Research Article, J Biochem Eng Bioprocess Vol: 6 Issue: 1
The Effect of Carbon Monoxide and Methane on the Oxidation of Ferrous Iron by Acidithiobacillus ferrooxidans
Beazley P and Karamanev D*
Department of Chemical and Biochemical Engineering, The University of Western Ontario, London, Ontario, N6A 5B9, Canada
*Corresponding Author: Karamanev D Department of Chemical and Biochemical Engineering, The University of Western Ontario, London, Ontario, N6A 5B9, Canada E-mail: dkaraman@uwo.ca
Received date: 03 November, 2022, Manuscript No. JBEBT-22-78995; Editor assigned date: 07 November, 2022, PreQC No. JBEBT-22-78995 (PQ); Reviewed date: 21 November, 2022, QC No. JBEBT-22-78995; Revised date: 03 January, 2023, Manuscript No. JBEBT-22-78995 (R); Published date: 10 January, 2023, DOI: 10.4172/jbebt.1000048
Citation: Beazley P, Karamanev D (2023) The Effect of Carbon Monoxide and Methane on the Oxidation of Ferrous Iron by Acidithiobacillus ferrooxidans. J Biochem Eng Bioprocess 6:1
Abstract
The impact of carbon monoxide and methane on ferrous iron biooxidation by Acidithiobacillus ferrooxidans was studied. A variety of concentrations of each gas was added to the headspace in equilibrium with the growth medium of the bacterium. The effects of carbon monoxide and methane were compared to parallel control samples. Results showed no inhibition caused by either gas. Not recognized before, this occurrence can be best explained by the variability and adaptability of Thiobacilli. This outcome is advantageous for the inclusion of At. ferrooxidans in applications such as removal of hydrogen sulfide from waste gas streams or others where residual carbon monoxide or methane may linger.
Keywords: Acidithiobacillus ferrooxidans; Ferrous iron oxidation; Carbon monoxide; Methane
Introduction
Refinery hydrocarbon streams typically contain sulfur compounds, which act as impurities that contaminate catalysts and can cause severe operational problems. One of these compounds is Hydrogen Sulfide (H2S) and if natural gas contains an excess of 1% H2S it is characterized as sour gas. H2S is a colorless, flammable, poisonous gas that is corrosive, water soluble and emits an unpleasant odor resembling that of rotten eggs. Due to its many adverse properties, hydrogen sulfide is stripped during the processing of natural gas, leaving any residual amounts to be incinerated to form Sulfur Dioxide (SO2), which is then released to the atmosphere.
The conventional method of hydrogen sulfide removal is called the claus process. This process consists of two stages: The thermal phase and the catalytic phase. The thermal phase partially oxidized hydrogen sulfide with air in a high temperature (1000°C-1400°C) reaction furnace, rendering sulfur, sulfur dioxide and unreacted hydrogen sulfide. The catalytic phase is conducted at lower temperatures (200°C-350°C) and reacts remaining hydrogen sulfide with the sulfur dioxide in the presence of a catalyst to produce additional sulfur. Still to achieve high conversion in the catalytic phase, two or three stages must often be employed.
Recently, microbial desulfurization solutions have been considered a viable alternative. Commonly, the acidophilic chemolithotroph Acidithiobacillus ferrooxidans is chosen for this application. Similarly, its effective removal of hydrogen sulfide is completed in two steps. First the gas is absorbed and reacted in ferric iron solution producing sulfur, ferrous ions and hydrogen ions. Then biological oxidation of ferrous iron occurs to regenerate the ferric solution. This has several advantages including complete conversion of sulfide to easily separated elemental sulfur, treatment of anaerobic gases and those with high hydrogen sulfide concentrations [1].
Where natural gas is involved, it is not only hydrogen sulfide that will likely affect bacteria, but the other trace gases present. After combustion is carried out residual gases including a variety of alkanes (majority being methane), carbon monoxide, oxygen, nitrogen, hydrogen and carbon dioxide often remain. With the latter four constituents regularly found in the aerobic atmosphere required for growth of At. ferrooxidans, it is chiefly the influence of methane and carbon monoxide that must be assessed.
The effect of CO on microorganisms can be quite diverse. It can be used as a substrate by carboxydotrophic bacteria that constitute a small but diverse group of aerobes, primarily within the α-Proteobacteria and the Firmicutes, which use CO as sole carbon and energy source at high concentrations while expressing relatively low affinity CO uptake systems [2]. The metabolism of carboxydotrophs is facultatively chemolithoautotrophic and they are unique within the classification of chemolithotrophs in being able to generate their own carbon dioxide for assimilation [3]. Furthermore, bacteria such as methanogens, acetogens, sulfate reducers and certain phototrophs all have the ability to oxidize carbon monoxide [4]. Under anaerobic conditions methanogenic bacteria, for instance Methanobacterium thermoautotrophicum, reduce CO with H2 to produce methane. Acetogenic bacteria (e.g. Clostridium thermoautotrophicum, Clostridium pasteurianum, Clostridium thermoaceticum) form acetate and carbon dioxide from CO. Sulfate reducing bacteria like Desulfovibrio desulfuricans and light deprived phototrophic bacteria like Rhodopseudomonas gelatinosa and Rhosospirill rubrum form CO2 and H2 while oxidizing CO anaerobically. All of the aforementioned bacteria have this capability because of the enzyme carbon monoxide dehydrogenase, which enables this by catalyzing the reversible reaction [5].
CO+H2O → CO2+H2
Although it is apparent that carbon monoxide can act as a substrate, there is also evidence showing that it can have a negative impact on bacterial growth and function. It has been shown that carbon monoxide may be an active biochemical and physiological regulator of cell functions, whereby small changes in its environmental concentration can greatly affect such features as energy transduction and biomass yield. Desulfovibrio vulgaris was found to only grow at CO concentrations of less than 4.5%, while Desulfovibrio desulfuricans could tolerate a greater amount of nearly 20%. Another example is Acetobacterium woodii, which was found to be able to grow at a gas phase concentration of up to 30% [6]. Therefore carbon monoxide can impact with varying sensitivity; however the mechanism of the inhibitory effect of CO on bacterial growth is still unknown.
Methylotrophs are aerobic bacteria that utilize organic compounds containing one carbon atom (e.g. methane, halomethanes, methylated sulfur compounds, methanol etc.) more reduced than formic acid as sources of carbon and energy and assimilate formaldehyde as a chief form of cellular carbon. Occurring naturally in both soil and water, methanotrophic bacteria are a subset of methylotrophs and are unique in their ability to use methane as a sole carbon and energy source [7]. The use of an enzyme called Methane Monooxygenase (MMO), which produces methanol upon the introduction of an oxygen atom into the methane molecule by way of the following reaction CH4+O2+2e-+2H+ → CH3OH+H2O, is a defining characteristic of methanotrophs [8].
Chemolithotrophic nitrifying bacteria such as Nitrosomonas oceanus and Nitrosomonas europaea, which typically oxidize ammonia, are also capable of oxidizing methane, yet its growth under such conditions has not been reported [9]. The effects of methane on iron or sulfur-oxidizing chemolithotrophic bacteria are not known.
The phylogenetic and taxonomic diversity of aerobic carbon monoxide oxidizing bacteria is continuing to expand, however as is the case for Acidithiobacillus ferrooxidans, the effects of carbon monoxide are still relatively unknown. This uncertainty also holds true for the relationship between methane and At. ferrooxidans. It is acknowledged that under anaerobic conditions At. ferrooxidans can be grown on elemental sulfur using ferric iron as the electron acceptor, signifying that it can be considered as facultative anaerobe. In addition, often in oxygen deficient surroundings methane is widespread, causing At. ferrooxidans to be exposed to it in all probability. Regarding carbon monoxide, it is unknown whether At. ferrooxidans possesses carbon monoxide dehydrogenase. As previously mentioned, the metabolism of carboxydotrophs is facultatively chemolithoautotrophic, similar to At. ferrooxidans, which is obligately chemolithoautotrophic. Furthermore, certain hydrogenoxidizing bacteria such as Ralstonia eutropha is known to be resistant to carbon monoxide and being that At. ferrooxidans is also capable of oxidizing hydrogen could act as another indicator. There is evidence however, that aside from the bacteria, the presence of iron could have an impact on both carbon monoxide and methane oxidation [10-13]. Consequently, this study was undertaken to evaluate what influence, if any, carbon monoxide and methane gas have on ferrous iron bio oxidation by Acidithiobacillus ferrooxidans.
Materials and Methods
Microorganism and essential chemicals
The original microorganisms were from a pure culture of Acidithiobacillus ferrooxidans, JCM3863, obtained from the Japan collection of microorganisms. It was used for the oxidation of ferrous iron in an inverse fluidized bed bioreactor. The inoculum used in the present work contained bacteria from the effluent of this bioreactor. The bacteria were then added to 9 K media, proposed by silverman and lundgren: (NH4)2SO4 3.0 gl-1; MgSO4 0.5 gl-1; K2HPO4 0.5 gl-1; KCl 0.1 gl-1. In addition, variable concentrations of FeSO4.7H2O (99+% pure), acquired from Merck KGaA (Darmstadt, Germany), were added depending on the experiment to be performed. All of the components present in the medium, including the ferrous sulfate, were of analytical grade. Sulfuric acid with purity of 95%-98% obtained from APC (Montreal, Quebec), was employed to adjust the pH of the medium accordingly.
The methane gas (99%) required for experimentation was purchased from Aldrich (Milwaukee, USA). Carbon monoxide (99%) was prepared from the dehydration of formic acid by sulfuric acid to produce carbon monoxide and water, which was then securely contained. Iron analysis involved 5-Sulfosalicylic Acid (SSA), which was produced by Merck KGaA and ammonium hydroxide from EMD (Gibbstown, New Jersey). The 5-sulfosalicylic acid and ammonia were used in aqueous solutions with concentrations of 10% w/v and 28%-30% w/v, respectively. Ferric sulfate, also obtained from Merck, was used to test for possible chemical interactions between ferric ions and the gases involved. The New Brunswick G25 gyratory shaker incubator was used throughout the experiment to ensure effective mixing of the solutions in the flasks.
Performance of spectrophotometric iron analysis
The analysis of iron in solution with the presence of both carbon monoxide and methane was executed by the spectrophotometric method using SSA as an indicator [14]. The procedure for determining the iron speciacion in solution included the following steps. 0.1 mL sample was taken by a micropipette and placed in a beaker glass. Then 3 mL of the 10% SSA solution was added, followed by 100 mL of deionized water. The spectrophotometer used was a varian cary 50 UV-Vis spectrophotometer (varian instruments, USA). The light absorbance was measured at 500 nm and using the established calibration equation, the ferric iron concentration was found. Once this was accomplished, the total iron concentration was found by adding 3 mL of ammonium hydroxide solution to the same flask and measuring the absorbance at a wavelength of 425 nm. This procedure was routinely used throughout the investigation to follow the progression of ferrous iron bio-oxidation in the presence of carbon monoxide and methane gas.
Headspace parameters and gas chromatography measurements
Rubber stoppers, wrapped in paraffin film, hermetically sealed the 125 mL flasks used for gas analysis. To enable samples to be injected and drawn from select flasks, a tube that could remain sealed or provide access was included in the body of the stopper. Liquid samples were taken using a glass pipette and bulb, while gas samples required the use of a 500 μL gastight Teflon resin luer lock syringe (Hamilton, USA).
The instrument used to interpret the gas samples was a varian saturn GC/MS, model 2200, which combines CP-3800 GC and 2000 series ion trap MS (Varian Instruments, USA). Equipped with a Mass Spectrometer (MS), Flame Ionization Detector (FID) and Thermal Conductivity Detector (TCD) system, the latter two components were needed for the current study. The FID was utilized to effectively measure the concentration of methane, while carbon monoxide was quantified by the TCD. The separation was carried out using a chrompack capillary column (CP-Sil 8 CB low bleed/MS with dimensions 30 m × 0.25 mm i.d.; film thickness 0.25 μm). The carrier gas was helium flowing at a constant rate of 1 mL min-1, while the FID gases (H2 and air) flow rates were 30 and 300 ml min-1 respectively. Prior to operation all gases were cleaned over gas filters in order to eliminate unwanted water, oil or other foreign material while aiding in chromatographic reproducibility [15]. Two filters were used in series to ensure effective removal, the labclear refillable gas filter (Model No. RGF-250-200) and the varian chrompack Gas-Clean GC-MS filter (Model No. CP 17973). The injector temperature was 80°C when the samples were introduced through insertion of the syringe via a septum. A customized method was developed and programmed with reference to the conditions to be maintained for experimental runs. The column oven was at 30°C, the middle FID was adjusted to 250°C and the front TCD at 100°C. The column flow was 5.0 ml min-1, column pressure was 1.2 psi, with total flow and linear velocity stabilized at 26.5 ml min-1 and 37.6 cm s-1, respectively. The entire length of the program was 7.325 minutes, which was sufficient for the elution of both gases.
Preparation of gas concentration standards
First, it was necessary to develop the relationship between the concentration of carbon monoxide or methane and the corresponding peak areas featured on the generated chromatogram. This was accomplished by preparing six different jars of known volume (43 mL) and fitting their lids with septums. Using a syringe, quantities of gas were injected into the jars so that each represented a chosen concentration (500, 3000, 5000 and 10,000 ppmv). Samples from each jar could then be chromatographically analyzed. Due to the elevated concentrations dilution may be essential to obtain positive chromatographic results. The concentrations and resultant peak areas were then tabulated. This set of data could then be plotted as standards, giving a representative mathematical equation that could then be manipulated to calculate gas concentration in future experimental runs.
Results and Discussion
Development of gas standards
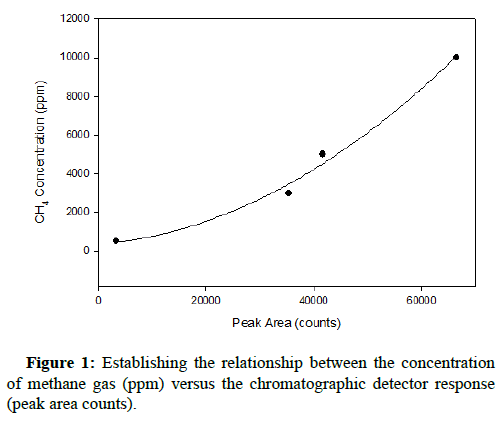
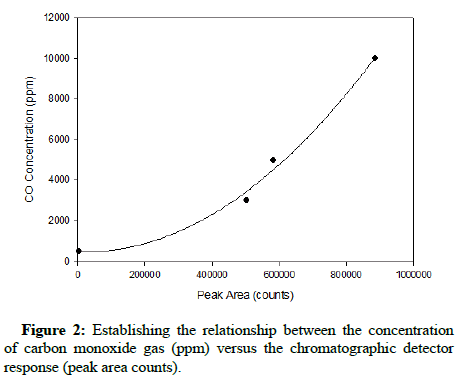
The standard relationship between gas concentration and the corresponding chromatographic peak area count results was completed prior to introducing either gas into the growth environment of At. ferrooxidans. After obtaining the results generated for both carbon monoxide and methane at concentrations of 500, 3000, 5000 and 10,000 ppm, representative plots were constructed. The instrument response to such elevated concentrations was non-linear; therefore the relationship between detector response and concentration was established by non-linear curve fitting, similarly reported by Rich and King. The quadratic curves characterizing the relationships with respect to methane and carbon monoxide are shown below, in Figures 1 and 2, respectively.
The R-squared values for both quadratic regressions exceeded 0.99, indicating that they have high relative predictive power as models and can reliably determine gas concentrations in successive runs.
Effect of ferric iron on carbon monoxide and methane gas under sterile conditions
Before introducing At. ferrooxidans, the behaviour of carbon monoxide and methane gas in the presence of ferric ions was examined. The significance of investigating this possibility is that as ferric iron is produced via oxidation, it would eliminate the hazard caused by the gases with regard to At. ferrooxidans. Pertaining to methane, ferric iron has been shown to inhibit methanogenesis, methane emissions and methane oxidation [16-18]. Similarly, hematite (α-Fe2O3) has proven to successfully promote catalytic oxidation of carbon monoxide. Consequently, there is supporting data to validate the evaluation of the influence of ferric iron on either gas.
Preceding the investigation of ferrous bio-oxidation in the presence of both gases, it was practical to check if ferric ions would oxidize carbon monoxide or methane chemically. Initially a batch of 9 K growth medium was prepared, with 2 g/L Fe3+ (approximately 7.16 g Fe2(SO4)3.7H2O) substituting for the ferrous sulfate produced biologically. Two 125 mL Erlenmeyer flasks were filled with 38 mL of ferric sulfate solution and 2 mL of the bacteria containing inoculum. The pH was adjusted to 1.80, the flasks were securely sealed using stoppers wrapped in parafilm that were then secured from the exterior and 200 μL of either pure carbon monoxide or methane gas was added. Gas samples were then drawn and chromatographically analyzed so the initial concentrations could be verified. The flasks were then incubated at 25°C on a gyratory shaker at a speed of 200 RPM and samples were analyzed by the same method every five hours to observe any apparent changes in gas concentration.
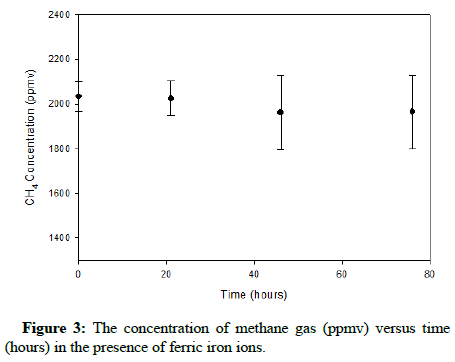
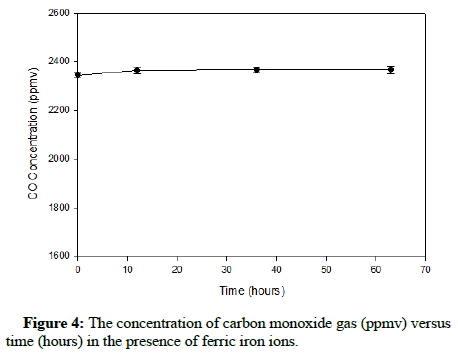
The plots showing the effect of ferric iron on methane and carbon monoxide are exhibited in Figures 3 and 4, correspondingly.
The results show that apparently ferric ions do not appreciably oxidize either methane or carbon monoxide. Figure 3 displays a minor decline in the concentration of methane; nevertheless, this change would not likely impact At. ferrooxidans during its process of ferrous iron oxidation. Therefore, during ferrous iron bio-oxidation, the bacteria will have to function while tolerating a relatively constant concentration of carbon monoxide or methane gas in the headspace.
Ferrous iron bio-oxidation in the presence of carbon monoxide and methane gas
The influence of carbon monoxide on ferrous iron bio-oxidation by At. ferrooxidans was examined. Ferrous iron based growth medium was prepared, containing 2 g/L Fe2+ (approximately 9.96 g FeSO4.7H2O) and having a pH of 1.80. Three 125 mL Erlenmeyer flasks were then charged with 38 mL of medium, followed by 2 mL of inoculum. With the absence of any added gases, one flask acted as the control, whereas the remaining flasks were subjected to equal quantities of the specified gas. From the flasks containing added gases, one remained sealed and undisturbed for the duration of the experiment, while the other provided samples for spectrophotometric and chromatographic analysis. The purpose of including the undisturbed flask was to observe experimental results with the assurance that they were unaffected by any potential escaping gas. With the exception of the control, initial concentrations of both ferric and total iron were ascertained spectrophotometrically before the appropriate amounts of carbon monoxide or methane gas were injected into the flasks. Once the initial conditions were recorded, the cultures were incubated at a temperature of 25°C on a gyratory shaker at a speed of 200 RPM. Ferric iron, total iron and gas concentrations were assessed periodically monitor the advancement of the oxidation.
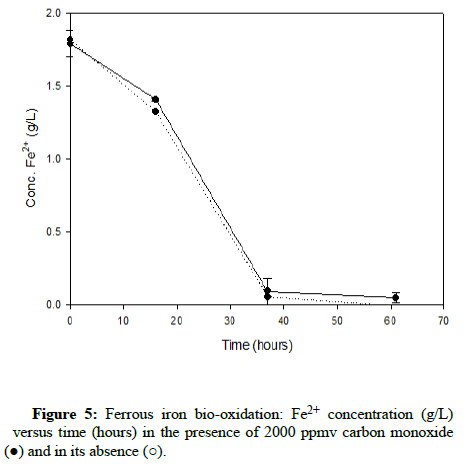
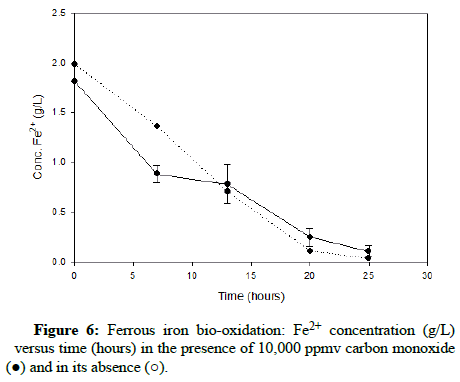
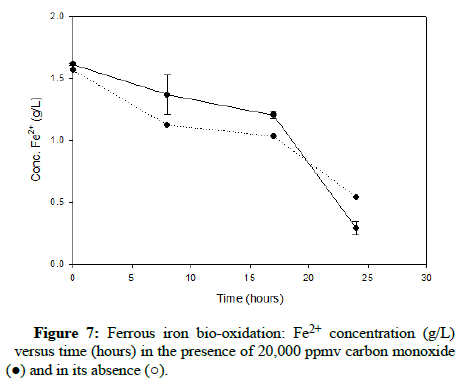
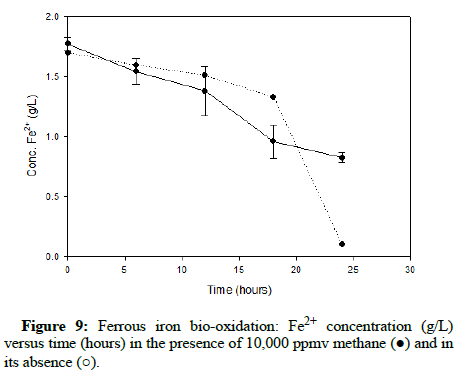
Numerous carbon monoxide concentrations were tested, with the following charts showing results that cover the complete range. (Figures 5-7) demonstrate results for carbon monoxide concentrations of 2000 ppmv, 10,000 ppmv and 20,000 ppmv in the gas phase, respectively.
As can be observed after reviewing the above plots, it is apparent that the ferrous iron bio-oxidation proceeds normally in the presence of the gas and the difference between the flasks containing carbon monoxide and the control flasks is minimal Using gas chromatography, the carbon monoxide concentration was measured during the experimental run, to ensure its continued presence within the flasks. This data showed that the gas concentration in the flask headspace remained nearly constant, so the results accurately describe the progression of the bio-oxidation under the desired conditions.
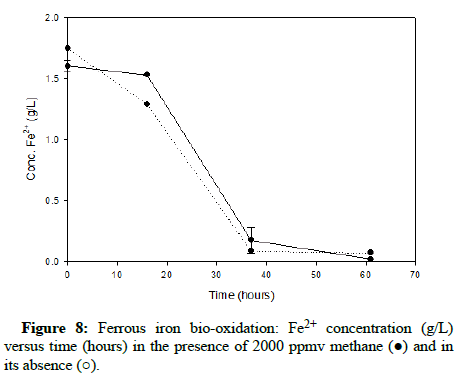
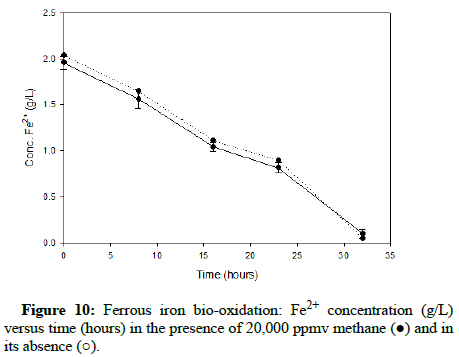
Comparable runs were performed using methane gas. (Figures 8-10) display results for methane concentrations of 2000 ppmv, 10,000 ppmv and 20,000 ppmv respectively. Similar to the findings of the trials involving carbon monoxide, methane appeared to have no impact on the process of ferrous iron bio-oxidation within the acceptable range of experimental error.
After testing within the range of gas concentrations that would probably occur in an industrial setting and acquiring positive results, more extreme conditions were tested to further inspect the tolerance of At. ferrooxidans to carbon monoxide and methane gas.
The ferrous iron bio-oxidation was examined also under more extreme concentrations. The medium was prepared and flasks were inoculated as outlined previously. Prior to gas addition, the initial concentrations of ferric and total iron were determined spectrophotometrically. With the exception of two control flasks, the remaining ones were injected with either carbon monoxide or methane gas. One set of flasks was injected with 10% (v/v), 20% (v/v), 30% (v/v), 40% (v/v), 50% (v/v) and 60% (v/v) of carbon monoxide, while the other contained the same quantities of methane. The flasks were then placed in the gyratory shaker, uninterrupted, until the average period of time needed for bio-oxidation had passed (~30 hours), whereby the final ferric and total iron concentrations were recorded. This ensured that no gas would escape from the flasks and skew the results.
Thus, ferrous iron bio-oxidation was carried out, while in an atmosphere of 10%, 20%, 30%, 40%, 50% and 60% (v/v) of either carbon monoxide or methane gas. Readings were only taken at the very start and finish of the trial to eliminate the possibility of gas leakage. The results collected are shown below in Table 1.
| % of gas (v/v) | Carbon monoxide | Methane | ||
|---|---|---|---|---|
| Initial Fe2+ Conc. (g/L) | Final Fe2+ Conc. (g/L) | Initial Fe2+ Conc. (g/L) | Final Fe2+ Conc. (g/L) | |
| 0 | 1.5675 | 0.292 | 1.6996 | 0.2837 |
| 10 | 1.695 | 0.575 | 1.984 | 0.427 |
| 20 | 1.704 | 0.733 | 1.76 | 0.451 |
| 30 | 1.568 | 0.786 | 1.673 | 0.605 |
| 40 | 1.512 | 0.412 | 1.552 | 0.605 |
| 50 | 1.568 | 0.511 | 1.572 | 0.582 |
| 60 | 1.566 | 0.766 | 1.578 | 0.236 |
Table 1: Initial and final ferrous iron concentrations while tolerating varying volume percentages of carbon monoxide and methane for duration of 30 hours.
As one can see, ferrous iron bio-oxidation still took place effectively despite the excessive amounts of gas added. This firmly shows that At. ferrooxidans has an exceptionally low sensitivity to both carbon monoxide and methane and can therefore be employed in applications where they may be included.
The high tolerance of At. ferrooxidans to carbon monoxide and methane has not previously been studied, but it appears that both can be added to the growing list of chemicals that the bacteria can thrive in spite of their presence. Naturally, At. ferrooxidans is aerobic and autotrophic, thus accepting oxygen and carbon dioxide, still it has been reported that a carbon dioxide concentration in excess of 10% (v/v) can cause inhibition of culture growth [19]. With respect to another prevalent gas, first reported strains of At. ferrooxidans that utilize molecular hydrogen as their sole energy source. The activity of the bacteria is also quite resistant to copper and nickel, withstanding concentrations of approximately 10.1 kgm-3 and 9.7 kgm-3, respectively. Conversely, while At. ferrooxidans has been known to exist in their presence, it does show increased sensitivity towards substances such as arsenic, silver, cobalt, uranium and mercury [20-22].
Evidently it is difficult to identify the precise biological basis for the results of the current study; however it is clear that At. ferrooxidans has the capacity to thrive in the presence of carbon monoxide and methane and can therefore be included in applications involving these gases.
Conclusion
The effect of carbon monoxide and methane gas on ferrous iron bio-oxidation by At. ferrooxidans was investigated in the current study. Determining the outcome was relevant not only to the use of the bacteria in hydrogen sulfide removal from waste gases, but any other application where carbon monoxide or methane may emerge. After establishing correlations from which to interpret the generated chromatographic data and examining if ferric iron ions impacted the headspace gas concentration, the process of ferrous iron bio-oxidation in the presence of carbon monoxide or methane was observed. Subsequent to testing an extensive range of gas concentrations, it was apparent that At. ferrooxidans was exceptionally resistant to both carbon monoxide and methane. This is best attributed to strain variability among At. ferrooxidans, however further research concerning physiological factors would unquestionably be useful. In any event, the positive findings are valuable for future use of At. ferrooxidans in operations involving hydrogen sulfide removal from waste gases or any other area where the potential presence of carbon monoxide or methane was formerly a concern.
References
- Pagella C, de Faveri DM (2000) H2S gas treatment by iron bioprocess. Chem Eng Sci 55:2185-2194.
- King GM (2003) Molecular and culture-based analyses of aerobic carbon monoxide oxidizer diversity. Appl Environ Microbiol 69:7257-7265.
[Crossref] [Google Scholar] [PubMed]
- Meyer O, Rajagopalan KV (1984) Molybdopterin in carbon monoxide oxidase from carboxydotrophic bacteria. J Bacteriol 157:643-648.
[Crossref] [Google Scholar] [PubMed]
- Rich JJ, King GM (1999) Carbon monoxide consumption and production by wetland peats. FEMS Microbiol Ecol 28:215-224.
- Davydova M, Sabirova R, Vylegzhanina N, Tarasova N (2004) Carbon monoxide and oxidative stress in Desulfovibrio desulfuricans B-1388. J Biochem Mol Toxicol 18:87-91.
[Crossref] [Google Scholar] [PubMed]
- van Houten RT, Pol LW, Lettinga G (1994) Biological sulphate reduction using gas-lift reactors fed with hydrogen and carbon dioxide as energy and carbon source. Biotechnol Bioeng 44:586-594.
[Crossref] [Google Scholar] [PubMed]
- Hanson RS, Hanson TE (1996) Methanotrophic bacteria. Microbiol Rev 60:439-471.
[Crossref] [Google Scholar] [PubMed]
- Madigan MT, Bender KS, Sattley WM, Stahl DA (2020) Brock Biology of Microorganisms. 15th edition, Pearson. 1064.
- Neufeld JD, Knowles R (1999) Inhibition of nitrifiers and methanotrophs from an agricultural humisol by allylsulfide and its implications for environmental studies. Appl Environ Microbiol 65:2461-2465.
[Crossref] [Google Scholar] [PubMed]
- Volova TG, Kalacheva GS, Altukhova OV (2002) Autotrophic synthesis of polyhydroxyalkanoates by the bacteria Ralstonia eutropha in the presence of carbon monoxide. Appl Microbiol Biotechnol 58:675-678.
[Crossref] [Google Scholar] [PubMed]
- Drobner E, Huber H, Stetter KO (1990) Thiobacillus ferrooxidans, a facultative hydrogen oxidizer. Appl Environ Microbiol 56:2922-2923.
[Crossref] [Google Scholar] [PubMed]
- Hung WT, Lin CF (2003) Use of regenerated ferric oxide for CO destruction and suppressing dioxin formation in flue gas in a pilot-scale incinerator. Chemosphere 53:727-735.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Otsuka K (1995) catalytic oxidation of methane to methanol with H2-O2 gas mixture at atmospheric pressure. J Catal 155:256-267.
- Karamanev DG, Nikolov LN, Mamatarkova V (2002) Rapid simultaneous quantitative determination of ferric and ferrous ions in drainage waters and similar solutions. Miner Eng 15:341-346.
- Eiceman GA (2006) Instrumentation of gas chromatography. Encyclopedia Anal Chem
- Jackel U, Schnell S (2000) Suppression of methane emission from rice paddies by ferric iron fertilization. Soil Biol Biochem 32:1811-1814.
- Kumaraswamy S, Ramakrishnan B, Sethunathan N (2001) Methane production and oxidation in an anoxic rice soil as influenced by inorganic redox species. J Environ Qual 30:2195-2201.
[Crossref] [Google Scholar] [PubMed]
- van Bodegom PM, Scholten JCM, Stams AJM (2004) Direct inhibition of methanogenesis by ferric iron. FEMS Microbiol Ecol 49:261-268.
[Crossref] [Google Scholar] [PubMed]
- Nemati M, Harrison STL, Hansford GS, Webb C (1998) Biological oxidation of ferrous sulphate by Thiobacillus ferrooxidans: A review on the kinetic aspects. Biochem Eng J 1:171-190.
- de GC, Oliver DJ, Pesic BM (1996) Effect of silver on the ferrous iron oxidizing ability of Thiobacillus ferrooxidans. Hydrometallurgy 41:211-229.
- Harvey PI, Crundwell FK (1996) The effect of As (III) on the growth of Thiobacillus ferrooxidans in an electrolytic cell under controlled redox potentials. Miner Eng 9:1059-1068.
- Sampson MI, Phillips CV (2001) Influence of base metals on the oxidising ability of acidophilic bacteria during the oxidation of ferrous sulfate and mineral sulfide concentrates, using mesophiles and moderate thermophiles. Miner Eng 14:317-340.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi