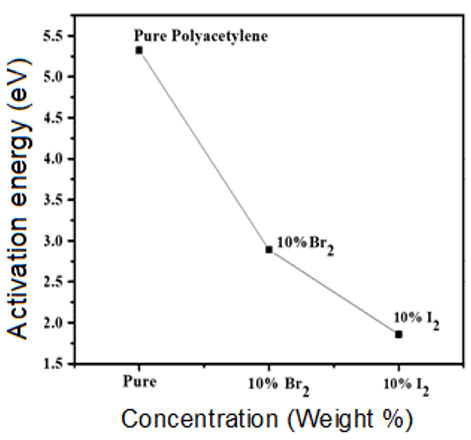
The activation energy values were obtained for polyacetylene, bromine doped polyacetylene and iodine doped polyacetylene from the Figure 6 and are given in Table 1. The activation energies are going to decrease from pure polyacetylene to doped polyacetylene. The values of activation energy for iodine doped polyacetylene composite is low as compared to pure polyacetylene as well as bromine doped polyacetylene composite.
Figure 5: Graph of Log σ vs. 1000/T for (a) Pure Polyacetylene (b) Polyacetylene-10% Bromine composite (c) Polyacetylene 10% iodine composite.
Figure 6: Graph for activation energy versus concentration of Polyacetylene, Polyacetylene-10% Bromine composite and Polyacetylene-10% iodine composite.
| Synthesized materials | Activation energy (Ea, eV) |
| Polyacetylene | 5.20 eV |
| Polyacetylene-10% Bromine | 2.75 eV |
| Polyacetylene-10% Iodine | 1.60 eV |
Table 1: Determination of activation energy for polyacetylene, bromine doped polyacetylene and iodine doped polyacetylene.
Conclusion
The polyacetylene was prepared by using Ziegler-Natta catalyst via in-situ polymerization method and doped it with iodine and bromine. From XRD patterns it was observed that polyacetylene has an amorphous nature because there was only a broad peak at angle 2θ range 16° to 26°. The XRD pattern of polyacetylene doped iodine and also polyacetylene doped bromine but iodine doped polyacetylene shows more crystallinity as compared to bromine doped polyacetylene. The SEM images of pure as well as iodine and bromine doped polyacetylene exhibited the granular as well as fibrous morphology. Electrical properties were observed for pristine and doped polyacetylene. Pure polyacetylene shows less electrical conductivity while iodine doped polyacetylene showed the higher conductivity as compared to the bromine doped polyacetylene. The electrical conductivity of iodine doped polyacetylene was much higher than pure polyacetylene which make theses composites material more useful for the potential application of devices.
References
- Ala O, Fan Q (2009) Applications of conducting polymers in electronic textiles. Res J Text Apparel 13: 51-68.
- Ates M (2013) A review study of (bio) sensor systems based on conducting polymers. Mater Sci Eng C Mater Biol Appl 33: 1853-1859.
[Crossref] [Google Scholar] [PubMed]
- Tomkiewicz Y, Schultz TD, Brom HB, Taranko AR, Clarke TC, et al. (1981) Solitons or inhomogeneous doping in As F 5-doped polyacetylene—EPR and dc conductivity evidence. Phys Rev B 24: 4348.
- SedláÄÂek J, Balcar H (2017) Substituted polyacetylenes prepared with Rh catalysts: from linear to network-type conjugated polymers. Polym Rev 57: 31-51.
- Gusakov PE, Andrianov AV, Aleshin AN, Matsushita S, Akagi K, et al. (2012) Electrical and optical properties of doped helical polyacetylene graphite films in terahertz frequency range. Synth Met 162: 1846-1851.
- Cao Y, Smith P, Heeger AJ (1991) Mechanical and electrical properties of polyacetylene films oriented by tensile drawing. Polymer 32: 1210-1218.
- Das TK, Prusty S (2012) Review on conducting polymers and their applications. Polym Plast Technol Eng 51: 1487-1500.
- Chiang CK (2003) The bromine doping of polyacetylene. Phys A: Stat Mech Appl 321: 139-151.
- Sichel EK, Knowles M, Rubner M, Georger Jr J (1982) Effect of dopant-molecule size on the electrical conductivity of polyacetylene. Phys Rev B 25: 5574.
- Chiang CK, Park YW, Heeger AJ, Shirakawa H, Louis EJ, et al. (1978) Conducting polymers: Halogen doped polyacetylene. J Chem Phys 69: 5098-5104.
- Shirakawa H (2001) The discovery of polyacetylene film–the dawning of an era of conducting polymers. Curr Appl Phys 1: 281-286.
- Shirakawa H, Louis EJ, MacDiarmid AG, Chiang CK, Heeger AJ, et al. (1977) Synthesis of electrically conducting organic polymers: halogen derivatives of polyacetylene,(CH) x. J Chem Soc ChemComm 16: 578-580.
- Epstein AJ, Rommelmann H, Fernquist R, Gibson HW, Druy MA, et al. (1982) Morphology of polyacetylene and doped polyacetylene. Polymer 23: 1211-1221.
- Chiang CK, Heeger AJ, MacDiarmid AG (1979) Synthesis, structure, and electrical properties of doped polyacetylene. Bunsen Socity Phys Chem 83: 407-417.
- Anderson M, Ramanan C, Fontanesi C, Frick A, Surana S, et al. (2017) Displacement of polarons by vibrational modes in doped conjugated polymers. Phys Rev Materials 1: 055604.
- Murthy NS, Shacklette LW, Baughman RH (1989) Structure and staging in polyacetylene charge-transfer complexes. Solid State Commun 72: 267-270.
- Rolland M, Cadène M, Bresse JF, Rossi A, Riviere D, et al. (1981) SEM and castaing microprobe studies of undoped and doped polyacetylene films. Mater Res Bull 16: 1045-1054.
- Chiang CK, Fincher JCR, Park YW, Heeger AJ, Shirakawa H, et al. (1977) Electrical conductivity in doped polyacetylene. Phys Rev Lett 39(: 1098.
- Ehinger K, Roth S (1986) Non-solitonic conductivity in polyacetylene. Philos Mag B 53:301-320.
- Heeger AJ, Kivelson S, Schrieffer JR, Su WP (1988) Solitons in conducting polymers. Rev Mod Phys 60: 781.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi