Review Article, J Plant Physiol Pathol Vol: 5 Issue: 4
Sucrose Metabolism and Regulation in Sugarcane
Jungang Wang, Tingting Zhao, Benpeng Yang and Shuzhen Zhang*
Institute of Tropical Bioscience and Biotechnology of Chinese Academy of Tropical Agricultural Sciences, Sugarcane Research Center of Chinese Academy of Tropical Agricultural Sciences, Key Laboratory of Biology and Genetic Resources of Tropical Crops, Ministry of Agriculture, Haikou Hainan–571101, China
*Corresponding Author : Shuzhen Zhang
Institute of Tropical Bioscience and Biotechnology of Chinese Academy of Tropical Agricultural Sciences, Sugarcane Research Center of Chinese Academy of Tropical Agricultural Sciences
Key Laboratory of Biology and Genetic Resources of Tropical Crops, Ministry of Agriculture, Haikou Hainan –571101, China
Tel: +86-898-66215109
E-mail: zhangshuzhen@itbb.org.cn
Received: August 11, 2017 Accepted: September 05, 2017 Published: September 12, 2017
Citation: Wang J, Zhao T, Yang B, Zhang S (2017) Sucrose Metabolism and Regulation in Sugarcane. J Plant Physiol Pathol 5:4. doi: 10.4172/2329-955X.1000167
Abstract
Sucrose Metabolism and Regulation in Sugarcane
Photo assimilates store in the form of sucrose in the stalks of sugarcane that provide 70% of world sugar. To meet the growing demand for sugar, it needs to increase the sugar yield. Sucrose accumulation is a complex physiological process controlled by network genes. This review summarized the development in the molecular regulation mechanism of sucrose accumulation in sugarcane. The molecular involving in source-sink communication, sucrose metabolism and storage were discussed. Although the sugar yield in sugarcane has not been increased by genetically modification, further researches to clarify the regulatory networks of sucrose accumulation will be helpful to breed high sugar content sugarcane.
Keywords: Sugarcane; Sucrose accumulation; Sucrose metabolism; Sugar transporter
Introduction
Sugars are carbon and energy resources for organisms. These sugars originate from photosynthesis which converts inorganic CO2 into carbohydrates. Generally, these carbohydrates store in the forms of polysaccharides, such as starch and cellulose, in plant sink organs. However, sugarcane can directly store assimilates in the form of sucrose in stalk parenchymatous cells which can directly supply sugar to humans. Hence sugarcane becomes an important worldwide sugar crop and provides more than 70% of sugars in the world. The world sugar demand is growing by years. It is necessary to increase sugar yield from sugarcane. The molecular genetics control mechanisms on sucrose content in sugarcane are complex, which limits the improvement on increasing sucrose yield. To clarify the molecular mechanisms on sucrose accumulation will be beneficial to improve sugarcane quality and understand sucrose accumulation regulation in plants.
Sucrose is synthesized in source mature leaves. Upon the phloem transportation, sucrose is unloaded into the sink for metabolism and storage. Sucrose accumulation in sugarcane stalks is affected by sucrose supply, metabolism and sink strength [1]. The photosynthesis activity in source leaves decides the amount of sucrose to sink organs. Sucrose metabolism enzymes control sucrose content in sugarcane stalks. Sink strength which decides by sink size and sink activity determines the amount of sugar storage and plant productivity [2-5]. The genes associated with sugar storage including sucrose metabolism enzymes, sucrose transporters and source-sink signal transduction molecular have been analyzed in plants [6-8]. Although the molecular regulation mechanism on sucrose accumulation is unclear yet in sugarcane, potential molecular and genes regulating sucrose accumulation have been identified. In this review, we summarized the developments of the molecular regulation mechanisms on sucrose accumulation in sugarcane from four aspects: (a) source/sink feedback regulation of sucrose accumulation; (b) molecular involving sucrose metabolism and transport in sugarcane; (c) candidate regulator genes relative to sucrose accumulation; (d) manipulations on improving sugarcane sugar yield. Finally, this review will point out further research directions to reveal molecular mechanisms underpinning sucrose accumulation in sugarcane.
Source-sink regulation of sucrose accumulation
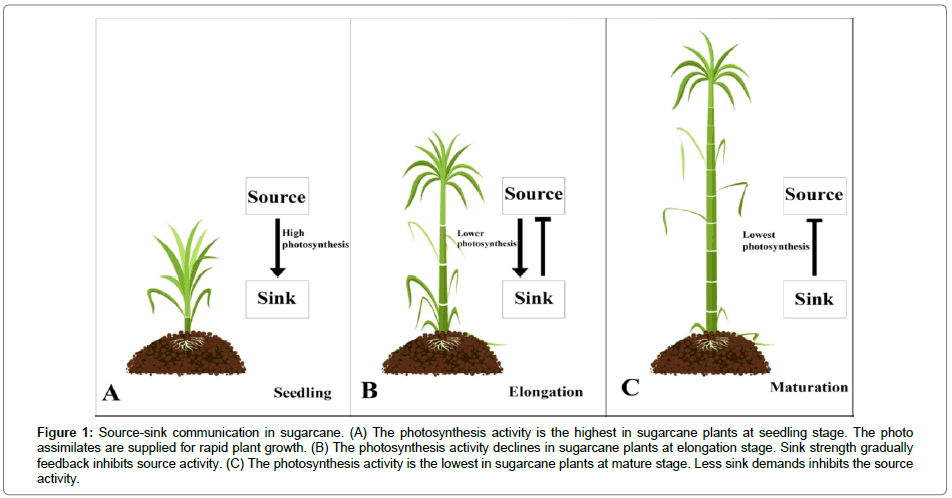
Sugar accumulations in plants are decided by source supply and sink demand. When source supply is not enough for sink demand, it defines as a source-limited plant. On the contrary, when source supply exceeds sink demand, it defined as sink-limited plant. For most of sugar plants, it seems to be sink-limited during sugar accumulation [9]. Sucrose demand in sugarcane stalks feedback regulates source leaves photosynthesis activity [2,10]. In general commercial sugarcane cultivars, photosynthesis activity decreased gradually with stalk maturation [10] (Figure 1). Moreover, high-sucrose genotypes (Saccharum officinarum L.) had lower photosynthesis activity than low-sucrose genotypes (Saccharum spontaneum L.) [10]. It demonstrates that the source-sink interactions control sucrose accumulation in sugarcane.
Figure 1: Source-sink communication in sugarcane. (A) The photosynthesis activity is the highest in sugarcane plants at seedling stage. The photo assimilates are supplied for rapid plant growth. (B) The photosynthesis activity declines in sugarcane plants at elongation stage. Sink strength gradually feedback inhibits source activity. (C) The photosynthesis activity is the lowest in sugarcane plants at mature stage. Less sink demands inhibits the source activity.
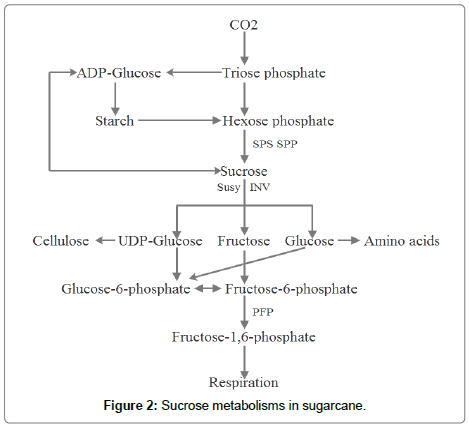
Sucrose supply in source leaves: In C4 plants, CO2 is fixed by phosphoenolpyruvate carboxylase (PEPCase) in mesophyll cells to form C4 acid, which is decarboxylated to release CO2 in bundle sheath cells. Then the released CO2 is fixed again by ribulose-1,5-biphosphate carboxylase:oxygenase (Rubisco) into Clavin-Benson cycle to synthesize triose phosphates, which is transported in the cytoplasm to synthesize sugars. These sugars, major in the form of sucrose, are immediately loaded into phloem for export to heterotrophic organs. When sugars in cytoplasm are excessive, they are transported into vacuoles for transient storage and triose phosphates are used to synthesize starch in chloroplasts (Figure 2). The activity of photosynthesis can be influenced by development and environmental signals. Although C4 photosynthesis was firstly reported in sugarcane leaves [11], the regulation mechanisms of sugarcane photosynthesis are far unknown.
The transcriptom and photosynthesis activity in segments of the first leaf were analyzed in sugarcane [12]. It showed that the middle segment had the highest photosynthesis rate, higher abundance and activity of PEPase and the most genes expression abundance including enzymes in Calvin-Benson cycle. However, there were no difference of Rubisco activity and the transcriptional expression of PEPase along the leaf segments. When exogenous sucrose was supplied on sugarcane leaves, the photosynthesis activity was decreased and the activity of Rubisco was also down-regulated, but the activity of PEPCase did not change [13]. Moreover, the activities of sucrose metabolism enzymes, invertase, sucrose phosphate synthase and sucrose synthase, were up-regulated under exogenous sucrose treatment [13]. These studies indicated that PEPCase and Rubisco were the key regulating points to control source supply in sugarcane source leaves. As a C4 plant, sugarcane leaves have two CO2 carboxylase, PEPCase and Rubisco, which cooperated to regulate photosynthetic activity [10]. The activity of PEPCase can be developmentally regulated and the activity of Rubisco can be regulated by sugar feedback in sugarcane.
Sucrose accumulation in stalks: The photo assimilates synthesized in source leaves will be transported by phloem to heterotrophic sink organs to supply plants growth and development. When the unloading assimilates exceed the metabolism demands, they will be stored in sink organs or inverted to other storage carbohydrates. Once more assimilates, major in the form of sucrose, accumulate in sink cells, it will induce high turgor pressure. Plant sink cells use two strategies to keep low turgor to drive continuous unloading. One is to compartment sucrose during cytoplasm, vacuole and apoplasm. The other is to synthesize low osmotic polymers such as starch, protein and oil to reduce turgor [6,9]. In sugarcane stems, sucrose is unloaded symplasmically firstly into storage parenchyma cells, then leak into apoplasm with sucrose concentration at 400- 700 mM [14,15]. The apoplastic sucrose cannot return into phloem preventing by a suberized cell walls barrier of bundle sheath cells [16]. Sucrose leakage in apoplast not only increases the amount of sucrose storage but also maintains a low turgor homoeostasis of parenchyma cells to accelerate sucrose accumulation [14]. Hence sucrose compartmentation between vacuole and apoplast is a control step for sucrose accumulation in sugarcane. It can be postulated that there is a low tugor maintence mechanism during sucrose accumulation and redistribution between apoplasm and symplasm in sugarcane stalks. Sucrose is actively metabolized to continuously hydrolyze and resynthesize and used for respiration, metabolism and storage in sugarcane internodes [1,17] (Figure 2). In immature internodes, 66% of carbon partitioned into respiration and synthesizes of proteins and fiber, while 34% of carbon was stored as sucrose [18]. But in more mature internodes, 66% of carbon partitioned into sucrose and carbon for respiration, proteins and fiber syntheses decreased [18]. The carbon assimilates mainly partitions between sucrose and fiber in mature sugarcane stalks. The compartmental distribution and partitioning of sucrose decide sink strength and sucrose yield in sugarcane stalks. However, the molecular networks to control sucrose content in sugarcane stalks are needed to be studied.
Source-sink communication: Photosynthesis consists of many metabolic processes, which maximize reasonably uses of light, carbon and nitrogen resources. Sink strength regulates the activity of photosynthesis not simply by a sugar feedback mechanism but through a signal pathway which senses the status of carbon/nitrogen in the whole plant and regulates the expression of photosynthetic genes and leaves development [4]. The leaf photosynthesis rates gradually decreasing with sugarcane maturation demonstrates that there exists a sink feedback regulation on source activity in sugarcane [10]. The source-sink relationship in sugarcane was studied by leaves shading treatment. When all leaves were shaded, except for a single leaf in sugarcane, the sink strength was increased which feedback up-regulated source activity [2,19]. During leaf shading treatment, sucrose content in immature internodes reduced to increase the sink demand and feedback up-regulated source activity. Although the majority of leaves were enclosed, the sucrose accumulation in sink stalks was not affected [2,19]. It appears that sucrose accumulation in sugarcane stalks is not source-limited and sink strength decides the sucrose content. During source-sink communication, genes relative to photosynthesis, mitochondria metabolism and sugar transport were regulated [19]. The up-regulated genes, especially for Rubisco and PEPase, may up-regulate the photosynthesis activity [19]. The down regulated gene, hexokinase, might sense the hexose signals to regulate source activity. It also demonstrated that CO2 carboxylases were the key regulated points during source-sink communication in sugarcane. Interestingly, the enhanced photosynthesis by leaf shading treatment could offset the photosynthesis inhibition caused by sucrose spraying in sugarcane through regulating the abundance and activities of Rubisco and PEPase [20]. More work is needed to clarify the signal molecular involving in source-sink communication in sugarcane, which is helpful to utilize the high efficient C4 photosynthesis to increase sucrose yield.
Sucrose metabolism and transport
Sucrose metabolism enzymes: Enzymes catalyzing sucrose synthesis and hydrolysis regulate sucrose content in sugarcane. Sucrose phosphate synthase (SPS) and sucrose phosphate phosphatase (SPP) are responsible for sucrose synthesis. SPS is the key enzyme regulating sucrose synthesis which can be active or inactive by protein phosphorylation under light and osmotic stress conditions [21]. The activity of SPS can be active by glucose-6-phosphate and inhibited by Pi [21]. In plants, SPS which expresses both in source and sink takes part in sucrose re-synthesis and regulate starch accumulation [22], protein storage [23] and cellulose synthesis [24]. Invertase (INV) and sucrose synthase (Susy) catalyze sucrose hydrolysis in plants. Invertases are classified as soluble acid invertase (SAI) and neutral invertase (NI) according to their optimum pH. Invertase involves in regulating carbon allocation and development and Susy involves in regulating syntheses of cellulose, starch and protein in plants sink tissues [7].
In sugarcane, sucrose metabolism enzymes, SPS, Susy, SAI and NI, were expressed both in leaves and stalks [25,26]. The activity of SPS was higher in high sucrose content sugarcane cultivars than low sucrose content sugarcane cultivars and higher in mature internodes than immature internodes [27]. The activities of Susy and SAI in sugarcane were higher in immature internodes than mature internodes [26,27]. The activities of high sucrose synthesis by SPS and low hydrolysis by SAI together decided sucrose content in sugarcane [25,26]. Although enzyme activities for sucrose metabolism were different in different sucrose content cultivars and internodes, the transcriptional differences of SPS, Susy and INVs were not found [28-30]. The activities of sucrose metabolism enzymes were regulated at post-transcriptional level in sugarcane. There are special signaling pathways to switch the activities of sucrose metabolism enzymes to control the sucrose content in sugarcane.
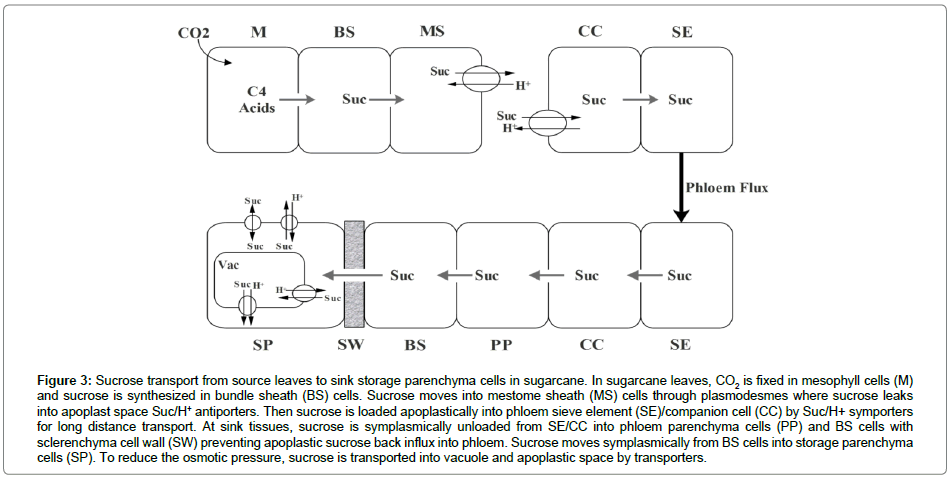
Sugar transporters: Sugar transporters regulate assimilates transportation, compartmentalization and storage in plants. They can be divided into monosaccharide transporters (MSTs), sucrose transporters (SUTs) and SWEETs. It has been proved that both plasma membrane and tonoplast of parenchyma cells could trans-membrane input and output sucrose [1]. However, few sugar transporters were identified in sugarcane. Sucrose apoplastic loading into phloem and storage in parenchyma cells need sugar transporters in sugarcane [31,32] (Figure 3). A sucrose transporter, ShSUT1, located into the periphery cells of vascular bundle and potentially functioned in sucrose retrieval during phloem long distance transportation [33]. The structure and expression of sucrose transporter family genes in sugarcane were also studied by comparative genomics and bacterial artificial chromosomes [34]. ShSUT1 and ShSUT4 were abundantly expressed both in leaves and stems. ShSUT2 was expressed at a similar level in leaves and stems. ShSUT5 and ShSUT6 were expressed highly in leaves. Transcriptom analysis of storage parenchyma, vascular bundles and rind revealed that sugar transporter genes, ShPST2a, ShPST2b and ShSUT4 were highly expressed in parenchyma cells and ShSUT1 was expressed highly in vascular bundles [35]. In Arabidopsis, tonoplast H+/sugar antiporters, AtTMT1 and AtTMT2, transfer glucose and sucrose into vacuoles [36]. ShPST2a and ShPST2b, highly homologous to AtTMT2 and AtTMT1, are likely to import sucrose into parenchyma cell vacuoles. SUT4 type sucrose transporters have been proved as tonoplast H+/sucrose symporters which export sucrose from vacuole into cytoplasm [37-39]. ShSUT4 highly homologous to rice OsSUT2 may function in tonoplast sucrose export [40]. In addition, sugar transporter SWEETs as sucrose effluxer might function in sucrose leakage from storage parenchyma cells to apoplast [41]. These sugar transporters may participate in regulating sucrose accumulation in sugarcane. More work is still needed to analyze the functions of sugar transporters in transgenic sugarcane plants.
Figure 3: Sucrose transport from source leaves to sink storage parenchyma cells in sugarcane. In sugarcane leaves, CO2 is fixed in mesophyll cells (M) and sucrose is synthesized in bundle sheath (BS) cells. Sucrose moves into mestome sheath (MS) cells through plasmodesmes where sucrose leaks into apoplast space Suc/H+ antiporters. Then sucrose is loaded apoplastically into phloem sieve element (SE)/companion cell (CC) by Suc/H+ symporters for long distance transport. At sink tissues, sucrose is symplasmically unloaded from SE/CC into phloem parenchyma cells (PP) and BS cells with sclerenchyma cell wall (SW) preventing apoplastic sucrose back influx into phloem. Sucrose moves symplasmically from BS cells into storage parenchyma cells (SP). To reduce the osmotic pressure, sucrose is transported into vacuole and apoplastic space by transporters.
Regulator genes during sucrose accumulation
Protein kinases: During sucrose synthesis, transport and storage, there are signal pathways to keep optimal sucrose level at different developmental stages under various grow conditions. Protein phosphorylation regulates sucrose accumulation and low inorganic phosphate level facilitated sucrose accumulation [1]. Protein kinases catalyzing protein phosphorylation are important signal molecules in plants. A type of Ser/Thr protein kinases, SNF1-related protein kinase (SnRK1) sensing the sugar and energy status regulate sourcesink balance in plants [42]. SnRK1 phosphorylated SPS to inhibit its activity [43]. Different expression genes of protein kinases in high and low sucrose content cultivars and internodes were analyzed by cDNA microarrays [30]. Sugarcane ScSnRK1-2 was lower expressed in mature internodes and could be induced expression by sucrose treatment [30]. SnRK1 may to be a key signal molecular to regulate sucrose accumulation in sugarcane. A sugarcane receptor kinase, ScBAK1, highly expressed in leaves vascular bundle sheath cells and high sucrose content individuals might regulate sucrose synthesis in source leaves [44].
Transcription factors: Transcription factors regulate gene expressions to control metabolism pathways in plant growth and development. The transcription factors potentially relative to regulate sucrose accumulation in sugarcane were also discovered by cDNA microarrays [30]. They were hormones and stresses response genes, such as DREB, ERF, NAC, MYB, auxin response factors ARF and ethylene regulator EIL. Sugar signaling usually interacts with other signaling pathways, for example, hormone and redox signals [45]. The Arabidopsis transcription factor bZIP11 with a sucrose control peptide and its translation can be repressed by sucrose to regulate amino acid metabolism [46]. The homologous of Arabidopsis bZIP11 in sugarcane still need to be identified. Over expression of Arabidopsis transcription factor AtDREB2A CA increased sucrose content and drought resistance in transgenic sugarcane plants [47]. It demonstrates that sucrose accumulation may interact with drought and hormone signal pathways.
Genes involving in cell war metabolism: In sugarcane mature stems, the unloaded sucrose is partitioned into polysaccharides synthesis. Cellulose is synthesized from UDP-glucose by cellulose synthase complex consisted of cellulose synthase (CesA) and accessory proteins. UDP-glucose is hydrolyzed from sucrose by sucrose synthase which may regulate the proportions of sucrose and cellulose [7]. Over expression of Susy in poplar increased the cell war cellulose content [48]. Genes relative to cell war synthesis were identified in sugarcane. The primary cell war synthesis related genes, ShCesA1, ShCesA7, ShCesA9 and Shbk2l3 were highly expressed in parenchyma and secondary cell war synthesis related genes, ShCesA10, ShCesA11, ShCesA12 and Shbk-2 were highly expressed in rind [35]. The correlative effect between sucrose accumulation and fiber synthesis in sugarcane stalk is still unknown. It needs more researches on different sugarcane cultivars with diverse sucrose and fiber contents to clarify the relationship between sucrose and fiber contents.
Molecular manipulations on improving sugar content in sugarcane
Sucrose metabolism is composed of synthesis, breakdown and conversion to other sugars, such as hexoses, oligosaccharides and polysaccharides. In order to increase sucrose content and sugar yields, studies have focused on increasing sucrose synthesis, reducing sucrose hydrolysis or converting sucrose to other sugar forms to increase total sugar yield. Nevertheless, these attempts to improve sugar yield in field-planted sugarcane plants by molecular genetic engineering have not been achieved due to the complex interactions during sucrose metabolism enzymes and the osmotic regulation of sugars in storage parenchyma cells. SPS is a key regulatory enzyme for sucrose synthesis. However, over expression of SPS in sugarcane didn’t increase the sucrose yield [49]. Reduced CIN activity in transgenic sugarcane plants led to a higher ratio of sucrose/hexose in culm internodes but the growth vigor of these plants decreased greatly than untransformed plants [50]. Pyrophosphate: fructose 6-phosphotransferase (PFP) is a key control point in glycolysis. Down-regulation of PFP activity in sugarcane increased sucrose concentrations in immature internodes [51]. Expression of a vacuolar-targeted expression of bacterial sucrose isomerase (SI) which converts sucrose into isomaltulose in sugarcane led to double sugar content accumulation under greenhouse growth condition [52]. Nevertheless, total sugar contents in SI transgenic sugarcane were not increased under field growth conditions [53].
Conclusion
The complex genomic structure of sugarcane hinders its genomic sequencing and genes discovery. Transcriptom data has been an effective way to discover functional genes involving in various physiological processes. Genes involve in sugarcane sucrose accumulation have also been analyzed through cDNA microarray and transcriptom sequencing techniques. Sucrose accumulation in sugarcane is a process integrating sucrose metabolism, transportation and partitioning. Enzymes of sucrose metabolism, sugar transporters and candidate regulator genes relative to sucrose content have been identified. However, the regulatory networks of these genes on regulating sucrose accumulation are not clear. More work still need to analyze the interaction regulation of sucrose metabolism enzymes activities and the regulatory mechanisms for sourcesink communication. In addition, it also needs further functional analysis of sugar transporters and regulator genes relative to sucrose accumulation in sugarcane. Due to that sugarcane is a type of allopolyploids plant, it is hard to prepare mutants for genetic complementary analysis. Therefore we can analyze genes function by up and down regulation gene expression in sugarcane genotypes with high or low sucrose content. Because sugarcane is sink-limited for sucrose accumulation, it needs to enhance sink strength to increase sucrose yield. To clarify the molecular regulatory networks of sucrose accumulation will facilitate us to change sink capacity through sugarcane breeding program.
Acknowledgments
This work was funded by the National High Technology Research and Development Program of China 863 Program Project (2013AA102604-1) Natural Science Foundation of Hainan province, China (20163124), National Nonprofit Institute Research Grant of CATAS-ITBB (ITBB2015RC04, ITBB2015ZY12).
References
- Moore PH (1995) Temporal and Spatial Regulation of Sucrose Accumulation in the Sugarcane Stem. Aust J Plant Physiol 22: 661-680.
- McCormick AJ, Cramer MD, Watt DA (2006) Sink strength regulates photosynthesis in sugarcane. New Phytol 171: 759-770.
- Osorio S, Ruan YL, Fernie AR (2014) An update on source-to-sink carbon partitioning in tomato. Front Plant Sci 5: 516.
- Paul MJ, Foyer CH (2001) Sink regulation of photosynthesis. J Exp Bot 52: 1383-1400.
- Smith AM, Stitt M (2007) Coordination of carbon supply and plant growth. Plant Cell Environ 30: 1126-1149.
- Bihmidine S, Hunter CT, Johns CE, Koch KE, Braun DM (2013) Regulation of assimilate import into sink organs: update on molecular drivers of sink strength. Front Plant Sci 4: 177.
- Ruan YL (2014) Sucrose metabolism: gateway to diverse carbon use and sugar signaling. Annu Rev Plant Biol 65: 33-67.
- Yu SM, Lo SF, Ho TH (2015) Source-Sink Communication: Regulated by Hormone, Nutrient, and Stress Cross-Signaling. Trends Plant Sci 20: 844-857.
- Patrick JW, Botha FC, Birch RG (2013) Metabolic engineering of sugars and simple sugar derivatives in plants. Plant Biotechnol J 11: 142-156.
- McCormick AJ, Watt DA, Cramer MD (2009) Supply and demand: sink regulation of sugar accumulation in sugarcane. J Exp Bot 60: 357-364.
- Hatch MD, Slack CR (1966) Photosynthesis by sugar-cane leaves. A new carboxylation reaction and the pathway of sugar formation. Biochem J 101: 111.
- Mattiello L, Riano-Pachon DM, Martins MC, da Cruz LP, Bassi D, et al. (2015) Physiological and transcriptional analyses of developmental stages along sugarcane leaf. BMC Plant Biol 15: 300.
- Lobo AK, de Oliveira Martins M, Lima Neto MC, Machado EC, Ribeiro RV, et al. (2015) Exogenous sucrose supply changes sugar metabolism and reduces photosynthesis of sugarcane through the down-regulation of Rubisco abundance and activity. J Plant Physiol 179: 113-121.
- Moore PH, Cosgrove DJ (1991) Developmental changes in cell and tissue water relations parameters in storage parenchyma of sugarcane. Plant Physiol 96: 794-801.
- Welbaum GE, Meinzer FC (1990) Compartmentation of solutes and water in developing sugarcane stalk tissue. Plant Physiol 93: 1147-1153.
- Welbaum GE, Meinzer FC, Grayson RL, Thornham KT, Welbaum GE, et al. (1992) Evidence for the Consequences of a Barrier to Solute Diffusion Between the Apoplast and Vascular Bundles in Sugarcane Stalk Tissue. Funct Plant Biol 19: 611-623.
- Whittaker A, Botha FC (1997) Carbon Partitioning during Sucrose Accumulation in Sugarcane Internodal Tissue. Plant Physiol 115: 1651-1659.
- Bindon KA, Botha FC (2002) Carbon allocation to the insoluble fraction, respiration and triose-phosphate cycling in the sugarcane culm. Physiol Plant 116: 12-19.
- McCormick AJ, Cramer MD, Watt DA (2008) Changes in photosynthetic rates and gene expression of leaves during a source-sink perturbation in sugarcane. Ann Bot 101: 89-102.
- Ribeiro RV, Machado EC, Magalhaes Filho JR, Lobo AK, Martins MO, et al. (2017) Increased sink strength offsets the inhibitory effect of sucrose on sugarcane photosynthesis. J Plant Physiol 208: 61-69.
- Huber SC, Huber JL (1996) Role and Regulation of Sucrose-Phosphate Synthase in Higher Plants. Annu Rev Plant Physiol Plant Mol Biol 47: 431-444.
- Cheng WH, Chourey PS (1999) Genetic evidence that invertase-mediated release of hexoses is critical for appropriate carbon partitioning and normal seed development in maize. Theor Appl Genet 98: 485-495.
- Weber H, Borisjuk L, Wobus U (1997) Sugar import and metabolism during seed development. Trends Plant Sci 2: 169-174.
- Babb VM, Haigler CH (2001) Sucrose phosphate synthase activity rises in correlation with high-rate cellulose synthesis in three heterotrophic systems. Plant Physiol 127: 1234-1242.
- Kalwade SB, Devarumath RM (2014) Functional analysis of the potential enzymes involved in sugar modulation in high and low sugarcane cultivars. Appl Biochem Biotechnol 172: 1982-1998.
- Zhu YJ, Komor E, Moore PH (1997) Sucrose Accumulation in the Sugarcane Stem Is Regulated by the Difference between the Activities of Soluble Acid Invertase and Sucrose Phosphate Synthase. Plant Physiol 115: 609-616.
- Verma AK, Upadhyay SK, Verma PC, Solomon S, Singh SB (2011) Functional analysis of sucrose phosphate synthase (SPS) and sucrose synthase (SS) in sugarcane (Saccharum) cultivars. Plant Biol (Stuttg) 13: 325-332.
- Carson DL, Huckett BI, Botha FC (2002) Sugarcane ESTs differentially expressed in immature and maturing internodal tissue. Plant Sci 162: 289-300.
- Casu RE, Grof CPL, Rae AL, Mcintyre CL, Dimmock CM, et al. (2003) Identification of a novel sugar transporter homologue strongly expressed in maturing stem vascular tissues of sugarcane by expressed sequence tag and microarray analysis. Plant Mol Biol 52: 371-386.
- Papini-Terzi FS, Rocha FR, Vencio RZ, Felix JM, Branco DS, et al. (2009) Sugarcane genes associated with sucrose content. BMC Genomics 10: 120.
- Robinson-Beers K, Evert RF (1991) Ultrastructure of and plasmodesmatal frequency in mature leaves of sugarcane. Planta 184: 291-306.
- Wang J, Nayak S, Koch K, Ming R (2013) Carbon partitioning in sugarcane (Saccharum species). Front Plant Sci 4: 201.
- Rae AL, Perroux JM, Grof CP (2005) Sucrose partitioning between vascular bundles and storage parenchyma in the sugarcane stem: a potential role for the ShSUT1 sucrose transporter. Planta 220: 817-825.
- Zhang Q, Hu W, Zhu F, Wang L, Yu Q, et al. (2016) Structure, phylogeny, allelic haplotypes and expression of sucrose transporter gene families in Saccharum. BMC Genomics 17: 88.
- Casu RE, Rae AL, Nielsen JM, Perroux JM, Bonnett GD, et al. (2015) Tissue-specific transcriptome analysis within the maturing sugarcane stalk reveals spatial regulation in the expression of cellulose synthase and sucrose transporter gene families. Plant Mol Biol 89: 607-628.
- Schulz A, Beyhl D, Marten I, Wormit A, Neuhaus E, et al. (2011) Proton-driven sucrose symport and antiport are provided by the vacuolar transporters SUC4 and TMT1/2. Plant J 68: 129-136.
- Endler A, Meyer S, Schelbert S, Schneider T, Weschke W, et al. (2006) Identification of a vacuolar sucrose transporter in barley and Arabidopsis mesophyll cells by a tonoplast proteomic approach. Plant Physiol 141: 196-207.
- Payyavula RS, Tay KH, Tsai C J, Harding SA (2011) The sucrose transporter family in Populus: the importance of a tonoplast PtaSUT4 to biomass and carbon partitioning. Plant J 65: 757-770.
- Schneider S, Hulpke S, Schulz A, Yaron I, Holl J, et al. (2012) Vacuoles release sucrose via tonoplast-localised SUC4-type transporters. Plant Biol (Stuttg) 14: 325-336.
- Eom JS, Cho JI, Reinders A, Lee SW, Yoo Y, et al. (2011) Impaired function of the tonoplast-localized sucrose transporter in rice, OsSUT2, limits the transport of vacuolar reserve sucrose and affects plant growth. Plant Physiol 157: 109-119.
- Chen LQ, Qu XQ, Hou BH, Sosso D, Osorio S, et al. (2012) Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 335(6065): 207-211.
- Polge C, Thomas M (2007) SNF1/AMPK/SnRK1 kinases, global regulators at the heart of energy control? Trends Plant Sci 12: 20-28.
- Sugden C, Donaghy PG, Halford NG, Hardie DG (1999) Two SNF1-related protein kinases from spinach leaf phosphorylate and inactivate 3-hydroxy-3-methylglutaryl-coenzyme A reductase, nitrate reductase, and sucrose phosphate synthase in vitro. Plant Physiol 120: 257-274.
- Vicentini R, Felix Jde M, Dornelas MC, Menossi M (2009) Characterization of a sugarcane (Saccharum spp.) gene homolog to the brassinosteroid insensitive1-associated receptor kinase 1 that is associated to sugar content. Plant Cell Rep 28: 481-491.
- Li L, Sheen J (2016) Dynamic and diverse sugar signaling. Curr Opin Plant Biol 33: 116-125.
- Rahmani F, Hummel M, Schuurmans J, Wiese-Klinkenberg A, Smeekens S, et al. (2009) Sucrose control of translation mediated by an upstream open reading frame-encoded peptide. Plant Physiol 150: 1356-1367.
- Reis RR, da Cunha BA, Martins PK, Martins MT, Alekcevetch JC, et al. (2014) Induced over-expression of AtDREB2A CA improves drought tolerance in sugarcane. Plant Sci 221-222: 59-68.
- Coleman HD, Yan J, Mansfield SD (2009) Sucrose synthase affects carbon partitioning to increase cellulose production and altered cell wall ultrastructure. Proc Natl Acad Sci USA 106: 13118-13123.
- Vickers JE, Grof CPL, Bonnett GD, Jackson PA, Morgan TE (2005) Effects of tissue culture, biolistic transformation, and introduction of PPO and SPS gene constructs on performance of sugarcane clones in the field. Crop Pasture Sci 56: 449-456.
- Rossouw D, Kossmann J, Botha FC, Groenewald JH (2010) Reduced neutral invertase activity in the culm tissues of transgenic sugarcane plants results in a decrease in respiration and sucrose cycling and an increase in the sucrose to hexose ratio. Funct Plant Biol 37: 22-31.
- Groenewald JH, Botha FC (2008) Down-regulation of pyrophosphate: fructose 6-phosphate 1-phosphotransferase (PFP) activity in sugarcane enhances sucrose accumulation in immature internodes. Transgenic Res 17: 85-92.
- Mudge SR, Basnayake SW, Moyle RL, Osabe K, Graham MW, et al. (2013) Mature-stem expression of a silencing-resistant sucrose isomerase gene drives isomaltulose accumulation to high levels in sugarcane. Plant Biotechnol J 11: 502-509.
- Basnayake SW, Morgan TC, Wu L, Birch RG (2012) Field performance of transgenic sugarcane expressing isomaltulose synthase. Plant Biotechnol J 10: 217-225.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi