Review Article, J Spine Neurosurg Vol: 8 Issue: 4
20 Years of Floseal Hemostatic Agent Use in Neurology and Spinal Surgery
Nitin Khanna1, Manuel G Ramirez2* and Mark P Connolly3,4
1Orthopedic Specialists of Northwest Indiana, Clinical Faculty Indiana University School of Medicine, USA
2Baxter Healthcare Corp. Associate Director, Global HEOR Advanced Surgery, USA
3Global Market Access Solutions Sarl, St-Prex, Switzerland
4Unit of Pharmacoepidemiology & Pharmacoeconomics, Department of Pharmacy, University of Groningen, Groningen, The Netherlands
*Corresponding Author: Manuel G Ramirez
Baxter Healthcare Corporation Global HEOR Advanced Surgery, One Baxter Parkway, Deerfield, IL 60015
Tel: +1 224.804.3347
E-mail: manuel_ramirez@baxter.com
Received: October 03, 2019 Accepted: November 08, 2019 Published: November 12, 2019
Citation: Khanna N, Ramirez MG, Connolly MP (2019) 20 Years of FloSeal Hemostatic Agent Use in Neurology and Spinal Surgery. J Spine Neurosurg 8:4.
Abstract
Studies have repeatedly demonstrated the importance of hemostasis in surgery which is essential for promoting positive outcomes and conserving healthcare resources. The need to minimize blood loss is also necessary for achieving good visibility of the operative site and preventing adverse physiological events associated with blood loss. To improve surgical outcomes hemostatic agents, including surgical sealants, have been used to achieve hemostasis in a wide range of specialties including orthopedics, traumatology, urology, neurosurgery, otorhinolaryngology, and gynecology. One of the early commercial products FloSeal for managing hemostasis overcame the limitations of earlier topical hemostatic agents, namely variable efficacy and lack of efficacy in heparinized patients, by combing the use of gelatin-based hemostatic agents (passive hemostasis) with the application of topical thrombin (active hemostasis). In this review we describe the range of prospective randomized clinical studies in spine, and neurological surgery demonstrating the superiority of FloSeal in achieving hemostasis more rapidly and with a higher success rate than other hemostatic agents. We also provide an overview of investigations demonstrating the economic benefits of reduced blood loss both by preventing the need for transfusions and future healthcare resources. In light of the 20 years of clinical use associated with FloSeal, this review provides both historical perspective and context to the role of this product for achieving hemostasis and improving clinical outcomes.
Keywords: Hemostasis; Thrombin; Blood replacement; Spinal surgery; Neurological surgery
Introduction
Hemostasis is of critical importance during all surgical procedures and effective management of bleeding is essential for promoting positive outcomes and conserving healthcare resources [1]. Studies have demonstrated that patients with bleeding-related complications and/or blood product transfusions experienced a longer length of stay compared to patients without bleeding-related complications and blood product transfusions [2]. A fundamental principle of good surgical technique is minimizing blood loss not only as it is necessary for good visibility of the operative site, but also to prevent adverse physiological events associated with blood loss [1]. Lack of successful remediation of bleeding can negatively impact a patient’s outcome and potentiate blood replacement, hemorrhagic shock, and longer hospital stays [1-3].
Topical hemostatic agents have been developed and used to promote hemostasis for over 70 years. Hemostatic agents can be classified as Passive or Active Hemostats based on their mechanism of action (MoA) and ability to effectively achieve hemostasis in different patient coagulation profiles. FloSeal (Baxter Healthcare Corporation, Fremont, CA 94555, USA) is an Active Flowable hemostatic agent (Hemostatic Matrix, bovine-derived Gelatin Matrix, human derived Thrombin Component) that has continuously evolved since its first introduction in 1999, by incorporating surgeons and/or staff needs into its design, improving its preparation process and safety profile. FloSeal is currently approved for use in surgical procedures (other than in ophthalmic) as an adjunct to hemostasis when control of bleeding by ligature or conventional procedures is ineffective or impractical [4].
This review discusses the efficacy, safety, and healthcare costs associated with the use of FloSeal as a hemostatic agent in surgery by using the accumulated knowledge of FloSeal gained over 20 years, with a focus on its use in, spine and neurosurgery. The paper summarizes the findings of comparative studies, with a focus on prospectively designed randomized controlled trials.
Hemostasis–An Overview
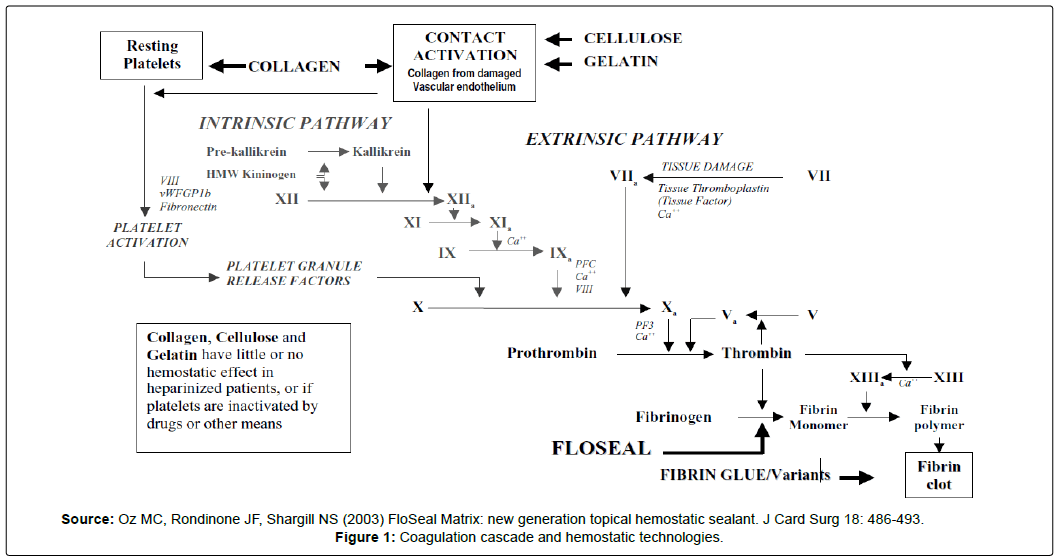
Hemostasis is a complex process requiring the coordinated activation of platelets and plasma clotting factors to form a plateletfibrin clot. Hemostasis can be divided into two distinct processes, primary and secondary hemostasis [5]. Primary hemostasis results in the formation of soft platelet plugs, which are consequently stabilized and cross-linked during secondary hemostasis. Secondary hemostasis is divided into two enzymatic pathways, the intrinsic (contact activation) and extrinsic (tissue factor) (Figure 1) [5]. The extrinsic pathway is activated by tissue damage activating coagulation factor VII, resulting in formation of the tissue-active factor VII complex which promotes the coagulation cascade [6]. In the intrinsic pathway, factor XII is activated through exposure to collagen due to surface damage [6,7]. Activated factor XII subsequently promotes proteolytic activation of clotting factors [6,7]. The extrinsic and intrinsic pathways converge into the common pathway, beginning the with the conversion of Factor X to Xa, and ultimately results in the conversion of prothrombin to thrombin, which is integral in clot stabilization via fibrin [7]. The common pathway is facilitated by Factor V [8].
Blood loss and hemostasis–An overview
Blood loss is an inevitable consequence of almost all surgical procedures. In many surgeries, success often depends on the ability to control bleeding and can influence both short and long-term outcomes [2]. The frequency and amount of blood loss depends on several factors including the type of surgery, medications administered, and the method of hemostasis [2]. Greater blood loss potentiates the likelihood of transfusion needs; blood products expose the patient to the possibility of disease transmission or transfusion reactions [2]. The relationship between surgical blood loss, transfusions, and morbidity and mortality has been consistently demonstrated across a range of different operational settings. One cardiac study reported it was associated with a 70% increase in mortality [9-11]. Significant blood loss also results in greater fluid shifts, which can affect cardiac, pulmonary, and renal status [12].
When surgical bleeding occurs, mechanical methods are used to achieve hemostasis, such as manual compression, compressive medications, ligatures, sutures and clips, as well as monopolar and bipolar cauterization [13]. These systems cause hemostasis to occur through the fusion of collagen and elastin contained in the intima of the vessel without the formation of the thrombin in the proximal vessel [13].
However, these methods may not be sufficient under certain circumstances, such as when the source of bleeding is difficult to identify, or when an inherent or intraoperative coagulopathy is present. Intraoperative bleeding can be induced by a number of factors including hemodilution and hypothermia [14]. Hemostasis can also be compromised by the presence of antiplatelet and anticoagulation agents, for example, heparin, especially in patients undergoing cardiac or vascular surgery [14]. Chronic use of analgesics such as non-steroidal anti-inflammatories, can decrease platelet function if not discontinued a week or two prior to surgery [15]. Herbal or naturalistic supplements, notably ginseng, ginkgo, and vitamin E, among others, have been shown to increase bleeding [13].
Topical hemostatic agents–A history
Topical hemostatic agents, including surgical sealants, have been used to achieve hemostasis in a wide range of specialties including orthopedics, traumatology, urology, neurosurgery, otorhinolaryngology, and gynecology [16,17]. Hemostatic agents include bone wax, absorbable gel sponges, microfibrillar collagen, oxidized regenerated cellulose, gelatin sponges with thrombin, gelatin-thrombin matrix sealant, and fibrin sealants (Table 1) [17].
| Material | Mechanism of Action | What it Needs to Work |
|---|---|---|
| Cellulose Surgicel Oxycel Tabotamp |
Contact activation of clotting cascade, swelling when saturated with blood | Functional clotting cascade and all clotting factors |
| Gelatin Gelfoam Surgifoam |
Contact activation of clotting cascade | Functional clotting cascade and all clotting factors |
| Collagen Avitene Actifoam Helistat Instat |
Contact activation and promotion of platelet aggregation to induce the clotting cascade | Functional clotting cascade and all clotting factors |
| Thrombin Thrombin-JMI |
Direct conversion of fibrinogen to fibrin | Circulating fibrinogen and a means of delivery (customarily used with Gelfoam sponges or powder) for use on active bleeding |
| Fibrin glue Tissucol Tisseel Fibrin Glue Variants Costasis Dynastat |
Generation of a clot is promoted by mixed fibrinogen, thrombin, and factor XIII delivered via a double barrel syringe; also includes aprotinin (to slow fibrinolysis) | – Fibrin glue must be warmed prior to use (20-to-40-minute process) – CoStasis/Dynastat requires patient’s blood to be drawn and centrifuged –Can only apply to a dry, stationary tissue surface |
| Aledhyde glues Bioglue |
Bovine serum albumin and glutaraldehyde cross-link with body proteins to form stable adhesive | –Dry thoracic aortic tissue –Tissue that can withstand exogenous cross-linking |
| Polysaccharide Arista HM |
Particles act as molecular sieve that rapidly dehydrate blood and concentrate clotting proteins, red blood cells, and platelets to promote clot formation | |
| Gelatin-thrombin matrix FloSeal Surgiflo |
Hemostatic effect is a combination of thrombin on a large surface, contact activation of clotting cascade by gelatin and to a lesser extend swelling following contact with blood. | Functional fibrinogen (last protein in the clotting cascade) |
Table 1: Composition and mechanism of action of hemostatic agents. (Source: Adapted from Fiss et al. [47] and Oz [14]).
Topical hemostatic agents are used as adjuncts to the conventional methods of achieving hemostasis, particularly in the case of life-threatening events, abundant bleeding, bleeding near critical anatomical structures, and for patients using anti-aggregant or anticoagulants or with congenital systemic coagulopathies [13,14]. In general, topical hemostatic agents actively or passively promote the activation of coagulation proteins and platelet adhesion [17] (Table 1).
In 1909, Bergel [18] first discussed the use of topical fibrin for hemostasis. Early fibrin sealants combined bovine thrombin with human plasma for topical application [19]. In 1938 purified thrombin became available through advancements in protein separation technology; this resulted in the rapid development of fibrin sealants [20,21]. Thrombin functions as an active hemostat, allowing for cleavage of fibrinogen to form fibrin (Figure 1).
Although, fibrin sealants were one of the first modern hemostatic agents, they were not used widely due to the slow development of the technology and restricted availability due to initial obstacles for FDA approval [7]. This limited availability for use afforded an opportunity for the development of novel hemostatic products, many of which are now common [7]. For example, gelatin-based hemostatic agents, such as Gelfoam, were first introduced in the 1940s, and currently are abundantly used in operating rooms [7]. Gelatin functions as a passive hemostat, inducing hemostasis via platelet activation and mechanical compression. For the most part, gelatin-based hemostatic agents have undergone very little evolution since their introduction; Gelfoam and Surgifoam are still offered in similar preparations as their initial release [7].
These types of products are limited by variable efficacy. Fibrin sealants can diffuse and cause unwanted clotting in other areas, and application of certain gelatin-based agents can negatively impact tissues due to excessive expansion of the gelatin matrix [22]. Moreover, these products often lack efficacy in heparinized patients and patients with adhesions from prior operations [22]. Hence, relatively early on, it was realized that a product that is not easily diffusible and that maintains a hemostatic plug and activates the clotting cascade could potentially overcome some of these problems [22].
FloSeal
A major advancement in the field of gelatin-based hemostatic agents was the development of FloSeal, which was approved for use in the United States in 1999 [7]. FloSeal combines the use of gelatinbased hemostatic agents (passive hemostasis) with the application of topical thrombin (active hemostasis) [7]. The two components of FloSeal act at the beginning and the end of the coagulation cascade to promote physical contact activation of platelets and facilitate fibrin formation [5,13]. The gelatin material conforms to the site’s surface and swells on contact with the blood to tamponade bleeding, while the high thrombin levels increase the rate of clot formation [5,13]. In addition, FloSeal uses a novel crosslinking of the gelatin matrix granules that reduce the in vivo expansion of the product; this property facilitates the application of the product, particularly in minimally invasive surgeries [7,23]. The action of FloSeal is rapid and can stop bleeding within 2 minutes of the site of action [24]. Unlike other hemostatic agents that tend to adhere to surgical gloves and instruments, FloSeal, due to its nature, does not adhere to gloves or instruments [22].
The original FloSeal product, called Fusion-Matrix-DRY or FloSeal-DRY, contained a gelatin matrix component with substantially dry cross-linked gelatin particles. This product required mixing with a thrombin component (bovine thrombin). The newer product, FloSeal VH S/D, was developed to replace the bovine thrombin component with human thrombin, which increased product safety by eliminating the risk of development of antibodies to bovine thrombin and transmission of bovine pathogens.
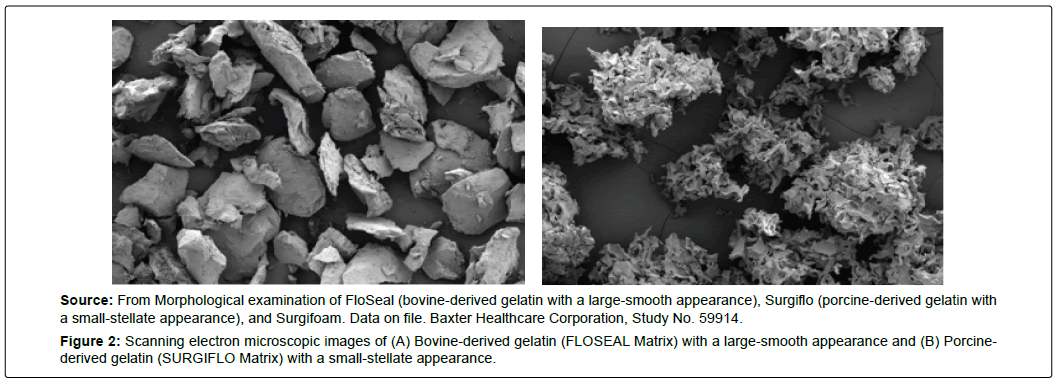
The cross-linked gelatin granules within FloSeal are smooth, distinct round particles of 500 to 600 μm in size [14,25]. The granular nature of the gelatin matrix enables the material to conform to different wound geometries and maintain thrombin on the tissue surface [14]. The smoothness of the FloSeal gelatin is in contrast with the stellate-like shape of gelatin matrices used in other products such as Surgiflo and Surgifoam (Figure 2). The shape and size of FloSeal is thought to better conform to irregular wound surfaces allowing intimate contact of the gelatin granules with the tissue surface at the site of bleeding [24,26]. These properties give FloSeal the advantage that it can be used with various surfaces, such as actively bleeding vascular anastomoses, and in a wide variety of surgical specialties [16,22,23,27-41].
Source: From Morphological examination of FloSeal (bovine-derived gelatin with a large-smooth appearance), Surgiflo (porcine-derived gelatin with a small-stellate appearance), and Surgifoam. Data on file. Baxter Healthcare Corporation, Study No. 59914.
Figure 2: Scanning electron microscopic images of (A) Bovine-derived gelatin (FLOSEAL Matrix) with a large-smooth appearance and (B) Porcinederived gelatin (SURGIFLO Matrix) with a small-stellate appearance.
In addition, the two hemostats demonstrated different in vivo performance, which is likely due to different ultrastructure. The reason for the different ultrastructures was not investigated and is of interest for future investigations. The extraction method can influence the isoionic point and viscosity of the gelatin, which may affect its ultrastructure. However, the likely difference is that porcine-derived gelatin has a higher isoelectric point, lower kinemetic viscosity, and different amino acid composition (i.e., decreased alanine, glycine, isoleucine, hydroxyproline, and increased tyrosine) than bovinederived gelatin. The species differences are likely the cause for the different appearances (i.e. stellate gelatin being porcine-derived and smooth gelatin being bovine derived) and subsequent performance differences.
FloSeal is typically prepared immediately before use and directly applied into the site of bleeding [16]. The average time for FloSeal preparation is about 168 seconds [16]. The granules swell by 10% to 20% upon contact with blood or body fluids, and the maximum swell volume is achieved within 10 minutes [24]. FloSeal is completely absorbed within 6 to 8 weeks of application [24].
Adverse events associated with the use of FloSeal include allergic reactions, perilesional edema, thromboembolism, compression of neural tissue, disseminated intravascular coagulopathy (DIC), factor V antibodies, and transmission of disease from human or bovine sources [17].
Summary of clinical studies of FloSeal
Approval of FloSeal in the US was based on safety and effectiveness findings from a multicenter, multispecialty, prospective, randomized clinical trial [14,22,23,42]. Patients (N=309) undergoing cardiovascular, vascular, or spinal operations were randomized to FloSeal or Gelfoam soaked with thrombin (Gelfoam-Thrombin [control]) following failure of standard surgical means to control bleeding.
Across the entire patient population, FloSeal demonstrated superior efficacy to Gelfoam-Thrombin when used as a topical hemostatic agent [14,22,23,42]. For all patients combined, FloSeal was significantly more effective than the control group in achieving hemostasis at the first lesion at 10 minutes (primary endpoint) (96% vs. 77%; p<0.001) (Table 2) [14,22]. In addition, the time to hemostasis was significantly shorter than control for the first treated site and for all bleeding sites (p values <0.001). At 3 minutes, FloSeal was also significantly more effective than the control at stopping the bleeding (85% vs. 48%; p<0.001). Moreover, FloSeal was effective within each surgical specialty subgroup, as described in more detail below [14,42].
| Surgical Specialty | Treatment Group [1] | Number of Patients | Number of Successes | Percent Successes | p value [2] |
|---|---|---|---|---|---|
| All Patients | FloSeal | 156 | 149 | 96 | <0.001 |
| Control | 153 | 118 | 77 | ||
| Cardiac | FloSeal | 48 | 45 | 94 | <0.001 |
| Control | 45 | 27 | 60 | ||
| Vascular | FloSeal | 43 | 40 | 93 | 0.036 |
| Control | 46 | 35 | 79 | ||
| Spinal | FloSeal | 65 | 64 | 98 | 0.042 |
| Control | 62 | 56 | 90 |
[2] p value from Cochran-Mantel-Haenszel test for raw mean scored, adjusted for investigational site.
Table 2: Treatment success by surgical specialty for first lesion. (Source: Oz et al. [14]).
The number and types of adverse events in the FloSeal and control groups were similar, indicating that FloSeal was as safe as the Gelfoam-Thrombin combination, which at the time of the study had been safely used as a hemostatic agent for >50 years [14,22,23,42].
As part of this registration trial, surgeons were asked three questions regarding product handling: (1) Ease of application, (2) Ability of material to conform to tissue surfaces, and (3) access to difficult-to-reach locations [22]. A greater percentage of surgeons rated FloSeal more favorably than Gelfoam-Thrombin in how well the material conformed to tissue surfaces and ease of delivery (p values ≤ 0.04). Ease of application was rated similarly between FloSeal and control by surgeons performing cardiac surgery, but FloSeal was rated higher by surgeons performing spinal surgery (p value<0.001) [23].
Summary of clinical studies of FloSeal in spinal surgery
As the complexity and number of spinal surgeries have evolved, the awareness of the impact of blood loss on treatment outcomes has increased [13]. Hemostasis in spinal neurosurgery is a delicate and difficult procedure due to the proximity of the Dural sac, the spinal cord and the nerve roots, and the frail nature of their afferent and efferent vascular system [13]. Numerous factors may impact surgical blood loss in spinal surgery including spinal/bone characteristics associated with older age, scoliosis, arthritis, type of surgical procedure [15,43,44]. A major concern regarding the use of use of hemostatic agents in spine surgery is the danger of compression to the neural structures [45]. Of note, FloSeal produces much less compressive effects and has shorter time to biodegradation than the other agents [45].
Analysis of the findings in patients undergoing spinal surgery from the registration study described above indicated that FloSeal was significantly better than Gelfoam-Thrombin in attaining hemostasis [23]. The time to hemostasis for the first bleeding site was significantly shorter for the FloSeal group (1.5 minutes) than the control group (3 minutes). In addition, a greater percentage of subjects treated with FloSeal had bleeding stopped within 10 min compared with control for the first bleeding site (98% vs. 90%, respectively; p=0.042) and for all bleeding sites (99% vs. 93%, p=0.001). Intraoperative blood loss drop in hematocrit, procedural times, and safety were similar between groups. There were no statistically significant differences in the estimated intraoperative blood Loss between the treatment and control groups for any of the procedure classes (p=0.22, ANOVA).
Several other studies have also evaluated the effectiveness of FloSeal in attaining hemostasis in spine surgery. A multicenter, randomized study (N=60) found that the addition of FloSeal to conventional surgical methods for achieving hemostasis, reduced both the intraoperative blood loss and the decrease in hemoglobin concentration postoperatively in adolescents undergoing posterior spinal fusion for idiopathic scoliosis [46].
Two non-randomized studies compared the ability of FloSeal and Surgiflo to achieve hemostasis in spinal surgery [13,26]. Surgiflo is similar to FloSeal in that it is a flowable gelatin with thrombin (Table 1). A prospective non-randomized study (N=149) compared the hemostasis effectiveness of FloSeal and Surgiflo [13]. The study found that, in patients with massive bleeding originating from the epidural venous plexus, FloSeal and Surgiflo stopped bleeding in a similar time (5 min 35 seconds for FloSeal and 5 minutes and 32 seconds for Surgiflo; p>0.05). The two products also had similar hemostatic properties in patients with traumatic or degenerative pathology, and time to hemostasis was comparable in patients with prior antiaggregant and/or anticoagulant therapy. Neither group had hemorrhagic complications observed during follow-up. The study suggests that FloSeal and Surgiflo are alike regarding hemostasis.
An observational study assessed the effectiveness of FloSeal and Surgiflo in the full range of spinal surgery indications, i.e., fusions/ repeat fusions, resections, stabilizations and corpectomies [26]. The study found that, in major spine surgeries, Surgiflo use was associated with significantly higher risk of blood product transfusion (odds ratio [OR]= 2.56; p<0.001), longer surgery time (8.8 min; p<0.0001), and increased product usage (1.9 mL; p<0.001) compared with FloSeal. In severe spine surgeries, Surgiflo was also associated with significantly longer surgery time (26.94 min; p<0.001).
Summary of clinical studies of FloSeal in neurosurgery
Immediate hemostasis is extremely important in brain surgery, as even the smallest amount of blood within the brain parenchyma may lead to permanent neurological damage or even death [47]. In addition, micro-neurosurgical interventions in the brain require a clear field of vision; obstruction of structures via bleeding may result in surgical-related tissue damage [17,47]. Hemostasis is paramount in treatment of brain hemorrhage because re-bleeding and hematoma expansion may occur after surgery; these are associated with poor outcomes [48].
Hemostasis is difficult to achieve in the case of diffuse capillary bleeding or in conditions where the use of cautery may cause vessel damage and thermal injury to adjacent tissues [17]. In addition, many patients have associated disturbances of plasmatic and/or thrombolytic coagulation caused by medications such as acetylsalicylic acid, warfarin, or phenprocoumon. Mechanical methods of hemostasis such as direct pressure and ligation have limited use in brain surgery [47].
A 6-month retrospective study investigated the effectiveness of FloSeal in neurosurgical procedures and estimated its economic value [49]. Information was collected from patients (N=78) undergoing neurosurgical procedures where bleeding was controlled with FloSeal (n=38) or per local bleeding control guidelines (control) (n=40). FloSeal was associated with shorter surgery duration (166 vs. 185 minutes; p=0.0839), lower estimated blood loss (185 vs. 250 mL; p=0.0017), shorter hospital stay (10 vs. 13 days; p<0.001), fewer ICU days (10 days/3 patients vs. 20 days/4 patients), and shorter time to recovery (3 vs. 4 weeks; p=0.0861). FloSeal patients had fewer postoperative complications compared with control, and none required blood transfusions, while 5 units were administered in the control group. The greater cost of FloSeal ($268.40/unit) was offset by the shorter surgery duration, and the economic values of better outcomes including shorter hospital stay, less blood loss/lack of need for transfusion, fewer days in the ICU and fewer complications.
Economic consequences of transfusions and blood loss
The need for blood transfusion due to blood loss is associated with significant risk and high costs. A study by Stokes et al. [2] evaluated the economic burden associated with bleeding related complications and/or transfusions due to surgery, and found that the incremental ICU and total hospital length of stay associated with a complication or transfusion was 2.8 days (3.3 vs. 0.5 days) and 6 days (10.4 vs. 4.4 days), respectively [2]. For each surgery type, patients with bleeding complications/transfusion had longer hospital stay, and higher costs compared with their counterparts with no surgical bleeding complications/transfusion. The incremental cost per hospitalization with bleeding complication/transfusion was 21,406$ for spinal surgery [2].
Health economic and outcomes studies have found that the use of FloSeal in spinal surgery is associated with better clinical outcomes, reduced use of healthcare resources and lower healthcare costs compared with the use of other hemostatic agents, including Surgiflo [26,50-52] (Table 3).
| Surgery type | ICU LoS (days) | Total Hospital LoS | Cost | |
|---|---|---|---|---|
| Spine | Complications | 1.7 | 7.8 | $41,917 |
| No complications | 0.3 | 3.3 | $20,511 | |
| Difference | (1.4) | (4.5) | (21,406) |
Table 3: Cost and length of stay with bleeding complications for spine surgery.
Impact of FloSeal on healthcare costs in spine surgery
Price et al. [26] evaluated comparative surgical outcomes in the full range of spinal surgery indications: fusions/repeat fusions, resections, stabilizations and corpectomies, relevant to flowable hemostatic agent use [26]. They found that in major spine surgeries, FloSeal use was associated with significantly lower risk of blood product transfusion, shorter surgery time and less product usage, and in severe spine surgeries, FloSeal was associated with shorter surgery time [26].
Price et al. also evaluated the clinical outcomes and health utilization between FloSeal and Surgiflo using the Premier Perspective hospital database [26]. Cases were classified as either major spine surgeries (n=12,782 for FloSeal and n=1,531 for Surgiflo) or severe spine surgeries (n=2,837 for FloSeal and n=174 for Surgiflo). For major spine surgeries, FloSeal, compared with Surgiflo, was associated with less blood product use (8.9 mL vs. 10.8 mL, respectively; p<0.0001), shorter surgical times (190 min vs. 199 min; p<0.0001) and risk of blood product transfusions (2.9% vs. 3.1%; p<0.0001). Surgery time was significantly less for severe spine surgeries with FloSeal compared to Surgiflo (266 min vs. 295 min; p<0.001).
A follow up study by Makhija et al. [53] evaluated the costconsequence of using FloSeal versus Surgiflo in major and severe spine surgery. A cost consequence model was constructed from a United States hospital provider perspective using clinical inputs from the published retrospective analysis, with supplemental analyses on annual spine surgery volume using the 2012 National Inpatient Sample database. The base case for a medium volume hospital showed that, compared with Surgiflo, patients receiving FloSeal required three fewer blood product transfusions and saved 27 hours of surgery time, resulting in annual savings of $151 per major surgery, and $574 per severe spine surgery. Probabilistic sensitivity analysis revealed FloSeal was cost saving in 76% of simulations in major spine surgery and 97% of iterations in severe spine surgery. These findings indicate that use of FloSeal instead of Surgiflo to induce hemostasis in both major and severe spine surgery could potentially lead to sizable cost savings in US hospitals (Table 4).
| Parameter | FloSeal | Surgiflo | Difference | Cost Differential |
|---|---|---|---|---|
| Major spine surgery | ||||
| OR surgery time (in hours | 576.33 | 603.59 | 27.26 | $52,122.20 |
| Number of blood product transfusions | 1.87 | 4.80 | 2.92 | $7,202.74 |
| Product acquisition cost | $69,126.08 | $37,212.83 | $31,913.25 | |
| Total (major spine surgery) | $27,411.70 | |||
| Severe spine surgery | ||||
| OR surgery time (in hours) | 261.57 | 287.98 | 26.42 | $50,511.66 |
| Number of blood product transfusions | N/A | N/A | N/A | N/A |
| Product acquisition cost | $29,694.40 | $13,021.70 | $16,672.74 | |
| Total (severe spine surgery) | $33,838.92 |
Note: Costs are in 2016 US dollars
Table 4: Summary of net cost savings-major and severe spine surgery, base case scenario.
FloSeal is often reserved as adjunctive therapy to non-flowable hemostatic agents, such as gelatin-thrombin, when bleeding is not well controlled. Based on the observed clinical benefit of flowable agents, Ramirez et al. [54] investigated whether FloSeal alone may result in better clinical and healthcare resource utilization than as adjunctive therapy [54]. The clinical and healthcare utilization outcomes were compared in a retrospective analysis of spine surgery cases with charges for FloSeal only, or FloSeal adjunct therapy with non-flowable hemostatic agents (i.e., gelatin-thrombin) (F+ G/T), using the United States Premier Hospital Database [54]. In the study, 40,335 spine surgeries, 15,105 FloSeal and F+ G/T matched pairs were compared. The study found significantly lower percentages of FloSeal than F+ G/T cases received intraoperative (1.4% vs. 2.5%), perioperative (1.6% vs. 2.8%), postoperative (1.6% vs 3.0%), and any transfusion (2.3% vs. 4.3%) (p values< 0.0001). In addition, FloSeal cases had significantly less blood loss complications than F+ G/T cases (0.5% vs. 0.8%; p=0.0022) and significantly shorter hospital LOS (-0.45 days), surgery time (‑39.0 min), and used less hemostatic agent (-12.5 mL) (p values<0.0001). These findings indicate that FloSeal use in spine surgery is associated with lower blood transfusion use, blood loss complications, less hemostatic volume, reduced surgery time, and shorter hospital stay compared to its use as adjunctive therapy with non-flowable hemostatic agents [54].
The cost-consequence of using FloSeal compared with F+G/T in minor, major, and severe spinal surgery from the US hospital perspective was also evaluated using the 2012 National Inpatient Sample database [55]. The study found a medium-volume hospital (130 spine surgeries per year) using FloSeal versus F+G/T for spine surgeries is expected to result in 85 less hours of surgery time, 58 fewer hospital days, and 7 fewer blood transfusions in addition to hemostat volume savings (FloSeal: 1 mL, Thrombin: 1,994 mL). The cost savings associated with the hospital resources for a mediumvolume hospital is expected to be $317,959 (surgical hours=$154,746, hospital days=$125,237, blood transfusions=$19,023, hemostatic agents=$18,953), or $2,445 per spine surgery. The results indicate that the use of FloSeal can result in cost savings in US hospitals.
Summary
Bleeding with surgical procedures is associated with increased morbidity, mortality and costs. In addition, blood transfusions during and after surgeries are known to increase morbidity and mortality and have been shown to impose a significant financial burden on the healthcare system. The need for effective hemostatic agents to control perioperative bleeding is only enhanced by the increasing number and complexity of spinal, and neurological surgeries.
The approval of FloSeal 20 years ago, was a major advance in hemostatic agents. FloSeal was the first hemostatic agent to promote coagulation by combining gelatin hemostatic agent with topical thrombin. FloSeal acts at the beginning and end of the coagulation cascade by incorporating platelet activation with promotion of the clotting cascade via thrombin. The smooth round gelatin particles of FloSeal enables the material to conform to irregular wound geometries and is thought to allow better wound penetration than the stellate shaped particles of Surgiflo.
Multiple prospective randomized clinical studies in spine, and neurological surgery demonstrated the superiority of FloSeal in achieving hemostasis more rapidly and with a higher success rate than other hemostatic agents. Although FloSeal and Surgiflo have similar mechanisms of action (MoA), FloSeal is consistently associated with greater effectiveness in achieving hemostasis. FloSeal is also associated with cost-savings compared with Surgiflo due to reduced need for transfusions, reduced hospital stays and fewer complications.
Author Contributions
Nitin Khanna, review materials, writing draft and final manuscript. Manuel G. Ramirez Lopez de Nava, review materials, discussing clinical studies, writing draft and final manuscript. Manuel G. Ramirez Lopez de Nava, literature searching, review materials, writing draft and final manuscript.
Disclosure Statement
Nitin Khanna is a paid advisor to Baxter and received payment for his work on this manuscript.
Manuel G. Ramirez Lopez de Nava is an employee of the company that manufactures FloSeal.
Acknowledgments
The authors would like to thank Therese De Serto-Cohen for her editorial assistance in preparing the manuscript.
Grants
The research performed was sponsored by Baxter Healthcare Corporation.
References
- Foundation A (2013) Management of Surgical Hemostasis, an Independent Study Guide.
- Stokes ME, Ye X, Shah M, Mercaldi K, Reynolds MW, et al. (2011) Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res 11: 135.
- Cho SK, Yi JS, Park MS, Hu G, Zebala LP, et al. (2012) Hemostatic techniques reduce hospital stay following multilevel posterior cervical spine surgery. J Bone Joint Surg Am 94: 1952-1958.
- Abu-Ghanem Y, Dotan Z, Kaver I, Zilberman DE, Ramon J, et al. (2016) The use of Haemostatic Agents does not impact the rate of hemorrhagic complications in patients undergoing partial nephrectomy for renal masses. Sci Rep 6: 32376.
- Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, et al. (2008) The coagulopathy of trauma: a review of mechanisms. J Trauma 65: 748-54.
- Behrens AM, Sikorski MJ, Kofinas P (2014) Hemostatic strategies for traumatic and surgical bleeding. J Biomed Mater Res Part A 102: 4182-4194.
- Sundaram CP, Keenan AC (2010) Evolution of hemostatic agents in surgical practice. Indian J Urol 26: 374-378.
- Franchini M, Lippi G (2010) Factor V Leiden and hemophilia. Thrombosis Research 125: 119-123.
- Engoren MC, Habib RH, Zacharias A, Schwann TA, Samuel J Durham (2002) Effect of blood transfusion on long-term survival after cardiac operation. The Annals of Thoracic Surgery. 74: 1180-1186.
- Rady MY, Ryan T, Starr NJ (1998) Perioperative determinants of morbidity and mortality in elderly patients undergoing cardiac surgery. Crit Care Med 26: 225-235.
- Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, et al. (2007) Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation 116: 2544-2552.
- Popovsky MA, Davenport RD (2001) Transfusion-related acute lung injury: femme fatale? Transfusion 41: 312-315.
- Landi A, Gregori F, Marotta N, Delfini R (2016) Efficacy, Security, and Manageability of Gelified Hemostatic Matrix in Bleeding Control during Thoracic and Lumbar Spine Surgery: FloSeal versus Surgiflo. J Neurol Surg A Cent Eur Neurosurg 77:139-143.
- Oz MC, Rondinone JF, Shargill NS (2003) FloSeal Matrix: new generation topical hemostatic sealant. J Card Surg 18: 486-493.
- Hu SS (2004) Blood loss in adult spinal surgery. Eur Spine J 13(Suppl 1):S3-S5.
- Echave M, Oyaguez I, Casado MA (2014) Use of Floseal(R), a human gelatine-thrombin matrix sealant, in surgery: a systematic review. BMC surgery 14: 111.
- Khanna P, Mahajan C, Gupta P (2015) Use of gelatin-thrombin matrix haemostatic sealant in neurosurgery: Anaesthetic implications and review of literature 2.
- S. B. Uber die wirkung des fibrins (1909) Dtsch Med Wochenschr 35: 663–665.
- Spotnitz WD (2010) Fibrin sealant: past, present, and future: a brief review. World Journal of Surgery 34: 632-634.
- Cronkite EP, Lozner EL, Deaver JM (1944) Use of thrombin and fibrinogen in skin grafting: Preliminary report. Journal of the American Medical Association 124: 976-978.
- Young J, Medawar P (1940) Fibrin suture of peripheral nerves. Lancet 275: 126-132.
- Oz MC, Cosgrove DM, Badduke BR, Hill JD, Flannery MR, et al. (2000) Controlled clinical trial of a novel hemostatic agent in cardiac surgery. The Fusion Matrix Study Group. Ann Thorac Surg 69: 1376-1382.
- Renkens KL Jr, Payner TD, Leipzig TJ, Feuer H, Morone MA, et al. (2001) A multicenter, prospective, randomized trial evaluating a new hemostatic agent for spinal surgery. Spine Aug 26: 1645-1650.
- Chung JP, Leung TY (2017) Uses of FloSeal((c)) in obstetric hemorrhage: Case series and literature review. Taiwan J Obstet Gynecol 56 827-830.
- Lewis KM, Atlee HD, Mannone AJ, Dwyer J, Lin L, et al. (2013) Comparison of two gelatin and thrombin combination hemostats in a porcine liver abrasion model. J Invest Surg 26: 141-148.
- Price JS, Tackett S, Patel V (2015) Observational evaluation of outcomes and resource utilization from hemostatic matrices in spine surgery. J Med Econ 18: 777-86.
- Gazzeri R, Galarza M, Neroni M, Alfieri A, Esposito S (2009) Minimal craniotomy and matrix hemostatic sealant for the treatment of spontaneous supratentorial intracerebral hemorrhage. J Neurosurg May 110: 939-942.
- Gill IS, Ramani AP, Spaliviero M, Xu M, Finelli A, et al. (2005) Improved hemostasis during laparoscopic partial nephrectomy using gelatin matrix thrombin sealant. Urology 65: 463-466.
- Bjorses K, Holst J (2009) Topical haemostatics in renal trauma--an evaluation of four different substances in an experimental setting. J of Trauma 66: 602-11.
- L'Esperance JO, Sung JC, Marguet CG, Maloney ME, Springhart WP, et al. (2005) Controlled survival study of the effects of Tisseel or a combination of FloSeal and Tisseel on major vascular injury and major collecting-system injury during partial nephrectomy in a porcine model. J Endourol 19: 1114-1121.
- Leixnering M, Reichetseder J, Schultz A, Figl M, Wassermann E, et al. (2008) Gelatin thrombin granules for hemostasis in a severe traumatic liver and spleen rupture model in swine. J Trauma 64: 456-61.
- Izzo F, Di Giacomo R, Falco P, Piccirillo M, Iodice R, et al. (2008) Efficacy of a haemostatic matrix for the management of bleeding in patients undergoing liver resection: results from 237 cases. Curr Med Res Opin 24: 1011-1015.
- Angioli R, Muzii L, Montera R, Damiani P, Bellati F, et al. (2009) Feasibility of the use of novel matrix hemostatic sealant (FloSeal) to achieve hemostasis during laparoscopic excision of endometrioma. J Minim Invasive Gynecol 16: 153-156.
- Nasso G, Piancone F, Bonifazi R, Romano V, Visicchio G, et al. (2009) Prospective, randomized clinical trial of the FloSeal matrix sealant in cardiac surgery. Ann Thorac Surg 88: 1520-1526.
- Ellegala DB, Maartens NF, Laws ER Jr (2002) Use of FloSeal hemostatic sealant in transsphenoidal pituitary surgery: technical note. Neurosurgery 51: 513-515.
- Gall RM, Witterick IJ, Shargill NS, Hawke M (2002) Control of bleeding in endoscopic sinus surgery: use of a novel gelatin-based hemostatic agent. J Otolaryngol 31: 271-274.
- Richter F, Schnorr D, Deger S, Trk I, Roigas J, et al. (2003) Improvement of hemostasis in open and laparoscopically performed partial nephrectomy using a gelatin matrix-thrombin tissue sealant (FloSeal). Urology 61: 73-77.
- Triaca V, Zagha RM, Libertino JA (2005) Does thrombin sealant allow nephron-sparing surgery with no renal artery occlusion? A description of technique and initial results. BJU International 95: 1273-1275.
- Wille AH, Tüllmann M, Roigas J, Loening SA, Deger S (2006) Laparoscopic partial nephrectomy in renal cell cancer--results and reproducibility by different surgeons in a high volume laparoscopic center. Eur Urol 49: 337-42.
- Bedi AD, Toms SA, Dehdashti AR (2011) Use of Hemostatic Matrix for Hemostasis of the Cavernous Sinus during Endoscopic Endonasal Pituitary and Suprasellar Tumor. Surgery 21: 189-92.
- Mathiasen RA, Cruz RM (2004) Prospective, randomized, controlled clinical trial of a novel matrix hemostatic sealant in children undergoing adenoidectomy. Otolaryngol Head Neck Surg 131: 601-5.
- Weaver FA, Hood DB, Zatina M, Messina L, Badduke B (2002) Gelatin-thrombin-based hemostatic sealant for intraoperative bleeding in vascular surgery. Ann Vasc Surg 16: 286-293.
- Moller H, Hedlund R (2000) Instrumented and noninstrumented posterolateral fusion in adult spondylolisthesis-a prospective randomized study: part 2. Spine 25: 1716-1721.
- Kuhns CA, Cook CR, Dodam JR, Leach SB, Kuroki K, et al. (2015) Injectable gelatin used as hemostatic agent to stop pedicle bleeding in long deformity surgical procedures: does it embolize? Spine. 40: 218-23.
- Yao HH, Hong MK, Drummond KJ (2013) Haemostasis in neurosurgery: what is the evidence for gelatin-thrombin matrix sealant? J Clin Neurosci 20: 349-356.
- Helenius I, Keskinen H, Syvänen J, Lukkarinen H, Mattila M, et al. (2016) Gelatine matrix with human thrombin decreases blood loss in adolescents undergoing posterior spinal fusion for idiopathic scoliosis: a multicentre, randomised clinical trial. Bone Joint J 98-b(3): 395-401.
- Fiss I, Danne M, Stendel R (2007) Use of gelatin-thrombin matrix hemostatic sealant in cranial neurosurgery. Neurologia medico-chirurgica 47: 462-467.
- Hsieh P-C, Cho D-Y, Lee W-Y (2005) Endoscopic evacuation of putaminal hemorrhage: how to improve the efficiency of hematoma evacuation. Surg Neurol 64: 147-153.
- Esposito F, Cappabianca P, Angileri FF, Cavallo LM, Priola SM, et al. (2016) Gelatin-thrombin hemostatic matrix in neurosurgical procedures: hemostasis effectiveness and economic value of clinical and surgical procedure-related benefits. J Neurosurg Sci 26.
- Tackett SM, Sugarman R, Kreuwel HT, Alvarez P, Nasso G, et al. (2014) Hospital economic impact from hemostatic matrix usage in cardiac surgery. J Med Econ 17: 670-676.
- Tackett SM, Calcaterra D, Magee G, Lattouf OM (2014) Real-world outcomes of hemostatic matrices in cardiac surgery. J Cardiothorac Vasc Anesth 28: 1558-1565.
- Krishnan S, Conner TM, Leslie R, Stemkowski S, Shander A (2009) Choice of hemostatic agent and hospital length of stay in cardiovascular surgery. Seminars in Cardiothoracic and Vascular Anesthesia 13: 225-230.
- Makhija D, Rock M, Ikeme S, Kuntze E, Epstein JD, et al. (2017) Cost-consequence analysis of two different active flowable hemostatic matrices in spine surgery patients. J Med Econ 20: 606-613.
- Ramirez MG, Deutsch H, Khanna N, Cheatem D, Yang D, et al. (2018a) Floseal only versus in combination in spine surgery: a comparative, retrospective hospital database evaluation of clinical and healthcare resource outcomes. Hospital Practice 23:1-8.
- Ramirez MG, Niu X, Epstein J (2018) Cost-consequence analysis of a hemostatic matrix (FLOSEAL) alone or in combination for spine surgery patients. J Med Econ 16: 1-11.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi