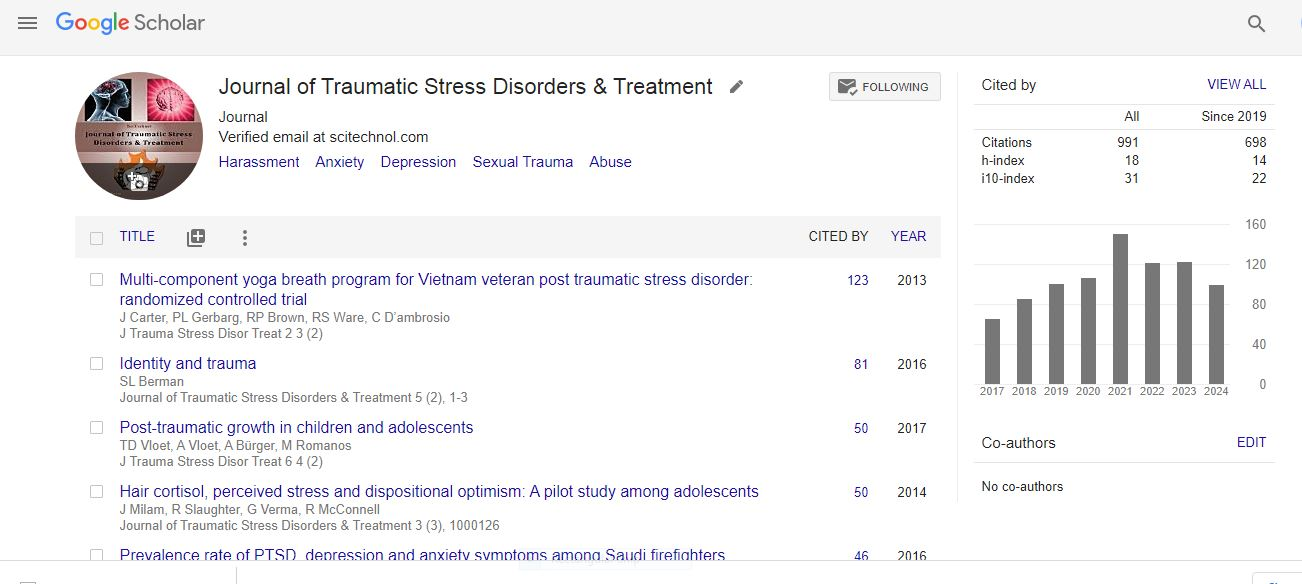
Research Article, J Trauma Stress Disor Treat Vol: 3 Issue: 1
How Does Lymphocyte Glucocorticoid Receptor Expression and Salivary Cortisol Relate to Trauma Exposure and Posttraumatic Stress Disorder?
| Claudia Liebscher, Oliver Grimm, Slawomira J Diener, Stephanie Ridder and Herta Flor* |
| Department of Cognitive and Clinical Neuroscience, Central Institute of Mental Health, Medical Faculty Mannheim, Heidelberg University, J5, Mannheim 69159, Germany |
| Corresponding author : Herta Flor Department of Cognitive and Clinical Neuroscience, Central Institute of Mental Health, Medical Faculty Mannheim, Heidelberg University, J5, 68159 Mannheim, Germany Tel: +49-621-1703-6302; Fax: +49-621-1703-6305 E-mail: herta.flor@zi-mannheim.de |
| Received: May 17, 2013 Accepted: November 28, 2013 Published: December 06, 2013 |
| Citation: Liebscher C, Grimm O, Diener SJ, Ridder S, Flor H (2013) How Does Lymphocyte Glucocorticoid Receptor Expression and Salivary Cortisol Relate to Trauma Exposure and Posttraumatic Stress Disorder? J Trauma Stress Disor Treat 3:1. doi:10.4172/2324-8947.1000117 |
Abstract
How Does Lymphocyte Glucocorticoid Receptor Expression and Salivary Cortisol Relate to Trauma Exposure and Posttraumatic Stress Disorder?
Hypothalamus-pituitary-adrenal axis functioning and glucocorticoid receptor number on lymphocyte subsets were investigated in patients with posttraumatic stress disorder related to type I trauma, trauma-exposed subjects without posttraumatic stress disorder and trauma-unexposed control subjects (n=13 per group) applying a detailed endocrinological assessment. Salivary cortisol profiles were obtained, first, as diurnal baseline cortisol (unstimulated), and second, after dexamethasone administration (stimulated cortisol) to investigate the negative feedback inhibition of the hypothalamus-pituitary-adrenal axis. For further assessment of glucocorticoid receptor binding, glucocorticoid receptor counts on lymphocytes were obtained from blood samples.
Keywords: Posttraumatic stress disorder; Trauma; Hypothalamic-pituitaryadrenal axis; Cortisol; Glucocorticoid receptor
Keywords |
|
| Posttraumatic stress disorder; Trauma; Hypothalamic-pituitaryadrenal axis; Cortisol; Glucocorticoid receptor | |
Abbreviation |
|
| HPA: Hypothalamic-pituitary-adrenal; GR: Glucocorticoid Receptor; PTSD: Posttraumatic Stress Disorder; NK cells: Natural Killer cells; CAPS: Clinician-administered PTSD Scale; CTQ: Childhood Trauma Questionnaire; CES-D: Center for Epidemiologic Studies Depression Scale; PDS: Posttraumatic Diagnostic Scale; FITC: Fluoresceinisothiocyanate; FACS: Fluorescence Activated Cell Sorting; AUCGunstim: Area Under the Curve with respect to the Ground, unstimulated; AUCGstim: Area Under the Curve with respect to the Ground, stimulated; meanincunstim: Mean Increase of cortisol level after awakening, unstimulated; meanincstim: Mean Increase of cortisol level after awakening, stimulated | |
Introduction |
|
| Posttraumatic stress disorder (PTSD) is a mental disorder that can occur when persons are exposed to severe traumatic events like accidents, rape or combat. The main symptoms include re-experiencing of the trauma, avoidance of trauma-related stimuli and hyperarousal [1]. Especially the later symptoms seem to be associated with a reduced cortisol awakening response in patients with PTSD [2]. Lower levels of salivary cortisol [3] as well as enhanced feedback inhibition of the HPA axis were found in PTSD patients when a dexamethasone suppression test was carried out [4,5]. Psychopathological symptom severity and lifetime trauma exposure were shown to be associated with the outcome of feedback inhibition post-dexamethasone in one study [6], while only basal salivary cortisol could be predicted from the number of PTSD symptoms in another study [7]. In the same study basal salivary cortisol (not suppressed cortisol) was predicted from the amount of peritraumatic distress and dissociation. Other studies neither found hypocortisolism in patients with PTSD nor an association between cortisol suppression and symptom severity [8]. In contrary, cortisol hypersuppression was found to be more related to PTSD or major depressive disorder than to a life history of traumatization per se [9]. Similarly, Young and Breslau [10] only found alterations in salivary cortisol of patients with lifetime PTSD and comorbid major depressive disorder, but not in persons with PTSD or MDD alone. Interestingly, in a recent meta-analysis [11] on daily cortisol in PTSD patients (with and without MDD) traumaexposed persons and healthy controls of, reduced cortisol levels were found to be a marker of PTSD while hypersuppression of cortisol by dexamethasone was shown to be a marker of trauma-exposure (not of PTSD vulnerability) while it made no difference if the PTSD patients suffered from a comorbid depression or not. The HPA effect sizes were rather moderated by age, sex, time since trauma and developmental timing of trauma exposure. | |
| Since the results are still conflicting, it is useful to measure GR levels on lymphocytes as a marker for the predicted negative feedbackloop because it causes inhibition of release of ACTH in the pituitary gland [12]. For example, when the ACTH response to dexamethasone was investigated in patients with PTSD and healthy controls, negative feedback inhibition in the patients was shown to result from pituitary glucocorticoid receptor binding and not from low adrenal output [13]. Furthermore, glucocorticoids are main modulators of the immune system. They inhibit lymphocyte proliferation and the release of proinflammatory cytokines by binding to the GR [14,15]. GR levels on lymphocytes might not only be seen as a surrogate marker but as a pivotal hub in mediating the linkage between brain, behavior and the immune system by the complex cytokine network [16]. A recent hypothesis speculates about the role inflammatory processes might contribute to PTSD-related hippocampal damage [17]. | |
| However, immunological parameters, especially the number of GR on lymphocytes in PTSD, also do not yet provide consistent results. For example, a greater population of GRs on lymphocytes was found when combat veterans were compared with healthy controls indicating enhanced GR sensitivity in PTSD [5]. This was supported by the observation that the GR number correlated positively with the severity of combat-related PTSD symptoms while no significant relationship with plasma cortisol levels could be observed. While here the total counts of GRs on lymphocytes were obtained, more recent studies also investigated subtypes of lymphocytes. One study found elevated total leukocyte counts as well as more absolute T cells in veterans with chronic PTSD as compared with veterans without mental disorder [18]. In the same study the number of lymphocyte and T cell counts was even more elevated in patients with a current anxiety disorder while depressed patients were less likely to show elevated B cell counts. Similarly, higher lymphocyte counts as well as a positive correlation of total lymphocyte GR expression with the number of years after trauma and with serum cortisol concentrations were found in male combat veterans with PTSD [19]. While here no significant differences in cortisol level or the number of GR on lymphocyte subpopulations were observed, another study reported higher cortisol levels in PTSD patients (Croatian combat veterans) when compared with healthy controls but no significant correlation between cortisol level and GR expression, or for the total number on lymphocytes or their subpopulations [20]. For both groups the GRs were unevenly expressed in the subpopulations: The patients displayed lower GR levels in each subpopulation, which was most pronounced in natural killer (NK) cells and less pronounced in B cells and T cells. Therefore, NK cells seem to have the highest GR expression on lymphocytes and may be most sensitive to GR changes (like in stress). This is in line with the finding that chronic combat-related PTSD is associated with decreased cytotoxicity of NK cells, which is suggested to impair immune function in PTSD patients [21]. | |
| The aim of our study was to investigate basal cortisol and negative feedback inhibition of the HPA axis as well as the GR expression on subsets of lymphocytes, namely T helper cells, T killer cells, B cells and NK cells in patients with PTSD compared to traumatized persons without PTSD and healthy controls. The last three studies [19-21] show a main strength of the subtyping of lymphocytes by FACS. Whereas our study is not the first utilizing FACS-techniques for investigating GR expression on lymphocytes, it is the first which combines this with an elaborated HPA-axis assessment and two control groups (trauma and healthy controls). In line with the findings mentioned above, we expected the PTSD patients to show lower levels of salivary cortisol and higher suppression of cortisol release after a dexamethasone suppression test compared with the healthy controls and the trauma group. As a potential underlying mechanism of the increased negative feedback inhibition of the HPA axis system, we expected the PTSD patients to display higher GR expression on lymphocyte subsets when compared to the control group, especially on NK cells, which seem to be especially sensitive to changes of GR expression. Furthermore, we investigated if the GR expression on lymphocyte subsets was linked to posttraumatic distress, the number of depressive symptoms, early life stress or the time since trauma-exposure. | |
Materials and Methods |
|
| Participants | |
| Thirty-nine Caucasian volunteers participated in the study, including 13 persons with PTSD after type I trauma (mean age (SD) 44.38 years (6.04), range 30 -57, 8 female, 5 male), 13 with trauma-exposure (type I) but no PTSD (mean age (SD) 37.31 years (12.19), range 21 – 63, 6 female, 7 male) and 13 control persons without trauma exposure (mean age (SD) 43.62 years (8.17), range 30 – 47 years, 7 female, 6 male). Participants were recruited by newspaper announcement and information days in fire and police departments. The history and current status of mental disorder was obtained from all participants using the German version of the Structured Clinical Interview for DSM-IV (SCID-I and SCID-II) [22,23]. | |
| Participants gave written informed consent and the study was approved by the Ethics Committee of the Medical Faculty Mannheim of Heidelberg University according to the Declaration of Helsinki. All participants had to be free of any psychotropic medication. Pregnant women and participants with severe somatic illness or a mental disorder other than mood or anxiety disorder were excluded. | |
| The current and lifetime diagnosis of PTSD symptom severity of all participants were tested by means of the German version of the Clinician-administered PTSD scale (CAPS) [24]. In contrast to the PTSD patients, the trauma-exposed persons fulfilled the trauma criteria A1 and A2 according to DSM-IV but not those of PTSD [1]. None of the unexposed control subjects fulfilled the A criteria of trauma-exposure. The traumatized groups were asked about peritraumatic fear, helplessness and loss of control on a scale from 0% to 100% to check for trauma severity and months that passed between the trauma and the assessment were recorded. The traumatic event dated back on average 114.92 months (SD=141.88, range 5-434 months) in the PTSD group and traumatic events involved car accidents, physical violence or gun-fights on duty. In the trauma group the mean time since trauma was 46.83 months (SD=39.63, range 6-120 months), which did not significantly differ from the PTSD group (see above) [t(24)=-1.74, p=.10]. Furthermore, on average both groups experienced a similar severity of the traumatic events: they did not differ significantly in experienced peritraumatic fear [t(24)=.69, p=.50], helplessness [t(24)=1.22, p=.24] or loss of control during the trauma [t(24)=-1.20, p=.24]. Finally, five persons of the PTSD group and 1 person of the trauma group were also diagnosed with the current DSM-IV-TR criteria for depression (Table 1). | |
| Table 1: Demographic and clinical characteristics of the three groups. Results of the questionnaire data and interviews. | |
| Questionnaires | |
| All subjects completed the Childhood Trauma Questionnaire (CTQ) [25], the German version of the Center for Epidemiologic Studies Depression Scale (CES-D) [26] and the Posttraumatic Diagnostic Scale (PDS) [27]. While the CTQ is a self-report retrospective inventory intending to measure childhood or adolescent abuse and neglect, the CES-D measures self-reported symptoms associated with depression experienced in the past week. The PDS is a self-report measure recommended for use in clinical or research settings to measure severity of PTSD symptoms related to a single identified traumatic event. This questionnaire was only employed in the trauma and PTSD groups. As expected, compared to the trauma group the PTSD patients reported significantly more posttraumatic distress symptoms in the PDS [t(24)=6.25, p<.001] and in the CAPS with significantly more symptoms [t(24)=12.23, p<.001] and higher symptom subgroup values such as reexperiencing [t(24)=10.06, p<.001], avoidance [t(24)=11.80, p<.001] and hyperarousal [t(24)=7.91, p<.001] (Table 1). | |
| Finally, all three groups differed significantly in the CTQ report of traumatic experiences in their childhood [F(2, 30)=11.76, p<.001]. In addition, the groups differed significantly in the number of depressive symptoms [F(2, 32)=29.77, p<.001]. In both cases, the PTSD group reported the most childhood traumata and the highest levels of depression followed by the trauma group and the control group (Table 1). | |
| Cortisol assessment | |
| We obtained the cortisol day profile of each participant by sampling saliva after awaking in the morning and at several time points during the course of the day. Each participant was provided with eighteen salivette tubes with roll-shaped synthetic swab (Sarstedt, Nümbrecht, Germany) and collected nine samples of salivary cortisol per day: after awakening and 30, 45 and 60 minutes later as well as at 11 a.m., 1 p.m., 3 p.m., 6 p.m. and 8 p.m. The day profiles were obtained at two following days (called A and B). After the first day of unstimulated salivary cortisol sampling (A), a low dose dexamethasone suppression test of 0.5 mg (Jenapharm, Jena, Germany) was carried out in the evening (11:00 p.m.) to investigate responsivity of the HPA axis the second day (B). The participants conducted the cortisol sampling as well as the intake of the dexamethasone pill by themselves at home before coming to the department. The returned salivette tubes were stored at -20ºC and cortisol levels were measured by radioimmunoassay. | |
| The participants were instructed not to smoke while sampling and they kept a diary about cortisol sampling and dexamethasone intake to control for their adherence. Furthermore, we assessed smoking status, intake of oral contraceptives and body mass index (BMI) of the participants and compared them between groups. There was 1 person with intake of oral contraceptives in the trauma group and none in the other groups. Furthermore, there were no significant differences in the BMI or in the number of cigarettes per day. Finally, in an analysis of covariance there was no influence of oral contraceptive intake or generell smoking status on the cortisol data (Table 1). | |
| Blood sample collection | |
| Blood samples were obtained at the Department by venipuncture at 8-9 a.m. into vacuum tubes (Becton Dickinson Vacutainer System Europe, Grenoble, France) and the exact time point of the blood collection was noted. Heparinized peripheral blood for immunophenotyping was processed immediately. The sera for cortisol determination were isolated by centrifugation after clotting of unheparinized blood samples and stored at −70°C until assayed. | |
| Immunophenotyping and intracellular GR determination | |
| Intravenously obtained blood samples were used for surface immunophenotyping and intracellular glucocorticoid receptor measurement. A modified three-colour staining method was used to simultaneously label surface markers of lymphocyte subpopulations and their cytoplasmic GRs. The antibodies consisted of fluoresceinisothiocyanate (FITC) conjugated anti-GR (IgG1, clone no. 5E4-B1), described in Berki and Németh [28] and mouse isotype control antibodies (Becton Dickinson, Heidelberg, Germany); phycoerythrin conjugated anti-CD4, anti-CD19, anti-CD56. | |
| The surface staining was performed by incubating 50 μl of heparinized whole blood with 10 μl of particular MoAbs for 30 min at 4°C temperature in the dark. Cells were washed with the staining buffer and fixed in 100 μl of 4% paraformaldehyde in PBS (fixation buffer 0.1% BSA, 0.1% NaN) for 20 min at 4°C. After one more washing, the erythrocytes were lysed for 20 min with 1 ml of 10×diluted lysing solution (Becton Dickinson) in the dark. Cells were washed again, resuspended in 50 μl of permeabilization buffer (0.1% saponin 10% FCS and 0.1% NaN3 in PBS) containing a predetermined optimal concentration (2.66 μg/ml) of anti-GR MoAb or 5 μl of isotype control, and incubated at 4°C for 20 min in the dark. Two additional washing steps with 350 μl washing buffer took place for removing of unspecific binding antibodies. | |
| We added 10 μl of the phycoerythrin -conjugated antibody (CD4, CD8, CD19 or CD56) to 50 μl heparinized whole blood and incubated it at 4°C in the dark for 30 min. 1 ml of lysis buffer was added and incubation took place for 10 min at 4°C in the dark. Suspension was washed two times with 350 μl cold cell wash, 100 μl cytofix buffer and incubation took place for 20 min at 4°C in the dark. An additional cell washing step (350 μl cell wash) took place. Afterwards 500 μl permeabilization sol. 2, 350 μl perm/wash buffer incl 20 μl 2nd antibody were incubated for 30 min at 4°C in the dark. After another washing step, 350 μl of staining buffer were added and the suspension was transferred in tubes for fluorescence activated cell sorting (FACS) analysis. | |
| The FACS analysis took place on a FACSCalibur flow cytometer (Becton Dickinson) and was consecutively analyzed with the CELLQuest software. At least 10000 events in the light-scatter (FSC/ SSC) lymphocyte region were acquired. The fluorescence intensity of FL-1 (GR) peak was compared by overlaying the histograms of different lymphocyte populations (by gating the FL-2+ cells). Lymphocyte populations were identified and gated on FITC versus phycoerythrin plots. The FITC-fluorescence intensities of GR-labelled lymphocyte populations and isotype controls were displayed and determined as mean channel values on a four-decade log scale in histogram plots. We subtracted the GR mean fluorescence intensity of the isotype antibody control from the GR mean fluorescence intensity obtained from each lymphocyte subpopulation. The instrument calibration was performed daily by FACSComp software using CaliBRITE™ 3 beads. | |
| Data analysis | |
| Statistical analyses were performed with the software program Statistical Package for the Social Sciences 15.0 for Windows (Chicago, Illinois). We checked the cortisol data for outliers and eliminated them by replacing them by group means (rate: 6.01 %). We could not obtain cortisol samples of four persons in the PTSD group, three persons in the trauma group and one (basal cortisol) or two (stimulated cortisol) persons in the control group. For the remaining data we calculated the area under the curve with respect to the ground (AUCG) for the first four salivettes in the morning, for the remaining five salivettes (afternoon) and of all nine salivates of the day profile [29]. Additionally, we calculated the mean increase of the curve at the morning for each group, a method used previously [30]. The cortisol data (time points, mean increase and AUCG), the number of GRs detected on lymphocytes and the questionnaire data of the CTQ and CES-D were averaged per group and groups were compared via an analysis of variance (ANOVA) for the three groups. The same counts for the questionnaire data and the BMI. In addition, in the traumaexposed groups we compared PTSD symptom severity (CAPS), posttraumatic distress (PDS) and time since trauma for both traumaexposed groups by a two-tailed Student t test. Finally, we looked for the respective influence of dimensional categories on GR expression with regression analysis. | |
Results |
|
| Unstimulated cortisol | |
| There were no significant differences in the basal cortisol day profiles the three groups when the area under the curve of unstimulated cortisol (AUCGunstim) of morning A [F(2, 28)=.05, p=.95], mean increase of morning A [F(2, 28)=1.27, p=.30], the area under the curve of stimulated cortisol (AUCGunstim) of afternoon A [F(2, 28)=.16, p=.86] and AUCGunstim of day A [F(2, 28)=.72, p=.49] were compared (Figure 1a). | |
| Figure 1a: Unstimulated diurnal cortisol profiles of the three groups after awakening (left) and from 11 a.m. to 8 p.m. (right): patients with posttraumatic stress disorder (PTSD), trauma-exposed and control subjects, obtained from saliva samples at different time points of the day. | |
| Stimulated cortisol | |
| After stimulation with dexamethasone, the three groups displayed significantly different means in the increase of morning B [F(2, 27)=3.83, p=.03] and in the AUCGstim of afternoon B [F(2, 26)=3.61, p=.04]. In addition, the groups showed a trend for different group means for the AUCGstim of day B [F(2, 23)=2.80, p=.08] (Figure 1b). | |
| Figure 1b: Stimulated diurnal cortisol profiles after administration of dexamethasone obtained from saliva samples of each group at different time points of the day. The groups displayed a significant difference in the mean increases of cortisol in the morning (left) as well as for the area under the curve with respect to the ground in the afternoon (right). | |
| Glucocorticoid expression on lymphocytes | |
| There were no significant differences for the GR number on T helper cells [F(2, 36)=.84, p=.44], T killer cells [F(2, 36)=1.12, p=.34], and the B cells [F(2, 36)=1.29, p=.29], but a trend for different means of the GR number on NK cells [F(2, 36)=2.90, p=.07] (Figure 2). | |
| Figure 2: The number of glucocorticoid receptors (GRs) on lymphocyte subpopulations obtained from the blood of the participants. | |
| A multiple regression analysis model with the number of GR on NK cells as dependent variable (F(4, 16)=5.32, p=.006, R²=.57) included posttraumatic distress (PDS; β=-4.35, t(16)=-3.52, p=.003), time since trauma (β=-.49, t(16)=-3.65, p=.002) and the number of depressive symptoms (CES-D; β=74.94, t(16)=2.25, p=.04) as significant predictors of the number of GR on NK cells. In Addition, there was a trend for the CTQ values to be positively associated with GR expression on NK cells (CTQ; β=5.05, t(16)=1.89, p=.08) (Table 2). | |
| Table 2: Results of the multiple regression analysis: predictors of the glucocorticoid receptor number on natural killer cells. | |
Discussion |
|
| In the present study we examined the diurnal salivary cortisol profile in PTSD patients, trauma-exposed persons and unexposed controls without stimulation and after a dexamethasone suppression test. Also, we investigated GR expression on lymphocyte subsets in these groups. There were no significant differences in the diurnal cortisol profile (AUCGunstim and mean increase) between the three groups. After stimulating the HPA axis with low dose dexamethasone, the three groups differed significantly in the mean increase of cortisol in the morning and in the cortisol level in the afternoon with the trauma group showing the highest increase in the morning and the highest cortisol levels in the afternoon, whereas the PTSD group displayed the lowest levels in both cases. Here, GR responsivity might have increased in response to the extremely challenging experience of the traumatic event in the patients with PTSD [13] confirming results of a meta-analysis that enhanced feedback inhibition seems to be a marker of trauma-exposure per se [11]. But looking at the GR expression on lymphocyte subsets, the three groups only showed a trend for different numbers of GRs on NK cells which have a high density of GR and thus are sensitive to GR changes [20]. This contradicts the idea that enhanced negative feedback inhibition by the HPA axis is linked with higher GR number to adjust for the low cortisol level in PTSD patiesnts [31]. Indeed, several other studies neither found an association of cortisol level and GR expression [5,32] nor increased numbers of receptors in PTSD patients [19]. On the contrary, in the two traumatized groups of the present study GR expression on NK cells highly covaried with a lower level of posttraumatic distress after trauma-exposure. Support for this finding comes from animal studies in which mice overexpressing GR where shown to be more stressresistant [33]. | |
| In addition, there was a strong and significant negative correlation of the GR number on NK cells with the duration since trauma. Although this contradicts recent findings [19] who reported a positive correlation between time since trauma and GR expression on lymphocytes in war veterans with PTSD, these results fit with our former finding that high GR expression on NK cells covaries negatively with posttraumatic distress, because especially in the trauma group posttraumatic symptoms might have been fading with the time since trauma. Furthermore, in the present study the sample of PTSD patients differed in trauma type from the former studies, which investigated exclusively war veterans [18-21]. Here, the PTSD patients (as well as the trauma-exposed controls) were traumatized by different kinds of single events like car accidents, physical violence, or gun-fights on duty that constitute type I traumata. PTSD in war veterans develops from complex forms of traumatization (type II trauma) [34] like being wounded plus witnessing wounding, torture and massacre always containing interpersonal violence and resulting in a higher “dosage” of trauma that enhances the risk of PTSD [35,36]. Furthermore, a higher trauma dose is associated with more severe forms of PTSD [37]. In the study of Vidovic et al. [19] the male combat veterans displayed on average much higher sums in the CAPS interview, especially more avoidance behavior, than our PTSD sample containing men and women who experienced type I trauma. | |
| Finally, GR expression was significantly affected by the number of depressive symptoms in the way that high depression levels were associated with a higher GR expression. This contradicts past findings [5], who did not find a significant correlation between the number of GRs and the depressive state of the PTSD patients (assessed with the Hamilton Depression Scale) but a trend for high urinary cortisol to be related to low GR number on lymphocytes [32]. In patients with severe fatigue and comorbid depression, a higher GR binding on lymphocytes was observed [38]. In line with that, our findings indicate that the level of depression in traumatized persons might have a stronger effect on GR expression after trauma-exposure than trauma per-se. | |
| This study has several limitations. First, because of small sample sizes and some missing cortisol data the power of detecting differences was limited. In addition, five PTSD patients also suffered from major depressive disorder. Depression alone was found to influence the suppression of salivary cortisol post dexamethasone stimulation in opposite directions than in PTSD patients [39]. But when comorbidity of PTSD and depression was investigated like in a recent review [11], comorbid depression had no influence on the main findings, e.g. that trauma-exposure enhances negative feedback inhibition of daily cortisol. In our study, we found a positive association of depression symptoms and GR number on NK cells, but compared to that posttraumatic distress and the time since trauma were much stronger predictors being negatively correlated with the numbers of GRs on NK cells. Nevertheless, because of the crosssectional investigation, it remains unclear whether the observed findings constitute predisposing factors for the development of PTSD or whether they are psychobiological changes resulting from the challenge of the neuronal system during confrontation with the traumatic event. | |
Conclusion |
|
| In sum, our study is the first which combines FACS-techniques for investigating GR expression on lymphocytes with a detailed HPA axis assessment containing basal salivary cortisol as well as salivary cortisol after dexamethasone administration. By comparing the negative feedback inhibition of salivary cortisol release between groups, we replicated findings of enhanced suppression of morning cortisol in PTSD patients. In contrast to former findings, no higher GR expression on lymphocyte subsets was found for the PTSD patients but only for the trauma-exposed controls. Hence, it cannot be assumed that there is a direct link to enhanced negative feedback inhibition observed in PTSD patients. Instead, more complex mechanisms (e. g. via lymphocytic cytokine networks or mineralocorticoid receptor feedback) must be considered in the alterations of HPA axis functioning in subjects who were challenged with traumatic events. | |
Acknowledgments |
|
| This work was supported by grant of the Deutsche Forschungsgemeinschaft to HF, (SFB636/C1). The help of S. Adam is gratefully acknowledged. | |
References |
|
|
|
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi