Research Article, J Plant Physiol Pathol Vol: 5 Issue: 2
The Ameliorative Effects of Silicon on Salt-Stressed Sorghum Seedlings and Its Influence on the Activities of Sucrose Synthase and PEP Carboxylase
Amani Abdel-Latif1* and Fatma M. El-Demerdash2
1Department of Botany, Faculty of Science, Alexandria University, Egypt
2Department of Environmental Studies, Institute of Graduate studies and Research, Alexandria University, Egypt
*Corresponding Author : Amani Abdel-Latif
Department of Botany and Microbiology, Faculty of Science, University of Alexandria, Alexandria 21526, Egypt
Tel: 00203/5922352
E-mail: krhamani@hotmail.com
Received: April 17, 2017 Accepted: May 13, 2017 Published: May 18, 2017
Citation: Abdel-Latif A, El-Demerdash FM (2017) The Ameliorative Effects of Silicon on Salt-Stressed Sorghum Seedlings and Its Influence on the Activities of Sucrose Synthase and PEP Carboxylase. J Plant Physiol Pathol 5:2. doi: 10.4172/2329-955X.1000164
Abstract
The Ameliorative Effects of Silicon on Salt-Stressed Sorghum Seedlings and Its Influence on the Activities of Sucrose Synthase and PEP Carboxylase
Under salt stress, Silicon is considered a beneficial element for the growth of higher plants as it alleviates the harmful effects caused by salt. In the present study, Sorghum bicolor L. seedlings were grown hydroponically in growth units, filled with continuously aerated half strength of Hoagland-nutrient solution. Different treatments were manipulated to examine the negative effects of NaCl and the combined effect of NaCl with Si on seedlings growth, chlorophyll content, soluble protein content, ion accumulation, phosphoenolpyruvate carboxylase (PEPCase) and sucrose synthase (SS) activities. The salt induced decrease in seedling growth was relieved upon treatment with Si. Meanwhile, the sucrose and glucose levels were significantly increased and there was a reduction in sodium concentrations in the salt-stressed plants treated with silicon. The PEPCase activity in sorghum seedlings subjected to salt-stress was higher in the treatment with Si than that without Si, but did not significantly differ from that of control.
Keywords: Sorghum bicolor L.; Sucrose synthase; PEP carboxylase; Silicon
Introduction
Salinity is the process of accumulation of soluble salts, it affects plant productivity especially in arid regions where evapotranspiration is high and the rain is not enough for leaching the salt out of the root zone. Salt stress can lower leaf water potential, leading to reduced turgor and some other responses and ultimately lower crop productivity. Plants have difficulties in absorbing water when they are subjected to salt, which quickly causes reduction in growth rate [1]. The response of plants to increased salinity is complex and included changes in plant morphology, physiology and metabolism [2,3]. Salt stress adversely affects plants and crops at all stages of their life cycle as reported by many researchers [4-6].
Salinity affects plants through osmotic stress and ion imbalance and toxicity [7,8]. Osmotic effects are due to salt-induced decrease in the soil water potential. High salts inside the plant take time to accumulate before they affect plant function. Plants have developed a wide range of mechanisms to sustain productivity under salt stress environment. These mechanisms are osmotic adjustment, Na+ and/or Cl− exclusion and tolerance [8]. Research on salt tolerance of various crops has indicated that salt tolerance depends largely on genera and species and even on cultivars within certain species. Sorghum bicolor (L.) Moench was characterized as moderately tolerant to salinity [9].
Phosphoenolpyruvate (PEP) carboxylase (PEPC; EC 4.1.1.31) is an important, highly regulated, cytosolic metabolic enzyme that plays a pivotal photosynthetic role in primary CO2 fixation by C4 and Crassulacean acid metabolism plants, but also has a variety of additional functions, including roles in seed development and germination germination.
Recently, Isabel Ruiz et al. [10] showed that PTPC monoubiquitination in germinating sorghum seeds always increases at the stage of radicle emergence is maintained during the aerobic period of rapid cell division and reserve mobilization, and remains relatively constant until the coleoptiles initiate the formation of the photosynthetic tissues. Monoubiquitination is inhibitory as it results in increased Km (PEP) values and enhanced sensitivity to allosteric inhibitors.
The second most prevalent element in the soil after oxygen is silicon. Si uptake by plants may alleviate biotic and abiotic stresses. Therefore, Silicon application could improve plant growth and production under adverse climate and soil conditions. Many reports of silicon application against NaCl stress have been reported in rice [11], wheat [12], soybean [13], maize [14] and barley [15-18].
Induction of tolerance by Si could be explained by the accumulation of this element in the leaves which may limit transpiration [11].
Silicon may protect the membranes hence the ultrastructure of organelles especially chloroplasts [15,16]. It could form complexes with sodium in the root zone and decreasing salt uptake [12]. Yeo et al. [19] suggested that Si minimize the water passing through the apoplastic way, which may reduce sodium uptake and in the meantime do not affect largely the transpiration rate of the plant.
Sorghum (Sorghum bicolor L.) is the crop of arid regions because it can withstand relatively low rainfall and remain alive under unfavorable environmental conditions as high temperatures and salts. It has been reported that Si accumulated in crops of Gramineae and Cyperaceae. Sorghum can take up and accumulate a large amount of silicon (2 to 3% Dry weight), and therefore in many reports it is considered as a silicon accumulator [20]. The present study aims to investigate the effect of silicon on minimizing the damage caused by salt in young Sorghum seedlings.
Materials and Methods
Sorghum seeds (Sorghum bicolor L. Moench) were obtained from the Crop Institute, Agricultural Research Center, Giza, Egypt. Before sowing, the seeds were surface sterilized by soaking for two minutes in 4% sodium hypochlorite, then, rinsed four times with distilled water. The seeds were arranged in 9-cm diameter Petri-dishes lined with two discs of Whatman No.1 filter paper under normal laboratory conditions at 28°C under dark condition (germination in the dark condition causes extension of shoot, which will facilitate the transplanting into the nutrition solution). 10 ml of distilled water were added daily to three replicates in a randomized complete block design. After one week, selected seedlings of equal size (shoot lengths reached about 2 cm) were transplanted in the growth units and subjected to different salinity levels.
Seedlings were grown hydroponically for 14 days in growth units consists of 4 polyethylene tubes with dimensions: 5 cm diameter, 51 cm length. Each tube has an out and inlet in order to pore the nutrient solution. The capacity of each tube is 1500 ml with 31 pores (8 mm) were made in each tube distributed in two alternation lines, where the seedlings should be settled. The plastic tips of micropipettes (Eppendorf) were used to support the seedlings during growth and when they were harvested. The growth units were placed in a growth room under normal laboratory conditions (20 ± 2°C temperature, 75 ± 2% relative humidity, and 14/10 h light/dark photoperiod). Seedlings were irrigated with half strength Hoagland solution. An air pump (200 ml/min.) was put in the inlet of the tube to provide good circulation and aeration of the solution. The pH was kept within the range of 6.0 to 6.5. The solutions were made using distilled water, and changed every 3 days.
Salinity treatments (0 and 50 mM NaCl) started upon transferring the seedlings to the tubes and went on until the end of the trial. Silicon was added to the nutrient solution as K2SiO3 (0 and 2 mM). The Si concentration (2 mM) used was comparable with those widely used in other studies investigating the ameliorative role of Si in abiotic stress [21-26]. Dry mass of seedlings was measured after their drying in oven at 75°C till constant mass.
Determination of sucrose synthase activity (EC 2.4.1.13)
Sucrose synthase activity was determined according to the method described by Egger [27]. Briefly; One gram of leaf tissue was homogenized with liquid nitrogen using a Microdismembrator. 100 mg polyclar was added to the leaf homogenate (4 mg) and extracted for 5 min. with 1 ml of ice-cold Tris/Borate/β-mercaptoethanol buffer (100/300/1 mM: pH 7.6 at melting ice temperature). Aliquots (15 μl) were introduced into a 2- step assay (the specific step: formation of UDPG by the enzyme; and the indicator step: determination of UDPG by UDPG dehydrogenase). The specific step was performed in 96-well microtiter plates (Figure 1) as described by Abdel-Latif [28].
Determination of PEP Carboxylase activity (EC 4.1.1.31)
Approximately 1.0 g of leaf tissue was ground in a prefilled mortar with purified sand and 10 ml of freshly prepared homogenization buffer (0.1M Tris HCl (pH 7.8), 0.5 mM EDTA, 1 mM Mg2SO4 and 1 mM DTE). The method is slightly modified from that described by Foyer et al. [29] to suit the quantities of the enzyme present in the leaves investigated. The extract was centrifuged in a Beckman-centrifuge (model 2-21) at 16.000 rpm for 25 min. Approximately 0.3 g of solid PVP was added to 3 ml of the supernatant and mixed vigorously with a stirrer for 3 sec., and then the mixture was centrifuged at 8000 rpm at 4°C for 10 min. The clear supernatant was used as the source of the enzyme. Activity of PEPCase (EC 4.1.1.31) was determined spectrophotometrically at 340 nm by coupling the reaction to the oxidation of NADH in the presence of malate dehydrogenase (MDH).
Determination of protein content
Protein content was determined using coomassive Brilliant Blue G-250 (Bio-rad reagent) as described by Bradford [30].
Measurement of ions concentration
For the determination of mineral elements, dried powder samples were digested in nitric acid. Triplicate determinations of each element were carried out using Atomic Absorption and expressed on dry weight basis.
Photosynthetic pigments
The leaf photosynthetic pigments (chlorophyll a, chlorophyll b and carotenoids) were determined spectrophotometrically according to Moran [31]. A known fresh weight of leaves was homogenized in 85% aqueous acetone for 5 min. The homogenate was centrifuged and the supernatant was made up to known volume with 85% acetone and measured against a blank of pure 85% aqueous acetone.
Quantification of non-structural carbohydrates
Carbohydrates were measured following the method of Irigoyen et al. [32], with appropriate adaptations. A sample of 0.5 g fresh matter was homogenized twice with 95% ethanol (v/v) and washed with 70% ethanol (v/v), followed by centrifugation at 1500 g at 2°C for 15 min. Glucose, fructose, and sucrose were determined in the resulting supernatant spectrophotometry at 650 nm [33].
Free proline content
The method of Bates et al. [34] was used in the determination of free proline.
Statistical analysis
Based on the data obtained from the experiment, data presented are the mean ± standard deviation (SD) gained from at least three replicate samples. All the data of the present study were subjected where appropriate; to standard one-way analysis of variance (ANOVA) and student’s t-test (t-value<0.05 was considered as significant) where a significant difference was detected by ANOVA test, pairwise comparisons of means were performed using Least Significant Differences (LSD) at 0.05 probability level.
Results and Discussion
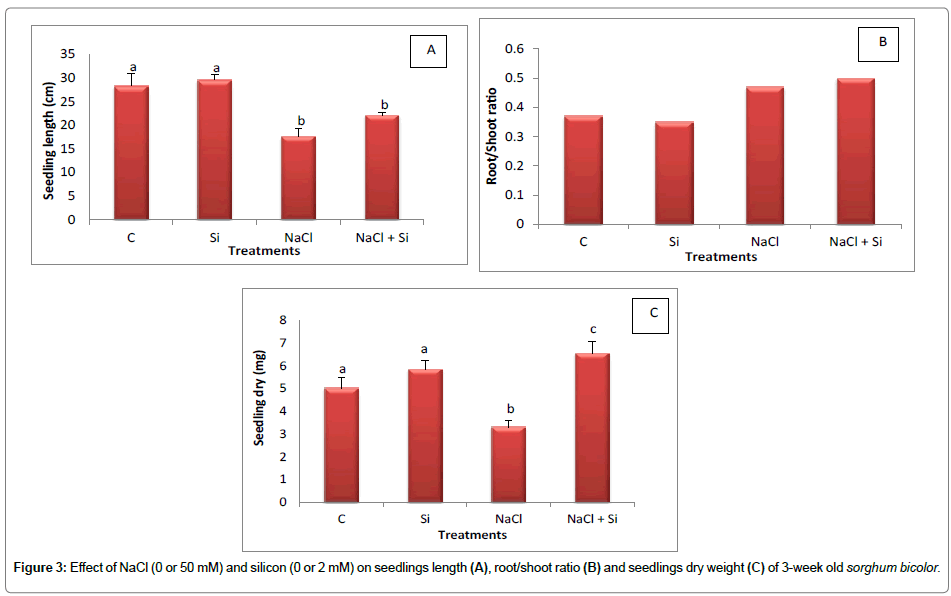
Soil salinization and alkalization is a cosmopolitan problem that affects environment and sustainable development of agriculture. Seed germination and early seedling growth are important stage for the establishment of plants population. Increasing salinity levels significantly decreased germination parameters, root and shoot length, shoot and root fresh and dry weights of some forage sorghum cultivars [35]. Salt stress as major factor can lower leaf water potential, leading to reduced turgor and some other responses and ultimately lower crop productivity in arid and semi-arid zones [1]. In the present study, seedlings lengths as well as dry weight were affected by salinity. The percent reduction in seedlings length and seedlings dry weight was 37.8% and 32.8%, respectively as compared to the control values. The decline in seedlings dry weight observed in this study may be caused by the reduced nutrients transportation from the soil to the growing shoots. Therefore, a decrease in shoot dry matter accompanied by a decline in root dry matter is a normal growth phenomenon under salt stress.
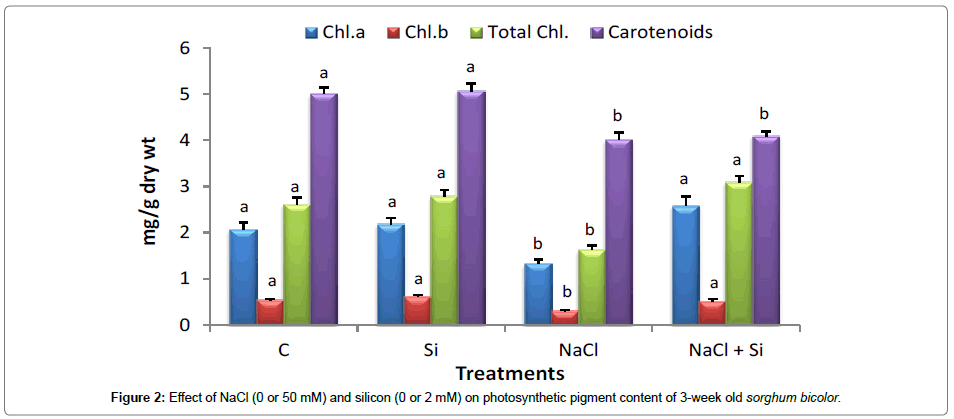
It has been reported that Si, regardless of its essentiality, is beneficial to higher plants subjected to stress [36]. The present experiment was an attempt to monitor the effects of Si application on salinity tolerance of young Sohrgum bicolor L. seedlings. Many authors reported the positive effects of this element on maintenance and growth of plants under saline conditions [37-39]. The decrease which appeared in seedling length was partial when silicon was supplied to the medium. Furthermore, inhibition of plant growth by salinity was associated with the appearance of leaf chlorosis or necrosis [40]. However, in the current work, the seedling remained healthy for the duration of experiment and the shoots showed almost no sign of senescence or salt injury. In the present study, salt stress significantly decreased both Chl a and b content, however, Si supplementation increased them under salt stress after two weeks of treatments (Figure 2). These results are in accordance with that of Al-aghabary et al. [41].
Recently, Moussa and El-Galad [14] reported that silicon (Si) treatment at 2.5 mM partially offset the negative impacts and increased tolerance of maize (Giza 2 and Trihybrid 321) to salinity stress by enhancing parameters such as chlorophyll (a+b), carotenoids, total protein contents, transpiration rate, relative water content (RWC), enzyme activity of ribulose-1,5-bisphosphate-carboxylase/oxygenase (RuBPCase).
It is known that roots allow a plant to absorb nutrients from the surrounding medium, and a healthy root system is a key to a healthy plant. It is therefore, necessary to consider that the root length, shoot length, root dry weight, and shoot dry weight will be important parameters to evaluate the salt tolerance of plants. An increase in root: shoot ratio, as observed in the current study, could be an indication of a healthier plant, provided that the increase comes from a greater root size and not from a decrease in shoot weight [42]. Our results also revealed that root/shoot ratios increased significantly in response to salt treatment (Figure 3B). A maximum increase for root shoot ratio (0.50) is recorded in the salt treated plants in combination with silicon. The results and data presented in this investigation confirm the view of other earlier reports [43-45].
Sorghum seedling length was reduced upon salt exposure by 37.8% compared to the corresponding control (Figure 3A). On the other hand, when seedlings were grown at 50mM NaCl with 2mM silicon, seedling length was reduced only 22%. Similarly, NaCl alone reduced seedling dry weight by 34% but applying 2 mM Si in the salt treated plants resulted in an increase in seedling dry weight (6.56 mg) compared to the control value (Figure 3C). Under non saline conditions silicon increased both seedling length and seedling dry weight compared to the control.
In a study by Cheng et al. [46] on aloe, they reported that addition of Si (2.0 mmol/L) in irrigation solution alleviated significantly the inhibition of NaCl stress at 100 or 200 mmol/L. They also reported that exogenously applied Si decreased significantly Na(+) and Cl(- ) contents, increased K(+) content and K(+)/Na(+) ratio. One of the mechanisms they explained this was the significant enhancement of H(+)-ATPase activities by the addition of silicate in plasma membrane and tonoplast and H(+)-pyrophosphatase activity in tonoplast isolated from aloe root tips under NaCl stress.
Several reports concluded that the positive effect of Si in salt treated plants is due to lowering sodium element in the shoots [39,47- 50]. This silicate-enhance salt tolerance is associated with decrease sodium concentration and increase potassium concentration in plants which uptake and transport is an active process [51].
Matoh et al. [11] reported that silica deposition in leaf walls of rice reduced transpiration leading to lowering sodium uptake.
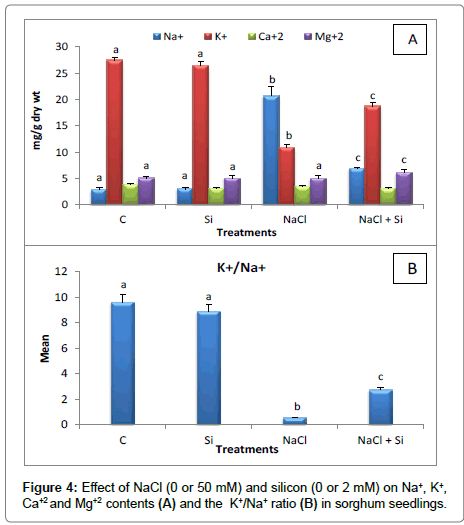
In this work, adding silicon reduced sodium uptake in salt treated seedlings. The Na+ content in salt-stressed plants supplemented with Si accounted for 6.89 mg/g dry weight compared to 20.72 mg/g dry weight in salt-stressed plants (Figure 4A).
In the study of Wang and Han [48] on alfalfa, they reported that potassium increased in leaves and shoots of tolerant alfalfa only. Tuna et al. [49] reported similar results on wheat that was subjected to salt stress. They showed enhanced potassium content upon treatment with silicon.
This is in agreement with the present results, as supplementary Si increased K+ content of sorghum seedlings to 18.76 mg/g dry weight compared to 10.91 mg/g dry weight in salt-stressed seedlings (Figure 4A).
Previous researches [39,47,49] concluded that silicon in salt treated plants decreases sodium uptake and as a result increases the K+/Na+ ratio. In the present study, this ratio increased (2.72) in salt stressed seedlings supplemented with Si (Figure 4B) compared to saltstressed ones (0.52).
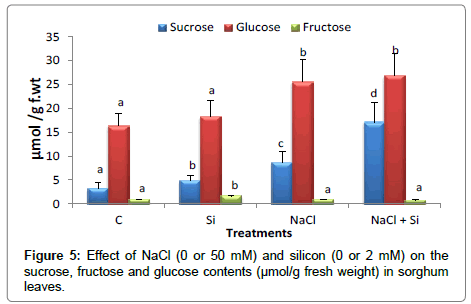
Compared to the corresponding control, silicon significantly increased the sucrose content under salt about 5.3-fold compared to an increase of 2.7-fold in salt-treated seedlings .Silicon increased the content of fructose and glucose. However, under salt stress, the increase in these sugars was more pronounces in glucose while fructose content remained more or less similar to the corresponding control value. Glucose content increased by salt application with a percent increase of 36% compared with the control value. Similarly, sucrose content increased in all treatments. However, no effect of Si was observed on fructose content either in salt treated seedlings or not (Figure 5). Increase of soluble sugars in silicon treated seedlings referred mainly to the changes in sucrose content of those seedlings.
Moussa [52] studied the influence of Silicon on salt- stressed maize. He concluded that Si offset the negative effects and increased tolerance of maize to NaCl stress by enhancing SOD and CAT activities, chlorophyll content and photosynthetic activity.
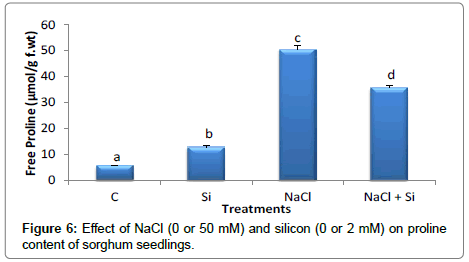
Proline is an osmolyte which plays an important role in osmotic adjustment. It usually accumulates as a response to osmotic stress [53]. However, its role in osmotic adjustment is still controversial. Some studies reported that its accumulation under stress is higher in stresstolerant plants than in stress sensitive ones [54,55]. Other research concluded that proline accumulation is due to the injury caused by stress rather than an indication tolerance [56,57]. In the current study, proline did not increase markedly upon silicon treatment,while seedlings subjected to salt treatment showed a significant increase in free proline content (Figure 6). Nonetheless, Si treatment resulted in a significant decrease in free proline level in salt treated plants compared to the seedlings treated with NaCl alone. It was reported that proline acts as a source of both carbon and nitrogen helping in the recovery of stress, as well as a membrane stabilizer and also a free radical scavenger [58]. Proline increased remarkably in Sorghum seedlings grown under saline condition and showed a marked decreased in seedlings treated with both Si and NaCl. Silicon therefore may play a protective role for Sohrgum seedlings to prevent them from being injured by salinity stress. Therefore, the level of proline accumulated in salt-stressed with Si treatment was not as high as it was in saltstressed without Si treatment. The results of this work show that silicon enhance the salt tolerance and this was not accompanied by increase in proline content [49,59].
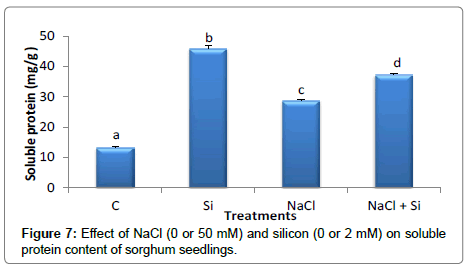
Soluble Protein content of sorghum seedlings increased significantly in all treatments in comparison to control group (Figure 7). In addition, exogenous application of Si significantly increased soluble protein content of sohrgum seedlings in both Si and Si+NaCl treated groups compared with control one. Early findings by Liang [16] and Al-aghabary et al. [41] showed similar results.
Growth reduction reflects the increased metabolic energy cost and reduced carbon gain, which is associated with salt adaptation [60]. PEPCase is expected to contribute the control of carbon flux in the photosynthetic pathway under saline condition [61]. Based on the data reported by Garcia-Mauriňo et al. [62], it can be hypothesized that both enzymes PEPCase and SS may have positive impact on osmotic adjustment. Different lines of evidence suggest that PEPCase enzyme contribute to the regulation of atmospheric CO2 uptake, whereas SS enzyme contributes to the control of sucrose production in leaves. There were some evidence that these enzymes changes may be related to the degree of many factors such as cell composition, the pattern of cation distribution, growth condition and certainly the age of the tissue is likely to be an important factor, although the photoperiod, temperature, salinity and nutritional status were widely appreciated [63].
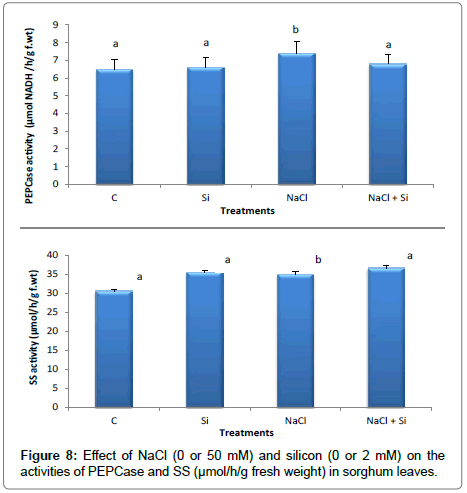
The effect of salt stress on sucrose metabolism was studied by Fernandes and Arrabaça [7] in Lupinus albus L. They found that Sucrose synthase (SS) activity increased under salt stress and sucrose phosphate synthase activity decreased. In the present study, the most significant response of S. bicolor plants to excess NaCl (with or without Si) was the increase of sucrose content in leaves, which was partially due to SS activity increase under salinity (Figure 8). It could be concluded that, there was a clear correlation between the increase in sucrose content in the different treatments and the SS activity.
Mudgal et al. [64] analyzed differences in sensitivity to saline stress among kabuli and desi cultivars of chickpea. They showed that PEPCase activity increased upon salt treatment and the degree of increase being concentration dependent. In the present investigation the PEPCase activity in sorghum seedlings subjected to salt-stress was higher in the treatment with Si than in the treatment without Si, but did not significantly differ from that of control treatment (Figure 8). Ahmad et al. [12] reported that Si enhanced the growth of salt treated barley by improving the chlorophyll content and photosynthetic activity of leaf cell organelles. This may indicate that silicon supplementation under salt stress can maintain photosynthesis efficiency in the leaves in a higher level.
Conclusion
The present results concluded that the growth inhibition caused by salt treatment of sorghum seedlings was partly improved by silicon supplementation. This study suggests that silicon may induce stress tolerance either by inhibiting Na+ accumulation and/or through the involvement of a metabolic or physiological regulator through the enhancement of both PEPCase and SS activities. Further studies should be done to understand the different roles of silicon in plant metabolism.
Acknowledgment
I am deeply grateful to Prof. Dr. R. Hampp who gave me the chance to do this work in his laboratory at the University of Tübingen, Germany.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Rani RJ (2011) Salt stress tolerance and stress proteins in pearl millet (Pennisetum glaucum L.). J Appl Pharma Sci 1: 185-188.
- Zhu J (2001) Plant salt tolerance. Trends Plant Sci 6: 66-71.
- Parvaiz M (2014) Response of Maize to salt stress a critical review. Intl J Healthc Sci p: 13-25.
- Mousavi SG, Jouyban Z (2012) Effect of salinity stress on germination and growth parameters of seedlings of basil (Ocimum basilicum L.). Tech J Engin App Sci 2: 84-87.
- Ashagre H, Hamza I, Fasika E, Temegen F (2013) Effect of salinity stress on germination and seedling vigour of chickpea (Cicer arietinum L.) cultivars. Academia J Agri Res 1: 161-166.
- Ali S, Idris A, Abdo M (2014) Effect of Salinity on Seed Germination and Seedling Growth of Pearl millet (Pennisetum glaucum L) and Sorghum (Sorghum bicolor L). J Plant Pest Sci 1: 01-08.
- Fernandes F, Arrabaça M (2004) Sucrose Metabolism in Lupinus albus L. Under Salt Stress. Biologia Plantarum 48: 317-319.
- Munns R, Tester M (2008) “Mechanisms of salinity tolerance”. Annu Rev Plant Biol 59: 651-681.
- Igartua E, Gracia M, Lasa J (1995) “Field responses of grain sorghum to a salinity gradient.” Field Crop Res 42: 15-25.
- Isabel R, Guillermo B, Jacinto G, Liqun W, Yi-Min S, et al. (2016) New insights into the post-translational modification of multiple phosphoenolpyruvate carboxylase isoenzymes by phosphorylation and monoubiquitination during sorghum seed development and germination. J Exp Bot 67: 3523-3536.
- Matoh T, Kairusmee P, Takahashi E (1986) Salt-induced damage to rice plants and alleviation effect of silicate. Soil Sci Plant Nutr 32: 295-340.
- Ahmad R, Zaheer S, Ismail S (1992) Role of silicon in salt stress tolerance of wheat (Triticum aestivum L.). Pl Sc 85: 43-50.
- Miao B, Han X, Zhang W (2010) The ameliorative effects of silicon on soybean seedling grown in potassium-deficient medium. Ann Bot 105: 967-973.
- Moussa HR, EL-Galad MAE (2015) Comparative Response of Salt Tolerant and Salt Sensitive Maize (Zea mays L.). Cultivars to Silicon. EuroJ Acad Essays 2: 1-5
- Liang Y, Shen Q, Shen Z, Ma T (1996) Effects of silicon on salinity tolerance of two barley cultivars. J Plant Nutr 19: 173-183.
- Liang Y (1998) Effects of Si on leaf ultrastructure, chlorophyll content and photosynthetic activity in barley under salt stress. Pedosphere 8: 289-296.
- Liang Y (1999) Effects of silicon on enzyme activity and sodium, potassium and calcium concentration in barley under salt stress. Plant Soil 29: 217-224.
- Liang Y, Ding R (2002) Influence of silicon on micro-distribution of mineral ions in roots of salt-stressed barley as associated with salt tolerance in plants. Sci China 45: 298-308.
- Yeo A, Flowers S, Rao G, Welfare K, Senanayake N, Flowers T (1999) Silicon reduces sodium uptake in rice (Oryza sativa L.) in saline conditions and this is accounted for by a reduction in the transpirational bypass flow. Plant Cell Environ 22: 559-565.
- Hattori T, Inanaga S, Araki H, An P, Morita S, et al. (2005) Application of silicon enhanced drought tolerance in Sorghum bicolor. Physiol Plant 123: 459-466.
- Hodson J, Evans D (1995) Aluminum/silicon interactions in higher plants. Journal of Experimental Botany 46: 161-171.
- Gong H, Zhu X, Chen K, Wang S, Zhang C (2005) Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Science 169: 313-321.
- Gunes A, Inal A, Baggi EG, Coban S, Pilbean DJ (2007) Silicon mediates changes to some physiological and enzymatic parameters symptomatic for oxidative stress in spinach (Spinacia oleracea L.) grown under B toxicity. Scientia Horticul 113: 113-119.
- Kaya C, Tuna L, Higgs D (2006) Effect of silicon on plant growth and mineral nutrition of maize grown under water-stress conditions. J Plant Nutri 29: 1469-1480.
- Liang Y, Chen Q, Liu Q, Zhang WH, Ding R (2003) Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt stressed barley (Hordeum vulgare L.). J Plant Physiol 160: 1157-1164.
- Bao-He M, Xing-Guo H, Zhang W (2010) The ameliorative effect of silicon on soybean seedlings grown in potassium-deï¬Âcient medium. Ann Bot 105: 967-973
- Egger B (1993) Invertase,sucrose synthase and sucrose phosphate synthase in lyophilized spruce needles, microplate reader assay. Trees 7: 98-103.
- Abdel-Latif A (2008) Activity of Sucrose Synthase and Acid Invertase in Wheat Seedlings During a Cold–Shock Using Micro Plate Reader Assay. Australian J Basic Appli Science 2: 53-56
- Foyer C, Noctor G, Lelandais M, Lescure M, Valadier J, et al. (1994) Short-term effects of nitrate, nitrite and ammonium assimilation, photosynthesis, carbon partitioning and protein phosphorelation in maize. Planta 192: 211-220.
- Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72: 248-254.
- Moran R (1982) Formulae for determination of chlorophyllous pigments extracted with N-N Dimethyl formamide. Plant Physiol 69: 1376-1381.
- Irigoyen J, Emerich D, Sánchez-Díaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol Plant 84: 55-60.
- Van Handel E (1968) Direct micro determination of sucrose. Anal Biochem 22: 280-283.
- Bates L, Waldren R, Teare I (1973) Rapid determination of free proline for water-stress studies. Pl Soil 39: 205-207.
- Kandil A, Sharief A, Abido W, Ibrahim M (2012) Effect of salinity on seed germination and seedling characters of some forage sorghum cultivars. Int J Agri Scien 4: 306-311.
- Epstein E (2001) Silicon in plants: facts vs concepts. Elsevier, Amsterdam: 1-16.
- Zhu J, Wei G, Li J, Qian Q, Yu J (2004) Silicon alleviates salt stress and increase antioxidant enzymes activity in leaves of salt stressed cucumber (Cucumis sativus L.) Plant Sci 167: 527-533.
- Romero-Aranda M, Jurado O, Cuarterp J (2006) Silicon alleviates the deleterious salt effect on tomato plant growth by improving plant water status. J Plant Physiol 163: 847-855.
- Ashraf M, Rahmatullah A, Bhatti A, Afzal M, Sarwar A Maqsood, et al. (2010) Amelioration of salt stress in sugarcane (Sacharum officinarum L.) by supplying potassium and silicon in hydroponics. Pedosphere 20: 153-162
- Tavakkoli E, Fatehi F, Coventry S, Rengasamy R, McDonald G (2011) Additive effects of Na+ and Cl–ions on barley growth under salinity stress. J Exp Bot 62: 2189-2203.
- Al-aghabary K, Zhujun Z, Qinhua S (2004) Influence of silicon supply on chlorophyll content, chlorophyll fluorescence, and antioxidative enzyme activities in tomato plants under salt stress. J Pl Nutr 27: 2101-2115.
- Aishah S, Saberi H, Halim R, Zaharah A (2011) Yield responses of forage sorghums to salinity and irrigation frequency. Afr J Biotechnol 10: 4114-4120.
- Bengum F, Karmoker J, Fattah Q, Maniruzzaman A (1992) The effect of salinity on germination and its correlation with K, Na, Cl accumulation in germinating seeds of Triticm aestivum L. Cv. Akbar. Plant Cell Physiol 33: 1009-1014.
- Muhling K, Laüchli A (2002) Effect of salt stress on growth and cation compartmentation in leaves of two plant species differing in salt tolerance. Plant Physiol 159: 137-146.
- Begdullayeva T, Kienzler K, Kan E, Ibragimov N, Lamers J (2007) Response of Sorghum (Bicolor varieties) to soil salinity and feed production in Karakalpakstan, Uzbekistan. Irri Drain Sys 21: 237-250.
- Cheng X, You L, Quing Z, Zhao P (2006) Silicate improves growth and ion absorption and distribution in aloe vera under salt stress. J Plant physiol Mol bio 32: 73-78.
- Gong H, Randall D, Flowers F (2006) Silicon deposition in the root reduce uptake in rice (Oryza sativa L.) seedling by reducing bypass flow. Plant Cell Environ 29: 1970-1979.
- Wang X, Han J (2007) Effects of NaCl and silicon on ion distribution in the roots, shoots and leaves of two alfalfa cultivars with different slat tolerance. Soil Sci Plant Nutr 53: 278-285.
- Tuna A, Kaya G, Higgs D, Murillo-Amador B, Aydemir S, et al. (2008) Silicon improves salinity tolerance in wheat plants. Environ Exp Bot 62:10-16.
- Kafi M, Rahimi Z (2012) Effect of salinity and silicon on root characteristics, growth, water status, propline contents and ion accumulation of purslane (Portulaca oleracea L.). Soil Sci Plant Nutr 57: 341-347.
- Rahimi R, Mohammakhani A, Roohi V, Armand N (2012) Effects of salt stress and silicon nutrition on cholorophyll content, and yield components in fennel (Foeniculum vulgar Mill.). Intl J Agri Crop Sci 4: 1591-1595.
- Moussa HR (2006) Influence of exogenous application of silicon on physiological response of salt-stressed maize (Zea mays L.). Inter J Agri Biology 293-297.
- Ashraf M, Foolad M (2007) Roles of glycine betaine and proline in improving plant abiotic stress and resistance. Environ Exp Bot 59: 206-216
- Madan S, Nainawatte H, Jain P, Chowdhury J (1995) Proline and proline metabolizing enzymes in vitro selected NaCl-tolerant Brassica juncea L. under salt stress. Ann Bot 76:51-57.
- Nayyar P, Wali P (2003) Water stress induced proline accumulation in contrasting wheat genotypes as affected by calcium and abscisic acid. Biol Plant 46:275-279.
- Lutts S, Nainawatee H, Jain R, Chowdhury B (1999) NaCl effects on proline metabolism in rice (Oryza sativa) seedling. Physiol Plant 105: 450-458.
- De-Lacerda C, Cambraia J, Oliva M, Ruiz Hand, Prisco J (2003) Solute accumulation and distribution during shoot and leaf development in two sorghum genotypes under salt stress. Environ Exp Bot 49: 107-120.
- Jain M, Mathur G, Koul S, Sarin N (2001) Ameliorative effects of proline on salt stressinduced lipid peroxidation in cell lines of groundnut (Arachis hypogea L.). Pl Cell Rep 20: 463-468.
- Lee S, Sohn E, Hamayun M, Yoon J, Lee I (2010) Effect of silicon on growth and salinity stress of soybean plant grown under hydroponic system. Agrofor Syst 80: 333-340
- Netondo GW, Onyango JC, Beck E (2004) Sorghum and salinity: I. Responses of growth, water relations, and ion accumulation to NaCl salinity. Crop Sci 44: 797-805.
- Soussi M, Liuch C, Ocaňa A (1999) Comparative study of nitrogen fixation and carbon metabolism in two chick-pea (Cicer arietinum L.) cultivars under salt stress. J Exp Bot 50: 1701-1708.
- García-Mauriño S, Monreal A, Alvarez R, Echevarría C, Vidal J (2003) Characterization of salt stress-enhanced phosphoenolpyruvate carboxylase kinase activity in leaves of Sorghum vulgare: independence from osmotic stress, involvement of ion toxicity and significance of dark phosphorylation. Planta 216: 648-655.
- Huber S, Huber J (1992) Role of sucrose-phosphate synthase in sucrose metabolism in leaves. Plant Physiol 99: 1275-1278
- Mudgal V, Madaan N, Mudgal A, Mishra S, Singh A, et al. (2009) Changes in growth and metabolic profile of Chickpea under salt stress. J Appl Biosci 23: 1436-1446.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi