Research Article, J Polym Sci Appl Vol: 2 Issue: 1
Synthesis and Use of PET Fibers Grafted with 4-Vinyl Pyridine and 2-Methylpropenoic Acid for Removal of Rhodamine B and Methylene Blue from Aqueous Solutions
Metin Arslan1* and Kübra Günay2
1Department of Chemistry and Chemical Processing Technologies, Kırıkkale Vocational High School, Kırıkkale University, Yahsihan,71450 Kırıkkale, Turkey
2Graduate School of Natural and Applied Sciences, Kırıkkale University, Yahsihan,71450 Kırıkkale, Turkey.
*Corresponding Author : Metin Arslan
Department of Chemistry and Chemical Processing Technologies, Kırıkkale Vocational High School
Kırıkkale University, Yahsihan,71450 Kırıkkale, Turkey
E-mail: marslan@hotmail.com
Received: August 16, 2017 Accepted: November 01, 2017 Published: November 06, 2017
Citation:Arslan M, Günay K (2017) Synthesis and Use of PET Fibers Grafted with 4-Vinyl Pyridine and 2-Methylpropenoic Acid for Removal of Rhodamine B and Methylene Blue from Aqueous Solutions. J Polym Sci Appl 1:3.
Abstract
Grafting of 4-vinyl pyridine (4-VP) and 2-methylpropenoic acid (MPA) onto poly (ethylene terephthalate) fibers (PET) was achieved via chemical grafting technique. A new fibrous adsorbent was used to the removal of rhodamine B (RB) and methylene blue (MB) from aqueous solutions through batch sorption method. The influence of pH, graft yield, removal time and initial concentration were investigated to study the adsorbing effect of 4-VP and MPA grafted PET fibers (4-VP/MPA-g-PET). The optimum pH values for RB and MB were found to be 12 and 10 respectively. The maximum adsorption capacities of grafted PET fibers were found to be 45.28 mg g-1 for RB and 56.72 mg g-1 for MB at 250 ppm. Almost all adsorbed dyes were eluted by acetic acid in methyl alcohol. Ten removal-desorption cycles indicated that the reactive fibers were favourable for use it again without a notable change in removal capacity. Consequently, the 4-VP/MPA-g-PET fibers have demonstrated potential as an effective adsorbent for the extremely effective removal of cationic dyes from aqueous media
Keywords: Poly(ethylene terephthalate) fibers; Grafting vinyl monomers; Removal; Cationic dye; Desorption; Reusing
Introduction
Water pollution has been a serious increasing problem in the recent years. This is due to both the increase in human population and the increase in industrial plants. There are many various kinds of water pollution [1,2]. Water has the capacity to dissolve organic compounds as synthetic dyes. Synthetic dyes are used to add a colour or to change the colour something. Such dyes are widely utilized in the production of user products, including paints, printing inks, food, plastics, textile, pharmaceutical, and paper industries [3-6]. Thence about 10.000, different dyes are used industrially. The greatest environmental concern with dyes is their reflection of sunlight entering water and absorption. Many dyes carcinogenic, mutagenic and toxic to life. Therefore, their removal is mandatory from waste before they are released into the environment [7]. Many methods have been used to eliminate of dyes from water [8]. The advantages and disadvantages are widely discussed in the previous work [9-12]. However, among these, Adsorption have a potential for removing dyes from water as a result of it’s understood for high efficiency and ability to removal, low disbursement, and simple operation and in most of the cases, regeneration of the adsorbent is feasible [2]. Several adsorbents are used for the removal of dyes from waste product [13-17]. However, some of these adsorbents have low adsorption capacities, need long adsorption time, difficult desorption and reuse. Recently, chelating fibers as grafted PET fibers have been applied for the removal of dyes from water. This mainly ascribes the relatively large external specific surface, active functional groups, high adsorption capacity, reuse and simple process. In addition to they have good resistance to most strong acids at room temperature, oxidizing agents, sunlight and microorganisms [18].
Grafting is an easy process to added desired active functional groups like -COOH, -CN, -C5H5N, CONH2, -NH2, -COOR, and epoxy on the synthetic fibers for the removal dyes. The radical graft polymerization of binary vinyl mixtures monomers has been noticed to introduce appropriate functional groups within the fibers for eliminating of dyes and heavy metals ions from water [19-21].
In particular, the carboxyl and amino groups on the surface of an adsorbent have been reported to be effective in the removal dyes [22]. Therefore, in this work, chelating PET fibers with carboxylic acid (MPA) and amino (4-VP) groups on the surface were prepared by graft copolymer. Then the 4-VP/MPA-g-PET fibers were used as a novel adsorbent for removal RB and MB. The optimal parameters to removal RB and MB were studied.
Experimental
Materials
The polyester (PET) fibers (122 dTex) were SASA Co. (Turkey) product. The models were cleared for 10 h by dimethyl ketone and dried in an oven at surroundings temperature. Benzoyl peroxide was doubly recrystallized from chloroform in methanol and dried. Other reagents were used like received. Total chemicals were Merck products.
Swelling procedure
The fibers were immersed in dichloroethane at 90 °C for 2 h. The swollen the fibers were wiped with a cleansing tissue to remove dichloroethane and place into the polymerization medium.
Graft copolymerization process
In the first step, graft copolymerization was allotted in a threenecked polymerization glass tube. The polymerization tube containing the PET fiber about 0.3 g, acceptable quantity of 4-VP monomer and benzoyl peroxide at a needed concentration in 2 mL dissolving agent (acetone) was created up to 20 mL with demineralized water. The polymerization tube was instantly placed into the water bathtub adjusted to the polymerization temperature (at 65°C). At the tip of the present chemical change time (2 h), the grafted fibers were taken out. Compound and free of the homopolymers were extracted with methanol for 8 h. Then the modified fibers were dried at 25°C for 24 h and weighed. In the second stage, with the above method, MPA monomers were grafted onto 4VP grafted PET fibers (at 85°C). Compound and free of the homopolymers were extracted with boiling water for 8 h. The modified fibers (4-VP/MPA-g-PET) were then dried at 25°C for 24 h and weighed. The graft yield (GY) was calculated from the weight increase in grafted fibers as follows:
![]() (1)
(1)
Where mi and mg represent the weights of the original and grafted fibers, severally
FTIR spectra
FTIR spectra of 4-VP/MPA grafted PET fibers were obtained. The fibers were cut into roughly 1 mm size, mixed with KBr, and then pressed. The spectra were recorded on a Bruker Vertex 70V FTIR photometer.
Scanning Electron Microscopy (SEM) analysis
SEM analysis was carried out to research the surface morphology of original and 4-VP/MPA-g-PET fibers employing a JEOL Model JSM 5600 scientific instrument. The fibers covered a thin evaporated layer of gold and were performed.
Sorption process
A volume of 25 mL of cationic dye solution (RB and MB) was additional onto a certain amount of 4-VP/MPA-g-PET adsorbent in a glass container. The ingredients were agitated on a shaker (Selectra) set at a 110 rpm for a fixed period at 25°C. The charged adsorbent was separated and cleaned politely. The solution was filtered and was adjusted to 6.5 pH value for RB and 6.8 pH value for MB, the remaining cationic dye was analyzed employing UV/Visible photometer (λ=554 nm RB, λ=665 nm MB, Perkin Elmer Lambda 25). The amount of cationic dye adsorbed by the adsorbent was analyzed by using Eq. 2.
![]() (2)
(2)
Where Co is the initial dye concentration (ppm), Ce is the last concentration of dye solution; V is the volume of the dye solution used and w is the quantity of the adsorbent used.
Desorption and Reuse of 4-VP/MPA-g-PET Fibers
Desorption of cationic dyes was studied with 25 mL of acetic acid in methyl alcohol adjusted to different concentrations of values. The dye was desorbed in a half-hour, then diluted with water and analyzed as above. The percent of desorption was calculated by employing Eq. 3.
![]() (3)
(3)
The sorption-desorption process was repeated ten times using 4-VP/MPA monomer mixture grafted PET fibers.
Result and Discussion
The aim of the current study is the novel practice of effective fibers containing amine and carboxylic acid groups to removal a hazardous RB and MB. Firstly, poly(ethylene terephthalate) (PET) fibers were grafted with 4-vinyl pyridine (4-VP-g-PET) after 2-methylpropenoic acid (4-VP/MPA-g-PET) using benzoyl peroxide as an initiator. Fibers were swelled in dichloroethane (DCE) for 2 h at 90°C to promote the incorporation and the subsequent polymerization of the 4-VP and MPA onto PET fibers. The graft copolymer chains grafted upon PET fibers. Functional groups coming from both 4-VP and MPA were placed into graft copolymer chains. Therefore, PET fibers contain chelating groups. Grafted PET fibers were characterized by scanning electron microscopy (SEM) and FT-IR spectra.
FT-IR spectrum
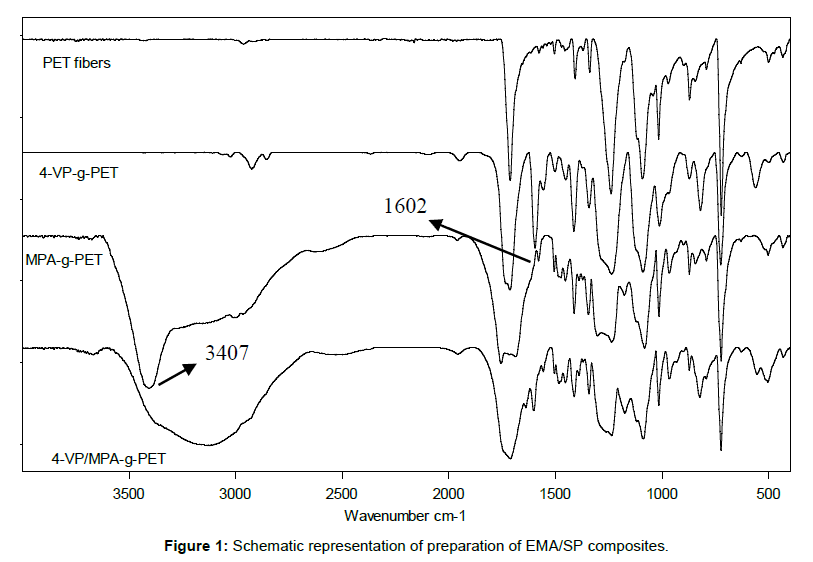
A FT-IR spectre of original, 4-VP-g-PET, an MPA-g-PET and 4-VP/ MPA-g-PET fiber have been analyzed and is displayed in Figure 1. The FT-IR spectra of original PET fibers displayed peaks owing to C=O (at 1712 cm-1), C=C and aliphatic C-H (at 1411 and 1578 cm-1) of PET fibers, while additional new peaks owing to the –OH groups of MPA (at 3407 cm-1) and the 4-vinyl pyridine groups (at 1602 cm-1) have been observed in Figure 1. New peaks (-OH and pyridine groups) have been shown in spectra of 4-VP/MPA-g-PET fibers.
Scanning Electron Microscopy (SEM) Study
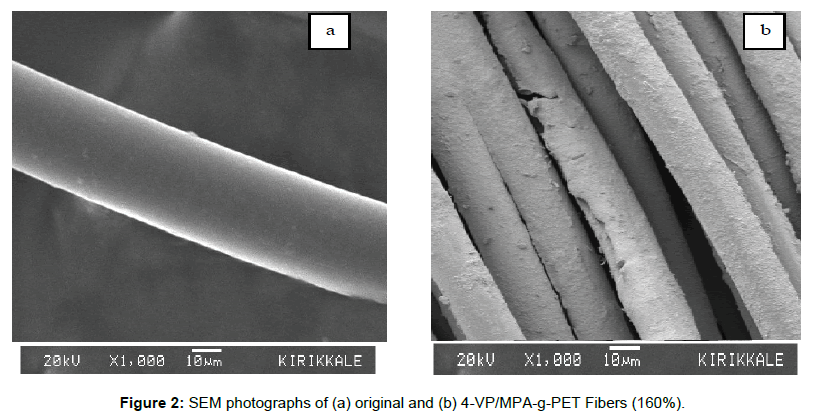
SEM study was carried out to analyse the surface morphology of original and 4-VP/MPA-g-PET fibers (160%) and are indicated in Figure 2. SEM photograph of the original PET fiber (Figure 2a) showed a smooth surface and relatively homogeneous surface. SEM photograph of 4-VP/MPA-g-PET fibers (at 160%), however, showed clear residual and cover of the grafting upon PET fibers.
Effect of pH
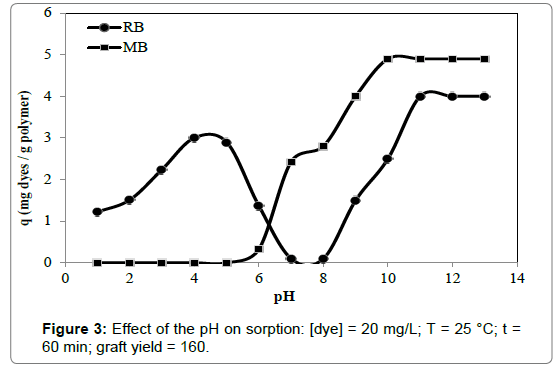
The pH is a significant parameter in the sorption study because the type of the functional groups, properties of grafted PET fibers and dyes can be changed with pH. Thus the effect of pH on cationic dyes removal was examined and Figure 3 was showed. The 4-VP/MPAg- PET fibers were incubated for 60 min. with aqueous basic dyes solution adjusted to required pH values of a range of 2-13 using buffer solutions (Briton-Robinson buffer).
It was clear from the plots that the pH values for optimum removal were 10 for MB, 12 for RB. The removal of RB ions at pH 7, 8 and 9 was the minimum, and a maximum in removal was shown at pH 4. However, when the pH of the solution was increased (> pH 9), the removal of RB ions was increased, and the removal of RB ions at pH 12 was higher than pH 4. Consequently, both acidic and basic solutions are suitable for removal RB ions.
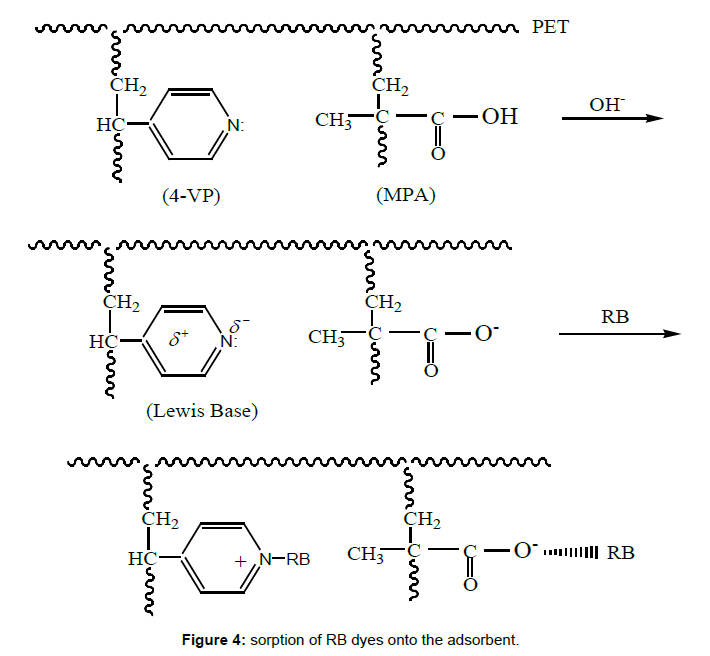
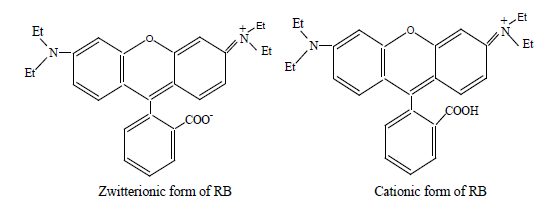
pH values effect ionization-dissociation of 4-VP/MPA-g-PET fibers and RB molecules. Because the adsorbent and RB ions have both amine and carboxyl groups. At pH values lower than 4, the RB ions are of cationic and monomeric molecular form and thus, removal decreased [23]. The electrostatic repulsion between cationic RB ions and positively charged 4-VP/MPA-g-PET fibers. On increasing the pH, the interaction between dye molecules and active centers on the grafted PET fibers is mainly effected by ionization states of the functional groups of both RB ions and the adsorbent surface. At pH values higher than 4 the decrease in removal may be due to the formation of the zwitterions form of RB which form has a net positive and negative charge [24]. It would not move towards the grafted PET fibers that they have both (+) and (-) charge. An increase in the removal of RB on the grafted PET fibers can be observed at pH>9. The preponderance of OH- compete with –COO- in binding with –N+ and it decreases the aggregation of RB, which causes an increase in the removal of RB ions on the fibers adsorbent [25]. This may be illustrated as in Figure 4.
Figure 3 shows that the removal of MB rises with increasing from pH 5 to 10, reaches a maximum at pH 10 and thereafter almost levelled off. The removal of MB on the adsorbent cannot be observed at pH ≤ 5. The important rise in elute efficiency with increasing pH value may be owing to deprotonation of the carboxyl groups upon the surface of the adsorbent and thus raises the removal of MB dye. When the pH of the solution increases, the free COO- upon the adsorbent surface favours the sorption of MB dye owing to the electrostatic attraction. As the low pH, the concentration of free COO- on the surface of modified PET fibers decreased, thus the surface site of the grafted fibers do not favor the removal of MB dye [26]. Most of the pyridine groups surface of the sorbent was loaded negative pole (at pH>6). Loaded negative pole pyridine groups (Lewis base) can, therefore, attract the MB dye. MB would be expected to have activity as a Lewis acid. In this way, MB interacts with grafted PET fibers by Lewis acid-base interactions [27].
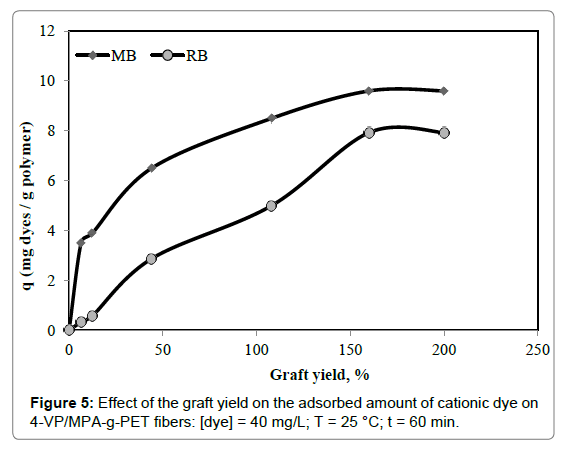
Effect of % grafting and sorption time
The effect of the graft yield on the adsorbed amount of RB and MB dyes was investigated at 25°C while keeping all other conditions constant. The results are shown in Figure 5. With the rise in grafting yield from zero to 160%, the amount of cationic dye adsorbed raised, thereafter leveled off. Original PET fibers have very low cationic dye sorption capacity owing to it has not included the active site. Increasing graft yield increases the number of functional groups thus, increases the number of negatively charged surface of 4-VP/MPA grafted PET fibers. The active sites of adsorbent became saturated to maximum sorption capacity on the chelating fibers. The increase in the adsorption with increasing graft yield may be attributed to a higher surface area and more active sites such as 4-VP and COO-. The similar results were observed in the previous works [28].
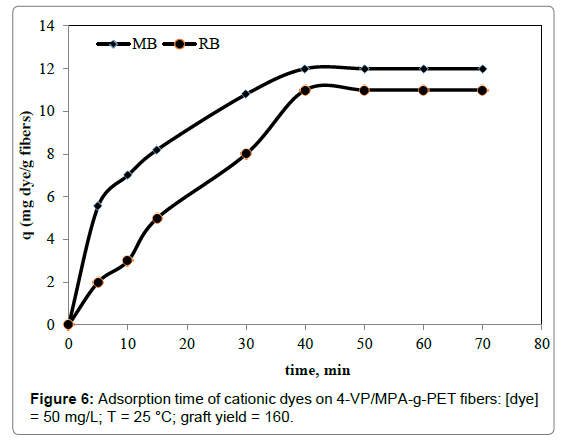
The rapid removal of RB and MB with the adsorbent will be advantageous for practical use. For this aim, the contact time of RB and MB on the 4-VP/MPA-g-PET fibers was studied. Figure 6 was shown that removal of the dyes might be considered very fast. After the removal became fast and saturation levels were reached within 40 min. for RB and MB. The removal of MB on the fibers was more positive than that of the RB. At first, faster rates of removal may be attributed to the presence of a large number of binding sites for removal.
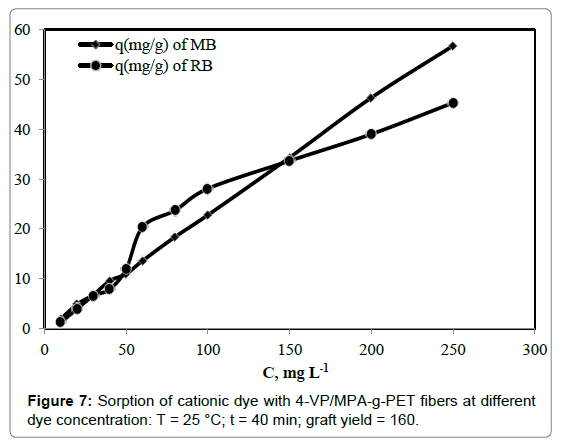
Effect of cationic dye concentration
Effects of initial dyes concentration on removal efficiency of RB and MB were investigated in the concentration range of 10-250 mg L-1. The results were shown in Figure 7. As the concentration increases, the removal values increased to 45.28 and 56.72 mg g-1 for RB and MB, respectively. The removal values steadily increased from 10-250 mg g-1. All cationic dyes present in the sorption environment could interact with the active functional groups on the grafted PET fibers, therefore higher dye removal percentage. The most common active groups attached to the fibers are the carboxylate, -COO- and amine, -4-VP. These sites have an anionic property that suitable for dyeing with cationic dyes. Thus, 4-VP/MPA-g-PET fibers showed very high adsorption capacity.
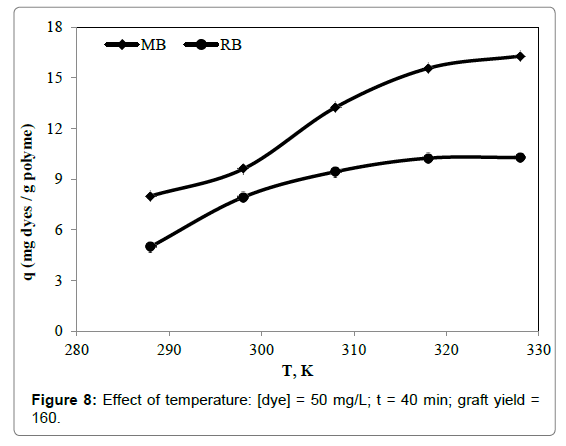
Effect of temperature
The temperature may be important in determining the capacity of removal. The experiments were carried out at the temperature in the range of 15-55°C. Figure 8 showed the influence of temperature on the removal of RB and MB dyes onto 4-VP/MPA-g-PET fibers. It was observed with increase in the temperature, removal of the RB and MB dyes increased, and indicating endothermic nature of the adsorption. The higher temperature was in favour of removal [29].
Desorption studies
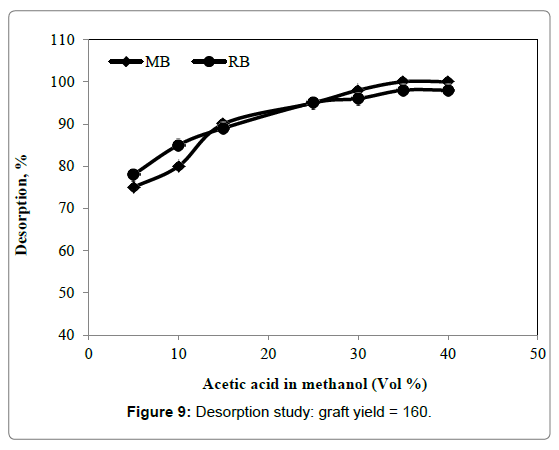
The desorption of RB and MB dyes are important for the regeneration of 4-VP/MPA-g-PET fibers. 4-VP/MPA-g-PET fibers adsorbent did not adsorb dye significantly at pH<3, that the adsorbed dye on the fibers adsorbent may be possibly desorbed in acidic solution with a low solution pH value. The adsorbed RB and MB dyes by 4-VP/MPA-g-PET fibers were recovered by using the different acetic acid concentrations in methyl alcohol as shown in Figure 9. At 30°C, in 30 min., as the acetic acid concentrations in methyl alcohol raised, from 5% to 35% the desorption% raised from 80% to almost 100% for MB and from 78% to 98% for RB adsorbed of 20 ppm. Desorption rate of RB and MB dyes from 4-VP/MPA-g-PET fibers were very high. 4-VP/MPA-g-PET fibers were eluted without losing its stability and activity. Thus, it should be effective an adsorbent for the removal of RB and MB dyes.
Reuse of grafted fibers
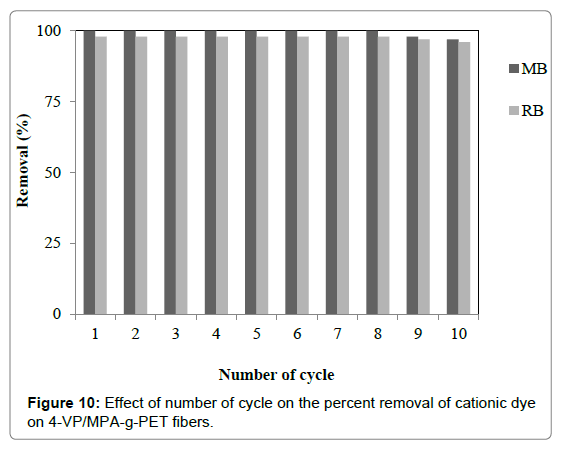
In order to indicate reusability of the 4-VP/MPA-g-PET fibers desorption cycles of RB and MB dyes were repeated ten times by using same the 4-VP/MPA-g-PET fibers. The regeneration of the fibers indicates that the fibers were used repeatedly for the removal RB and MB dyes without loss of dyes removal capacity as indicated Figure 10. Thus, due to the good recycling efficiency, 4-VP/MPA-g- PET fibers are used for practical applications.
Conclusions
4-VP/MPA-g-PET fibers were prepared as a new adsorbent and the removal of rhodamine B and methylene blue from water was achieved successfully using the adsorbent. The adsorption capacities were investigated in different conditions with various operating parameters such as pH, grafting yield, contact time, initial dye concentration and temperature. The adsorption process was highly pH dependent and a maximum adsorption achieved at pH 12 and 10 for RB and MB, respectively. The removal of cationic dyes increased with the increasing percent grafting. The sorption of cationic dyes very depended on active groups as 4-VP and MPA that inserted in the PET fibers. The maximum adsorption capacities of the grafted fibers are 45.28 mg g-1 for RB and 56.72 mg g-1 for MB at 250 ppm. The process has considerably fast kinetic, which achieve equilibrium at 40 min. Cationic dyes adsorbed onto 4-VP/MPA-g-PET fibers was desorbed by using the acetic acid in methyl alcohol. The adsorbent can be used repeatedly in the sorption-regeneration cycle without any loss and stability of the removal capacity. 4-VP/MPA-g-PET fibers were found to be effective in removal of cationic dyes, such as RB and MB.
Acknowledgment
The authors are grateful to the Kırıkkale University Research Fund for the ï¬Ânancial support (2015/88) for this work.
References
- Ratnamala GM, Shetty KV, Srinikethan G (2012) Removal of Remazol Brilliant Blue Dye from Dye-Contaminated Water by Adsorption Using Red Mud: Equilibrium, Kinetic, and Thermodynamic Studies. Water Air Soil Poll 223: 6187.
- Zhu L, Wang Y, He T, You L, Shen X (2016) Assessment of Potential Capability of Water Bamboo Leaves on the Adsorption Removal Efficiency of Cationic Dye from Aqueous Solutions. J Polym Environ 24: 148.
- Muthanna JA (2016) Journal of Environmental Chemical Engineering 4: 89.
- Li F, Chen Y, Huang H, Cao W, Li T (2015) Removal of rhodamine B and Cr(VI) from aqueous solutions by a polyoxometalate adsorbent. Chem Eng Res Des 100: 192.
- Mahmoodi N M, Arami M (2009) Degradation and toxicity reduction of textile wastewater using immobilized titania nanophotocatalysis. J Photoch Photobio B 94: 20.
- Shah J, Rasul JM, Haq A, Khan Y (2013) Removal of Rhodamine B from aqueous solutions and wastewater by walnut shells: kinetics, equilibrium and thermodynamics studies. Front Chem Sci Eng 7:428
- Wang S, Boyjoo Y, Choueib AA (2005) A comparative study of dye removal using fly ash treated by different methods. Chemosphere 60: 1401.
- Alkan M, Demirbaş Ö, Çelikçapa S, Doğan M (2004) Sorption of acid red 57 from aqueous solution onto sepiolite. J Hazard Mater 116: 135.
- Jai R, Mathur M, Sikarwar S, Mittal A (2007) Removal of the hazardous dye rhodamine B through photocatalytic and adsorption treatments. J Environ Manage 85:956.
- Muinde VM, Onyari JM, Wamalwa B, Wabomba J, Nthumbi RM (2017) Adsorption of Malachite Green from Aqueous Solutions onto Rice Husks: Kinetic and Equilibrium Studies. J Environ Prot 8:215.
- Yagub MT, Sen TK, Afroze S, Ang HM (2014) Dye and its removal from aqueous solution by adsorption: a review. Adv Colloid Interfac 209: 172.
- Gupta VK, Suhas (2009) Application of low-cost adsorbents for dye removal--a review. J Environ Manage 90: 2313.
- Coşkun R (2011) Removal of cationic dye from aqueous solution by adsorption onto crosslinked poly(4-vinylpyridine/crotonic acid) and its N-oxide derivative. Polym Bull 67: 125.
- Idan IJ, Abdullah LC, Mahdi DS, Obaid MK, Jamil SNAB (2017) Unique Common Fixed Point in b2 Metric Spaces. Open Access Library Journal 4: 1.
- Zeng GM, Cheng M, Huang DL, Lai C, Xu PA, et al. (2015) Study of the degradation of methylene blue by semi-solid-state fermentation of agricultural residues with Phanerochaete chrysosporium and reutilization of fermented residues. Waste Manage 38: 424.
- Abou-Gamra ZM, Ahmed MA (2015) TiO2 Nanoparticles for Removal of Malachite Green Dye from Waste Water. Advances in Chemical Engineering and Science 5: 373.
- Arslan M (2011) Immobilization horseradish peroxidase on amine-functionalized glycidyl methacrylate-g-poly(ethylene terephthalate) fibers for use in azo dye decolorization. Polym Bull 66: 865.
- Arslan M, Yigitoglu M, Sanli O, Unal HI (2003) Kinetics of swelling assisted grafting of 4-vinyl pyridine onto poly(ethylene terephthalate) fibers using a benzoyl peroxide initiator. Polym Bull 51: 237.
- Doke SM, Yadav GD (2014) Novelties of combustion synthesized titania ultrafiltration membrane in efficient removal of methylene blue dye from aqueous effluent. Chemosphere 117: 760.
- Yuan Q, Chen L, Xiong M, He J, Luo SL, et al. (2014) Cu 2 O/BiVO 4 heterostructures: synthesis and application in simultaneous photocatalytic oxidation of organic dyes and reduction of Cr (VI) under visible light. Chem Eng J 255: 394.
- Mohammadi T, Razmi A, Sadrzadeh M (2004) Effect of operating parameters on Pb2+ separation from wastewater using electrodialysis. Desalination 167: 379.
- Arslan M, Yigitoglu M, Soysal A (2006) Removal of chromium (VI) from aqueous solutions using poly (4â€Âvinyl pyridine) beads. J Appl Polym Sci 101:2865.
- Deshpande AV, Kumar U (2002) Effect of method of preparation on photophysical properties of Rh-B impregnated sol-gel hosts. J Non-Cryst Solids 306: 149.
- Guo Y, Zhao J, Zhang H, Yang S, Qi J, et al. (2005) Use of Rice Husk-Based Porous Carbon for Adsorption of Rhodamine B from Aqueous Solutions. Dyes Pigments 66: 123.
- Li L, Liu S, Zhu T (2010) J Environ Sci-China 22: 1273.
- Arslan M, Yigitoglu M (2008) Use of methacrylic acid grafted poly (ethylene terephthalate) fibers for the removal of basic dyes from aqueous solutions. J Appl Polym Sci 110: 30.
- Yigitoglu M, Arslan M (2009) Selective removal of Cr(VI) ions from aqueous solutions including Cr(VI), Cu(II) and Cd(II) ions by 4-vinly pyridine/2-hydroxyethylmethacrylate monomer mixture grafted poly(ethylene terephthalate) fiber. J Hazard Mater 166: 435.
- Yigitoglu M, Arslan M (2007) Adsorption behaviour of methylene blue from aqueous solution on poly (ethylene terephthalate)-g-4-vinylpyridine/2-hydroxyethylmethacrylate fibers. E-Polymers 55: 1.
- Singh V, Sharma AK, Tripathi DN, Sanghi R (2009) Poly(methylmethacrylate) grafted chitosan: An efficient adsorbent for anionic azo dyes. J Hazard Mater 161: 955.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi