Research Article, J Aging Geriatr Med Vol: 2 Issue: 2
The Effect of Serum Sex Steroidal Hormones, on Prostate Volumes of Benign Prostatic Hyperplasia Patients with Different Levels of Serum Testosterone
Masanori Nukui1*, Hitosi Takada2 and Shuichi Nakagawa3
1Nukui Urological Clinic, Kameoka, Japan
3Nakagawa Clinic, Kyoto, Japan
*Corresponding Author : Masanori Nukui
Nukui Urological Clinic, Kameoka, Japan, Kyoto, Japan
E-mail: ev5km2@bma.biglobe.ne.jp
Received: January 08, 2018 Accepted: March 02, 2018 Published: March 07, 2018
Citation: Nukui M, Takada H, Nakagawa S (2018) The Effect of Serum Sex Steroidal Hormones, on Prostate Volumes of Benign Prostatic Hyperplasia Patients with Different Levels of Serum Testosterone. J Aging Geriatr Med 2:2. doi: 10.4172/2576-3946.1000120
Abstract
Introduction: The effect of serum steroidal sex hormones on benign prostatic hyperplasia was evaluated with different levels of serum testosterone epidemiologically.
Methods: Between April 2015 and February 2017, 194 patients attending our urological clinic were assessed for the relationship between prostate volume (PV) and, serum sex hormones (testosterone (T), dihydrotestosterone (DHT), estradiol (E2), dehydroepiandrosterone sulfate (DHEAS)), and between PV and physical size, personal habits, etc. PV was measured by using trans–abdominal ultrasonography. Statistically, the Pearson correlation coefficients test was used.
Results: Total cases (194), the cases of T<3.5 ng/ml (51), the cases of 3.5 �?� T<5.5 ng/ml (85) and the cases of T �?� 5.5 ng/ml (58) were evaluated respectively. Total cases and the cases of T<3.5 showed a positive significant correlation with E2, but the cases of 3.5 �?� T<5.5 and T �?� 5.5 showed no such correlation. The cases of T �?� 5.5 had a positive significant correlation with T/DHEAS, DHT/ DHEAS, E2/DHEAS and age, but the cases of 3.5 �?� T<5.5 showed only correlation with E2/DHEAS.
Conclusions: The PV of lower serum T level was affected especially by serum E2. The PV of higher serum T level was correlated with age or DHEAS. Basically, androgen affects PV, however, it seems to work in cooperation with other sex hormones in different ways depending on the serum androgen level.
Keywords: Prostate volume; Benign prostatic hyperplasia; Testosterone; Estradiol; DHEAS; DHT
Introduction
The etiology of benign prostatic hyperplasia (BPH) is still controversial. However, it is well accepted that androgens are essential for the maintenance of BPH, and many lines of evidence support that androgens are permissive but insufficient for the induction and maintenance of BPH [1]. Estrogens also have been implicated as a cause of BPH [2]. In our previous study which evaluated the correlation between prostate volumes and serum sex hormones [3], we reported the possibility that the serum testosterone (T) and estradiol (E2) had positive effects on BPH growth, and that DHEAS, decreasing with age, had a negative effect. DHEA and its sulphated form DHEAS produced by the adrenal cortex, are the most abundant steroids in humans. DHEA has been shown to exert many of its effects via the androgen receptor (AR) and/or estrogen receptor (ERβ or ERα) following its enzymatic conversion to androgenic or estrogenic ligands, at the same time direct effects of DHEA on the AR and ER have also been demonstrated [4]. Actually, after our previous study we treated quite a few patients with large BPH in the lower serum T level. This might suggest some differences in the mechanism of BPH growth according to their serum T levels. This time therefore, we reexamined the relationship between PV and serum sex hormones and also included dihydrotestosterone (DHT), and furthermore, we evaluated the correlation between them and the PV of three serum T levels epidemiologically.
Methods
Subjects
The study concerned 194 ambulatory patients aged 48 to 98 years (mean: 71.7, SD: 8.76), who attended the urological clinic located in rural areas in Kyoto prefecture from April 2015 to February 2017. Patients who had prostate cancer or other critical diseases, or were prescribed 5α–reductase inhibitor were excluded. The patients studied complained of lower urinary tract symptoms (LUTS) had their kidney, bladder and prostate checked by ultrasonography. At the same time, blood examinations were performed for prostate–specific antigen (PSA), renal function and sex hormones. Permission for these examinations was received from the patients.
Measurement of sex hormones
Blood sampling was performed during clinic hours between 9:00 a.m. to 12:30 p.m.. Serum T, DHT, E2, and DHEAS, were measured. These analyses were conducted in the KYOTO BISEIBUTU KENKYUSHO (Kyoto Microbiological Institute). T, E2, and DHEAS were measured by enzyme immunoassay using the following kits: COBAS (Roche Diagnostics, Tokyo, Japan), ADVIA Centaur (Siemens Healthcare Diagnostics, Tokyo, Japan), and (BECKMAN COULTER, Tokyo, Japan), respectively. DHT was measured by radioimmunoassay (SRL, Tokyo, Japan).
Prostate measurement
Prostates were scanned by trans-abdominal ultrasonography by the same operator and the size was calculated from the longitude, the transverse, and the height of prostates following the method of Stamey [5,6].
Statistical method
The correlation between PV and sex hormones was analyzed by the Pearson correlation coefficients test. The correlation between other factors such as age, BMI were also considered. A P value of <0.05 was considered statistically significant on the two–sided test. All statistical analyses were carried out with the IBM SPSS Statistics version 24.
Results
The total number of patients was 194, and they were divided into three groups (T<3.5 ng/ml: 51 patients, 3.5 ≦ T ≧ 5.5 ng/ml: 85 patients, T>5.5 ng/ml: 58 patients) which were evaluated respectively for the correlation between PV and sex hormones. Patient`s characteristics are showed in the Table 1.
| All patients (N=194) | T<3.5 (N=51) | 3.5 ≦ T<5.5 (N=85) | T ≧ 5.5 (N=58) | |
|---|---|---|---|---|
| Testosterone(ng/ml) | 4.72 ± 1.79 | 2.79 ± 0.56 | 4.40 ± 0.58 | 6.88 ± 1.38 |
| DHT(ng/ml) | 0.59 ± 0.27 | 0.35 ± 0.11 | 0.56 ± 0.16 | 0.84 ± 0.27 |
| E2(pg/ml) | 30.8 ± 8.6 | 27.5 ± 8.2 | 30.2 ± 7.7 | 34.6 ± 9.4 |
| DHEAS(μg/dl) | 117.0 ± 62.5 | 107.1 ± 63.5 | 122.7 ± 61.4 | 117.5 ± 63.1 |
| BMI | 23.3 ± 2.7 | 23.7 ± 3.0 | 23.5 ± 2.5 | 22.6 ± 2.7 |
| Age | 72.4 ± 8.5 | 73.5 ± 8.6 | 71.5 ± 8.1 | 72.8 ± 8.9 |
| PV | 42.7 ± 24.8 | 43.5 ± 29.3 | 42.3 ± 22.3 | 42.7 ± 24.5 |
Table 1: Characteristics of patients.
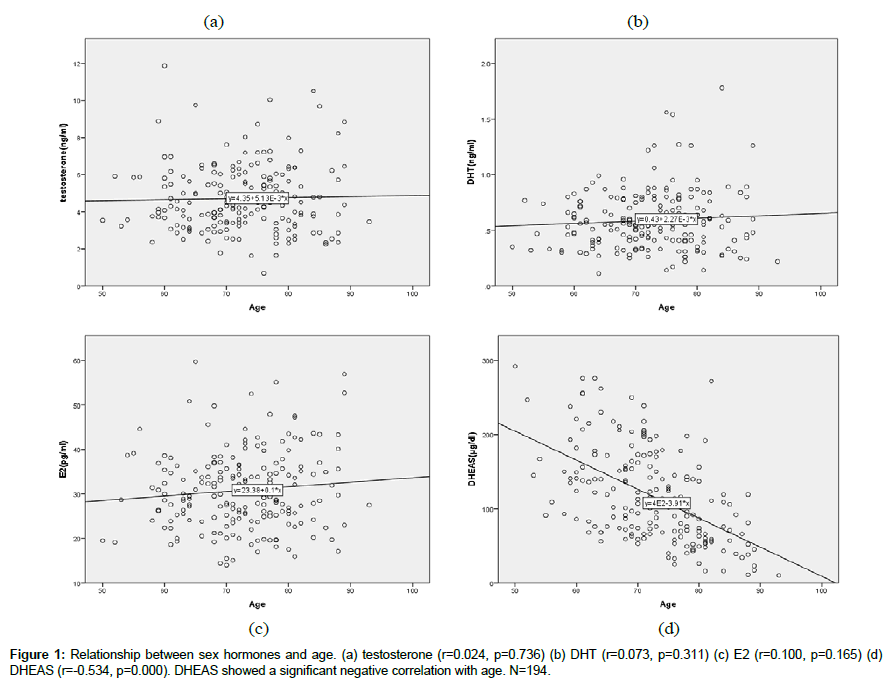
Sex hormones and age (Figure 1).
T (r=0.024, p=0.739), DHT (r=0.073, p=0.311), E2 (r=0.100, p=0.165) did not show any correlation with age. Only DHEAS (r=- 0.534, p=0.000) showed significant negative correlation with age.
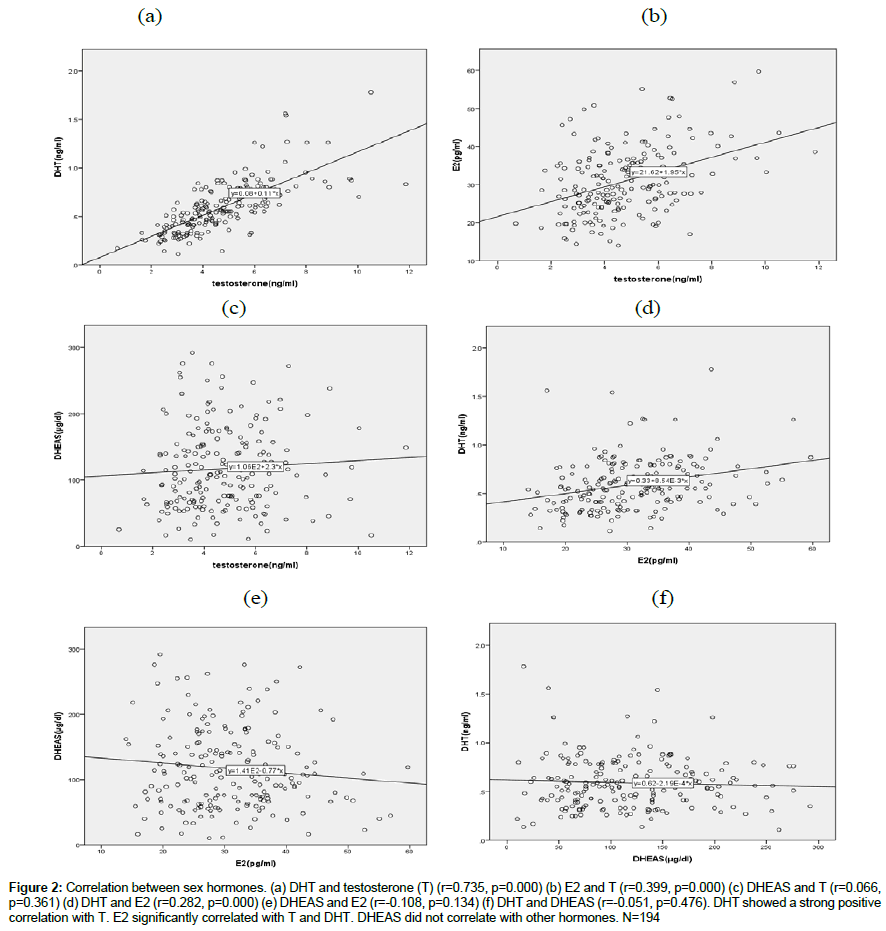
Relationship between sex hormones (Figure 2)
Figure 2: Correlation between sex hormones. (a) DHT and testosterone (T) (r=0.735, p=0.000) (b) E2 and T (r=0.399, p=0.000) (c) DHEAS and T (r=0.066, p=0.361) (d) DHT and E2 (r=0.282, p=0.000) (e) DHEAS and E2 (r=-0.108, p=0.134) (f) DHT and DHEAS (r=-0.051, p=0.476). DHT showed a strong positive correlation with T. E2 significantly correlated with T and DHT. DHEAS did not correlate with other hormones. N=194
T showed a positive significant correlation with E2 (r=0.399, p=0.000) and a strong correlation with DHT (r=0.735, p=0.000). DHT also correlated with E2 (0.282, p=0.000). DHEAS did not show any correlation with other hormones (T(r=0.066, p=0.361), DHT(r=-0.051, 0.476), E2(r=-0.108, p=0.134)).
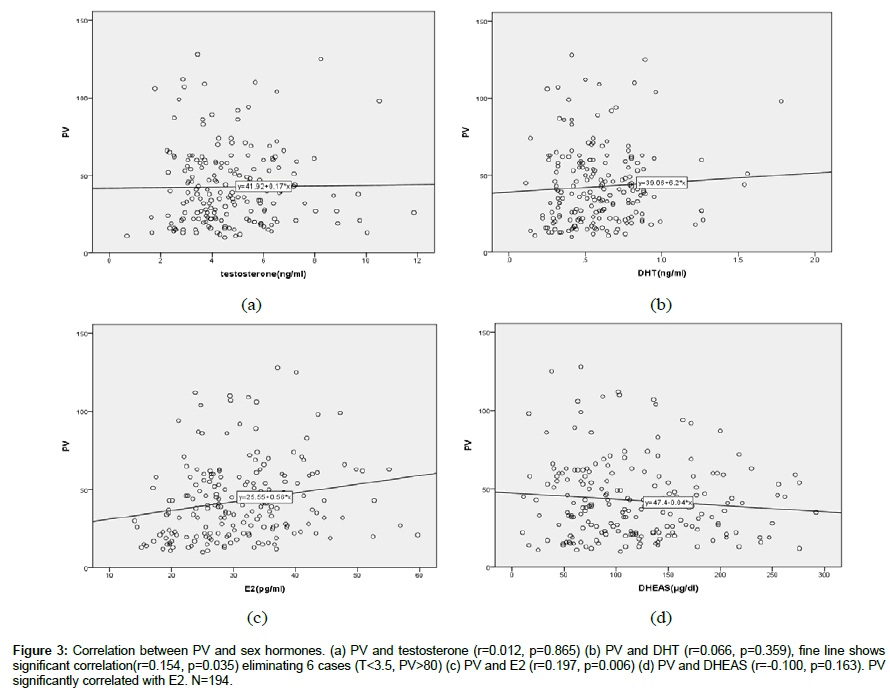
PV and sex hormones (Figure 3).
Figure 3: Correlation between PV and sex hormones. (a) PV and testosterone (r=0.012, p=0.865) (b) PV and DHT (r=0.066, p=0.359), fine line shows significant correlation(r=0.154, p=0.035) eliminating 6 cases (T<3.5, PV>80) (c) PV and E2 (r=0.197, p=0.006) (d) PV and DHEAS (r=-0.100, p=0.163). PV significantly correlated with E2. N=194.
PV showed a significant positive correlation with E2 (r=0.197, p=0.006), however, did not show any correlation with T (r=0.012, p=0.865), DHT (r=0.066, p=0.359), and DHEAS (r=-0.100, p=0.163). Concerning DHT, if 6 cases which were T<3.5 ng/ml and PV>80 are eliminated, DHT shows a positive correlation.
The correlation between PV and variables in each group (Table 2).
| PV | total (N=194) | T<3.5 (N=51) | 3.5 ≦ T<5.5 (N=85) | T ≧ 5.5 (N=58) |
|---|---|---|---|---|
| Testosterone | 0.012 (0.033) | 0.199 (0.116) | 0.049 (0.046) | 0.011 (-0.063) |
| DHT | 0.066 (.084) | 0.155 (0.081) | -0.017 (-0.007) | 0.191 (0.103) |
| E2 | 0.197** (0.180**) | 0.343** (0.283**) | 0.207* (0.183*) | 0.097 (0.050) |
| DHEAS | -0.100 (-0.077) | 0.144 (0.089) | -0.171 (-0.155) | -0.232* (-0.127) |
| E2/T | 0.108 (0.079) | 0.108 (0.044) | 0.204 (0.185) | 0.097 (0.097) |
| DHT/T | 0.034 (0.031) | -0.009 (-0.064) | 0.036 (-0.023) | 0.155 (0.104) |
| DHT/E2 | -0.054 (-0.031) | -0.148 (-0.163) | -0.149 (-0.127) | 0.049 (0.016) |
| T/DHEAS | 0.135* (0.140*) | -145 (-0.041) | 0.149 (0.132) | 0.316**(0.200) |
| DHT/DHEAS | 0.162** (0.166**) | -0.103 (-0.074) | 0.119 (0.103) | 0.328**(0.225*) |
| E2/DHEAS | 0.146** (0.140*) | 0.065 (0.013) | 0.222** (0.204*) | 0.287**(0.162) |
| BMI | 0.081 | 0.283** | 0.091 | -0.17 |
| Age | 0.053 | -0.189 | 0.079 | 0.268** |
Table 2: Pearson correlations between PV and variables with different levels of testosterone.
In the group of PV (T<3.5), E2 showed a significant positive correlation with PV (r=0.343, p=0.014). In the group of PV (T ≧ 5.5), T/DHEAS (r=0.316, p=0.016), DHT/DHEAS (r=0.328, p=0.012), and E2/DHEAS (r=0.287, p=0.029) showed a significant positive correlation with PV. This might mean PV becomes larger depend on deceasing DHEAS with age, because the serum level of T, DHT, and E2 do not change with age. PV in this group also showed a significant positive correlation with age (r=0.268, p=0.042) and a negative trend with DHEAS (r=-0.232, p=0.080). In the group of PV (3.5 ≦ T<5.5), PV showed a positive correlation with E2/DHEAS (r=0.222, p=0.041) and a positive trend with E2 (r=0.207, p=0.058). Altogether, the correlation coefficient of E2 became smaller depending on T level, on the contrary, that of DHEAS became progressively negative.
Discussion
The etiology of BPH is presumed to be multifactorial [7]. Therefore, it is still in discussion. In our previous study we assumed PV is affected positively by T and E2, but negatively by DHEAS in serum. However, after that study, we found quite a few BPH patients with signs of hypogonadism, i.e. with serum T levels under 3.5 ng/ml. Consequently, we investigated the correlation between PV and the variables of sex hormones for different levels of serum T. The results were, PV in low T level corresponded to E2, and PV in high T level corresponded to age or DHEAS. These results might mean the etiology of BPH is diverse in the prostate itself rather than multifactorial. Such diversity might also make it difficult to ensure a consistent etiology of BPH. In the literature, it is considered that there are different types of BPH nodules, which are likely to be initiated by different molecular mechanisms, and as such multiple theories may explain the distinct types of BPH [1].
Androgen is basically needed for the growth of BPH with estrogen as indicated by Walsh`s experiment on dogs [8]. There is also the hypothesis that estrogens play a role in the pathogenesis of BPH, characterized predominantly by stromal overgrowth [2], and also the report that proliferation of normal stroma cell of human prostate is stimulated by an increasing estrogen/androgen ratio in vitro [9]. Considering these studies, our finding that the PV of patients in low serum T level correlates with serum E2 level, does not seem to contradict the previous research. However, epidemiologically, there are some studies where PV in the higher bioavailable serum T level showed positive correlation with E2 [10]. On the contrary, there is a study where the risk of surgical treatment of BPH associated with E2 was found in men with relatively low androgen levels [11].
In the cases of therapy in hypogonadism patients, PV increases a little or not, and does not grow to BPH over several years [12,13]. In our study, PV correlated positively with age and had a negative trend with DHEAS in the high T level group. This might mean that the prostates with high serum T level need a lot of time to grow to BPH, in addition, the patients with high T level and also high DHEAS level do not tend to grow to BPH. DHEA has been shown to exert direct agonist effects on ERβ, for example, as observed in competitive receptor binding assays, where DHEA displayed a higher affinity for ERβ than for AR or ERα [4,14]. The precise roles of ERα and ERβ in the pathogenesis of BPH are not fully understood. In general, ERα stimulation in the prostate results in hyperplasia, inflammation, and dysplasia, on the other hand, ERβ inhibits proliferation in the prostates. ERβ knockout mice can develop prostatic hyperplasia with age, which is not observed in wild-type or ERα knockout mice [1,15,16]. Epidemiologically, DHEA was considered a positive risk for BPH in the case-control study in Athens [17], but then again no correlation has been detected between DHEAS and PV of BPH patients in a study evaluating the correlation between sex hormones and PV of BPH, in resected prostates after operations for small prostate cancer [18]. In our present and previous studies, the correlations were evaluated using the ratio of DHEAS and other hormones, therefore, the significant correlations between PV and DHEAS in ratio were detected. This might mean DHEAS works relatively to other sex hormones, and not in the absolute value, when affecting the growth of BPH, depending on the patient.
Conclusion
Androgen is essentially considered to affect the BPH growth as shown in Figure 3, evaluating the correlation between PV and serum DHT, excepting 6 special cases with large PV of BPH and low serum testosterone. In our previous study, we presumed DHEA decreasing with age might be one of the important factors in BPH growth with aging. However, the prostatic growth to BPH seems to have some diversity in the correlating factors. In our present study, E2 seemed to have a positive effect on BPH growth in the patients with lower serum T, and aging seemed to have similar effect in the patients with higher serum T. However, DHEAS seemed to have a negative effect on BPH growth at higher serum T level. These findings might aid the strategy of BPH patients, for example, using aromatase inhibitors to the BPH patients with serum low T, or DHEA to those with high serum T, as alternatives to the prevalent use of 5α-reductase inhibitors.
References
- Nicholson TM, Ricke WA (2011) Androgens and estrogens in benign prostatic hyperplasia: past, present and future. Differentiation 82: 184-199.
- Ho CK, Nanda J, Chapman K, Habib FK (2008) Oestrogen and benign prostatic hyperplasia: effects on stromal cell proliferation and local formation from androgen. J Endocrinology 197: 483-491.
- Nukui M, Takada H, Nakagawa S (2016) Cross sectional analysis between prostate volumes and serum sex hormones in Japanese men attending a urological clinic. The Aging Male 19: 148-154.
- Arnold JT (2009) DHEA metabolism in prostate: For better or worse? Mol Cell Endocrinol 301: 83-88.
- Homma Y, Ishizuka O, Ozono S (2011) Clinical guide line for benign prostatic hyperplasia. The Japanese Urological Association 36-37.
- Terris M, Stamey T (1991) Determination of prostate volume by transrectal ultrasound. J Urol 145: 984-987.
- Carson C, Rittmaster R (2003) The role of dihydrotestosterone in benign prostatic hyperplasia. Urology 61: 2-7.
- Walsh P, Wilson J (1976) The induction of prostatic hypertrophy in the dog with androstanediol. J Clin Invest 57: 1093-1097.
- King KJ, Nicholson HD, Assinder SJ (2006) Effect of increasing ratio of estrogen: Androgene on proliferation of normal human prostate stroma and epithelial cells, and the malignant cell line LNCaP. Prostate 66: 105-114.
- Roberts RO, Jacobson DJ, Rhodes T, Klee GG, Leiber MM, et al. (2004) Serum sex hormones and measures of benign prostatic hyperplasia. Prostate 61: 124-131.
- Gann PH, Hennekens CH, Grodstein F, Stampfer MJ, Longcope C, et al. (1995) A prospective study of plasma hormone levels, nonhormonal factors, and development of benign prostatic hyperplasia. Prostate 26: 40-49.
- Minnmann T, Schubert M, Hübler D, Berthold G, Schumann FC, et al. (2007) A four-year efficacy and safety study of the long-acting parenteral testosterone undecanoate. Aging Male 10: 155-158.
- Meuleman EH, Legros JJ, Bouloux PMG, Lenovas AOJ, Kaspers MJGH, et al. (2015) Effects of long-term oral testosterone undecanoate therapy on urinary symptoms: data from a 1-year, placebo-controlled, dose-ranging trial in aging men with symptomatic hypogonadism. Aging Male 18: 157-163.
- Chen F, Knecht K, Birzin E, Fisher J, Wilkinson H, et al. (2005) Direct agonist/antagonist functions of dehydroepiandrosterone. Endocrinology 146: 4568-4576.
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, et al. (1998) Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA 95: 15677-15682.
- Ellem SJ, Risbridger GP (2009) The dual, opposing role of estrogen in the prostate. Ann NY Acad Sci 1155: 174-186.
- Lagiou P, Montzoros CS, Tzonou A, Signorello LB, Trichopoulos D, et al. (1997) Serum steroids in relation to benign prostatic hyperplasia. Oncology 54: 497-501.
- Partin AW, Oesterling JE, Epstein JI (1991) Influence of age and endocrine factors on the volume of benign prostatic hyperplasia. J Urol 145: 405-409.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi