Review Article, Jrgm Vol: 13 Issue: 2
A Brain-Eating Amoeba: Naegleria Fowleri In Focus
Dr. Fatima Khurshid1* & Ayesha Khurshid2
1Department of Radiation Oncology, Shifa International Hospital Ltd. Islamabad. Pakistan. Medical Doctor
2Department of Physiotherapy, Mirpur University of Science and Technology. DPT student
*Corresponding Author: Khurshid F
Department of Radiation
Oncology, Shifa International Hospital Ltd. Islamabad. Pakistan
E-mail: fatimakhurshid61@yahoo.com
Received: 07-Feb-2024, Manuscript No. JRGM-24-127164; Editor assigned: 09-Feb-2024, PreQC No. JRGM-24-127164 (PQ); Reviewed: 23-Feb-2024, QC No. JRGM-24-127164; Revised: 27-Feb-2024, Manuscript No. JRGM-24-127164 (R); Published: 5-Mar-2024, DOI:10.4172/2325-9620.1000296
Citation: Khurshid F & Khurshid A. (2024) A Brain-Eating Amoeba: Naegleria Fowleri in Focus. J Regen Med 13:2.
Copyright: © 2024 Khurshid F. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Primary Amoebic Meningoencephalitis (PAM) is a deadly infection caused by the free-living amoeba Naegleria fowleri. This article provides an overview of the epidemiology, pathophysiology, clinical symptoms, diagnosis, therapy, and prevention of PAM. N. fowleri primarily affects children and young adults who are exposed to warm freshwater habitats. The amoeba enters the body via the nasal passages and causes significant harm to the central nervous system. Diagnosis is difficult, and treatment choices are scarce. Preventive measures, such as avoiding watery activities and utilising protective equipment, are critical in lowering the risk of infection. A thorough understanding of N. fowleri and PAM is required for accurate diagnosis, effective treatment, and successful prevention of this deadly disease.
Keywords: Brain Eating Ameoba, Naeglaria Fowleri, primary ameobic Meningoencephalitis, Amphotericin B, Public Health.
Keywords: Brain Eating Ameoba, Naeglaria Fowleri, primary ameobic Meningoencephalitis, Amphotericin B, Public Health.
Introduction
In 1899, a protist pathogen called Naegleria fowleri was identified as the major cause of amoebic meningoencephalitis. The death rate of the infection still exceeds 95% despite improvements in supportive care and antimicrobial treatment. The first case was originally documented in Australia in 1965 by Fowler and Carter. It listed three fatal cases from 1965 and one from 1961. The earliest infections in the United States were discovered in 1937 in Virginia, according to further research using archived autopsy tissue samples. Infections were initially discovered in Florida in 1962 [1, 2].
The genus Naegleria is made up of a collection of Free-Living Ameboflagellates (FLA), which may be discovered in a variety of environments around the globe, such as freshwater lakes, ponds, domestic water sources, swimming pools, thermal pools, soil, and dust. Only Naegleria fowleri has been discovered in humans, and while over 30 different species of Naegleria have been isolated from environmental sources, PAM is caused by this particular species. Children and young adults with a history of swimming and diving in freshwater are more likely to be affected by this swiftly deadly disease that affects the Central Nervous System (CNS). Naegleria fowleri infection rates increase in the summer since this is when the amoeba thrives in warmer climates where temperatures can reach 115°F (46°C).
Over the past five years, several high-profile instances with Naegleria fowleri have attracted a lot of media attention. The public and the major national news organisations closely followed a few of these cases. Unfortunately, a lot of the media coverage did more to spread panic among the populace than it did to educate it [3, 4].
N. fowleri is a category B priority pathogen, the second-highest category of priority organisms/biological agents, according to the National Institute of Allergy and Infectious Diseases (NIAID). High fatality rates, ease of transmission, the potential for widespread panic and social unrest, and the need for particular precautions are all characteristics of category B viruses, which call for urgent public health preparation. It is clear why N. fowleri shouldn’t continue to be an underappreciated organism given its horrific mortality rate, the effects of climate change, and the speed with which it spreads. Utilising a combination of well-known antifungals, antibiotics, and microbicides, the available treatment options are exceedingly limited and poorly defined [5].
Epidemiology
The first case of free-living amoebae, in particular Naegleriafowleri, causing human disease was documented in 1965. The Central Nervous System (CNS) is a tropism for N. fowleri, a pathogenic organism. It comes in three different shapes: the spherical cyst, the pear-shaped motile flagellate form, and the invasive, reproducing trophozoite (ameboid-form). Temperatures between 27°C and 37°C are sufficient to maintain the motile form, while temperatures between 35°C and 46°C are ideal for the trophozoite form. The trophozoite form can return in the presence of favourable conditions, while the cyst form can endure lower temperatures. Trophozoites consume bacteria in their free-living stage, but when they are inside of tissues, they phagocytose red and white blood cells, causing tissue damage. While unfavourable circumstances result in cyst formation, changes in ionic concentration can cause the trophozoites to change into a biflagellate form [6,7].
More than 30 different Naegleria species have been found, but only N. fowleri is known to infect humans. Other species, such N. italica and N. australiensis, have not been identified to infect humans but are capable of infecting experimental animals. Due to the prevalence of these amoebae in warm, stagnant bodies of water and even chlorine swimming pools, contact with people is unavoidable [8,9].
According to the disease’s epidemiology, the majority of primary amoebic meningoencephalitis (PAM) cases were found in nations like Australia, the United States, Great Britain, the Czechoslovak Republic, Thailand, and Mexico. These occurrences often took place in warm, muggy climates throughout the summer. Cases have been documented in both the USA and Mexico in North America, with the majority of illnesses there happening following a swim in naturally warm or geothermal waters. Cases were documented in Venezuela and Brazil in South America, with swimming in untreated or natural water being a common risk factor. Other nations like Cuba, Guadeloupe, Australia, New Zealand, India, China, Japan, Nigeria, Namibia, Madagascar, and Egypt have also reported isolated cases [10,11].
Pathogenesis
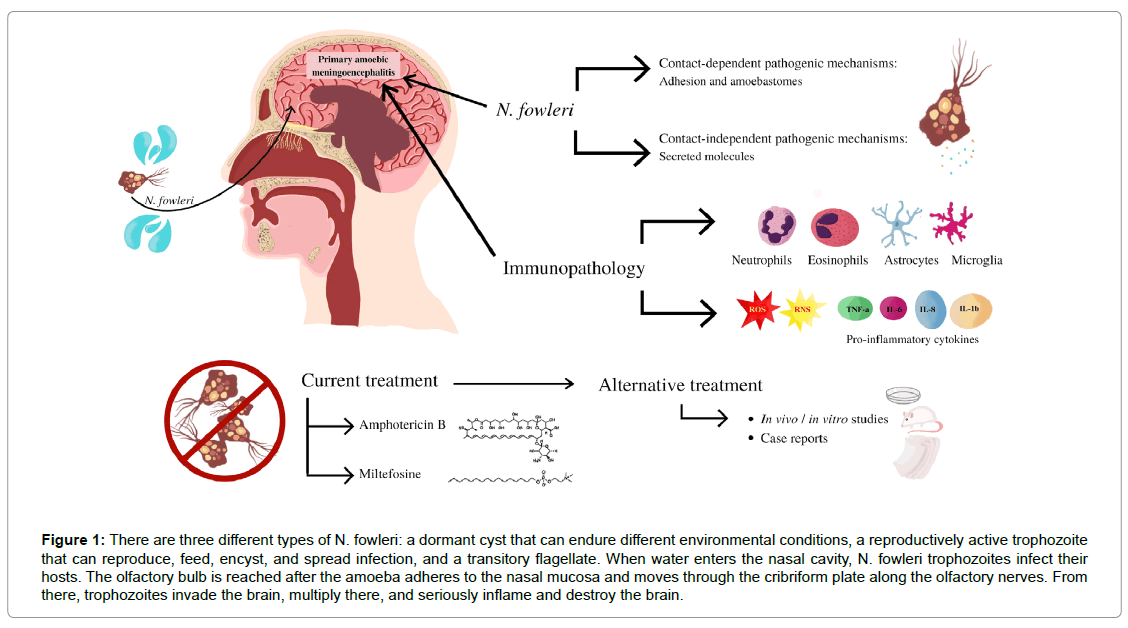
The amphizoic amoeba Naegleria fowleri can live in the human Central Nervous System (CNS), soil, or water in a free-living condition.7 Naegleria fowleri infections have been reported in healthy people, especially in kids and adults who participate in water sports including swimming, diving, and water skiing. The amoeba is thought to enter the human body through the nose when water is forced or splashed into the nasal canal. Naegleria fowleri attaches to the nasal mucosa to start the infection, then moves through the porous cribriform plate and along the olfactory nerve until it reaches the CNS’s olfactory bulbs.8 When Naegleria fowleri enters the olfactory bulbs, the innate immune system, which is activated by macrophages and neutrophils, results in a strong immunological response (Figure 1). The amoeba enters the body in its trophozoite form, where it can devour bacteria, fungus, and human tissue thanks to food cups on its surface 9, 10. Furthermore, Naegleria fowleri pathogenicity is dependent on the release of a variety of cytolytic molecules, including as acid hydrolases, phospholipases, neuraminidases, and phospholipolytic enzymes, which help destroy host cells and neurons.7 Due to the pathogenicity of Naegleria fowleri and the strong immunological reaction it elicits, the CNS suffers substantial nerve injury and subsequent tissue damage that frequently leads in death. The infection advances quickly, producing swelling, necrosis of brain tissue, and the symptoms typical with Primary Amebic Meningoencephalitis (PAM), including a strong headache, a fever of more than 100 degrees, a stiff neck, and altered mental status. Haemorrhage, brain enlargement, and coma may happen in extreme circumstances. The severity and lethality of PAM are attributed to Naegleria fowleri’s capacity to enter the CNS through the nasal passages and its aggressive destruction of brain tissue [12].
Figure 1: There are three different types of N. fowleri: a dormant cyst that can endure different environmental conditions, a reproductively active trophozoite that can reproduce, feed, encyst, and spread infection, and a transitory flagellate. When water enters the nasal cavity, N. fowleri trophozoites infect their hosts. The olfactory bulb is reached after the amoeba adheres to the nasal mucosa and moves through the cribriform plate along the olfactory nerves. From there, trophozoites invade the brain, multiply there, and seriously inflame and destroy the brain.
Clinical Manifestation
Naegleria fowleri infection commonly exhibits symptoms 2 to 8 days after exposure. However, symptoms may appear right away in some circumstances. Severe headache, fever, chills, Brudzinski and Kernig signs that are positive, photophobia, confusion, seizures, and perhaps coma are typical symptoms. Additionally recorded cases include myocardial necrosis and irregular heartbeats. Increases in cerebral spinal fluid pressure and intracranial pressure have been linked to fatalities. Anorexia, nuchal stiffness, lethargy, and vomiting are possible progressions of the clinical signs of Naegleria fowleri infection, which might resemble viral or bacterial meningitis. Because there are no identifiable symptoms, patients often pass away 1 to 2 weeks after exposure, and post-mortem diagnosis is routine. The results of autopsies frequently show enlarged cerebral hemispheres, demyelination of the brain and spinal cord, and trophozoites in multiple organs [10, 13].
Diagnosis
Cerebro Spinal Fluid (CSF) examination can yield important diagnostic data in N. fowleri infections. Due to an increase in red blood cells, abnormalities in CSF colour are seen, ranging from grey in the early stages of the disease to red in the late stages. Up to 26,000 mm3 of polymorphonuclear cells can frequently be seen in abundance. Additionally, trichrome or Giemsa stain can be used to confirm the presence of trophozoites in the CSF. Isolation of trophozoites from either the CSF or brain tissue is required for the diagnosis of an N. fowleriinfection. The diagnosis can be aided by CSF analysis, which includes measurements of glucose and protein concentration. Microscopic analysis of a CSF wet mount may reveal trophozoites that are actively moving and have distinctive features such visible nuclei, cytoplasmic vacuoles, and pseudopodia. Non-nutritional agar can be used as a culture medium to isolate trophozoites [10, 13, 14, 15].
N. fowleri can be found in environmental and clinical samples thanks to recent developments in molecular methods like PCR and real-time PCR. These methods can identify certain genotypes and find N. fowleri DNA in cerebrospinal fluid and brain tissue samples. Some techniques enable prompt identification in a matter of hours. These developments have also aided in the distinguishing of distinct Naegleria species and the detection of N. fowleri in diverse types of brain tissue.
PAM imaging characteristics are frequently non-specific and have not frequently been discussed before. Despite initially seeming normal, CT and MR imaging can eventually reveal signs of brain edoema and basilar meningeal enhancement. Imaging has occasionally shown signs of brain edoema, hydrocephalus, cistern obliteration with increased basilar exudates, and infarction of brain tissue. Although noted, there is little imaging evidence of cerebral infarctions in PAM. Amphotericin B, rifampicin, and miconazole are a few of the medications used as the primary treatment for PAM. Immunofluorescence, immunohistochemistry, or DNA probes are frequently necessary for amoeba detection. However, neither these methods were available nor used on the study’s participants, which is one of the drawbacks [15, 16].
The N. fowleri CNS disease progresses quickly, and quick diagnosis methods make it possible to administer fast therapy, which is crucial for a positive patient result.
Treatment
Due to significant brain damage and inadequate bloodbrain barrier penetration, the mortality rate in primary amoebic meningoencephalitis (PAM) caused by Naegleria fowlerisurpasses 95% with the current treatment with amphotericin B. Other substances that have been tested against N. fowleri have had minimal success. The most extensively used drug is amphotericin B, which is backed by case reports and in vitro research. Additional medications such rifampin, fluconazole, miconazole, miltefosine, and azithromycin have been used with varying degrees of success. The execution of clinical trials to assess treatment modalities has been hampered by the rarity of N. fowleri infections. It is essential to identify substances with blood-brain barrier permeability to increase patient survival and neurologic recovery [16, 17].
The main medication used to treat N. fowleri-caused primary amoebic meningoencephalitis (PAM) is amphotericin B. According to studies, amoeba proliferation can be totally suppressed at a concentration of 0.39 g/ml, whereas 90% of growth can be completely stopped at a concentration of at least 0.1 g/ml. Adults should take amphotericin B at a dose of 0.25 to 1.5 mg/kg every day by intravenous injection. Additionally advised is intrathecal administration. The CDC suggests a course of therapy lasting 10 days intrathecally and 14 days intravenously. Although amphotericin B works, it also has negative side effects, such as kidney damage. Although human studies are required, corifungin, a novel medication with improved solubility, has demonstrated encouraging effects in animal models of PAM. [18, 19, 20].
In 2013, two patients infected with N. fowleri survived after being treated with miltefosine. A phospholipid called meltefosine has the potential to be both an anticancer and an antiparasitic. Its precise mode of action is an inhibitory effect on protein kinase B (PKB or Akt). Miltefosine has demonstrated favourable oral bioavailability in animals (>80%) and widespread tissue distribution, including potential entry into the central nervous system (CNS). Human metabolism studies have not been conducted, however there is little chance of drug-drug interactions. Miltefosine has shown to be effective in vitro against N. fowleri, and it is amoebicidal at doses of at least 55 g. For the treatment of infections caused by free-living amoeba, including those brought on by N. fowleri, the CDC makes miltefosine available under an Investigational New Drug (IND) programme. For a total of 28 days, a dose of 50 mg taken orally twice to three times each day is advised. [7, 21].
Some N. fowleri infections have been treated with fluconazole, an azole antifungal, in addition to amphotericin B. Since it penetrates the Central Nervous System (CNS) more deeply, it offers extra advantages. Fluconazole and amphotericin B work together to eradicate the infection by drawing in neutrophils, which is known as synergy. The CDC advises using intravenous fluconazole once daily for 28 days at a dose of 10 mg/kg. At doses of less than 1 g/ ml, voriconazole, another azole antifungal, has also demonstrated efficacy in killing N. fowleri. [7, 20, 22].
In vitro investigations using azithromycin with a minimum inhibitory concentration (MIC) of 10 g/ml shown its effectiveness against N. fowleri. In vivo trials on mice infected with N. fowleri showed that a daily dose of 75 mg/kg avoided death. The CDC suggests administering azithromycin intravenously for 28 days at a rate of 10 mg/kg/day. Other drugs that have been studied for effectiveness against N. fowleriinclude miconazole, hygromycin, clarithromycin, erythromycin, roxithromycin, chlorpromazine, and rifampin [18, 20, 23].
Rifampin may or may not be effective in treating primary amoebic meningoencephalitis (PAM). Although it has been applied in surviving cases, there are still concerns regarding its capacity to sufficiently penetrate the central nervous system (CNS) at conventional doses. Rifampin concentrations in the CNS have been shown in some studies to be favourable, although other studies indicate large variability. Concentrations may not be enough to completely eradicate N. fowleri, although exceeding the minimum inhibitory concentration (MIC) for susceptible bacteria. Rifamycin, a similar substance, was first shown to have some growth-inhibitory effects on N. fowleri, but these effects gradually faded. Rifampin’s MIC against N. fowlerihas not been clearly demonstrated by later research. As a result, there is no evidence to back up the use of rifampin at recommended dosages to treat PAM. The significant risk of drug-drug interactions with rifampin, especially when taken in combination therapy, is another issue. Rifampin is known to stimulate several enzymes, such as CYP2C9, CYP2C19, and CYP3A4, which can impact how other medicines are metabolised. There have been reports of interactions between rifampin and 14-demethylase inhibitors like fluconazole. These interactions may significantly alter fluconazole’s pharmacokinetics and potentially lessen its efficacy. Since 14-demethylase inhibitors and amphotericin B have been shown to work in concert to treat N. fowleri, adding rifampin to the mix may not be of much use and may even prevent other drugs from exerting their full therapeutic potential [20, 22, 24,25 ].
Prevention
It’s critical to take specific precautions in warm climates to lower the danger of N. fowleri infection. If at all possible, stay away from freshwater areas like lakes, rivers, and ponds. Choose saltwater or chlorinated settings instead because they are safer. Avoid dipping the head or splashing water if engaging in freshwater activities since these actions can prevent N. fowlerifrom entering the nasal passages. Infection risk can be reduced by using nasal clips while engaging in water-related activities. Some people advise cleaning the nose with clean water after swimming in freshwater, however it’s unclear whether this is actually useful. Use of commercially available distilled or purified bottled water is advised for sinus rinsing. The risk of N. fowleri infection can be further decreased by boiling or filtering water with pores of 1 m or smaller. In order to avoid contracting N. fowleri, it is crucial to be aware of and abide by local water safety regulations as well as to inform oneself and others about the dangers of this organism. [20, 23].
Conclusion
Primary Amoebic Meningoencephalitis (PAM), sometimes known as the “brain-eating amoeba,” is an infection that can be fatal and is brought on by the free-living amoeba Naegleriafowleri. Children and young adults who are exposed to warm freshwater, such as polluted lakes, rivers, and swimming pools, are most at risk. The amoeba migrates to the Central Nervous System (CNS) after entering the body through the nose and wreaking havoc there.
N. fowleri is harmful because it can adhere to the nasal mucosa, travel to the CNS via the olfactory nerve, and incite a potent immunological response. The trophozoite forms of N. fowlerifeed on bacteria, fungi, and human tissue, destroying the host’s tissue and producing cytolytic chemicals in the process. Severe headache, fever, chills, confusion, seizures, and coma are just a few of the infection’s symptoms. Diagnosis involves cerebrospinal fluid analysis, MRI imaging, and trophozoitedetection.
Amphotericin B is the major medication used to treat N. fowleri-induced PAM, however this is still a difficult condition to treat. The effectiveness of other drugs varies, including miltefosine, fluconazole, azithromycin, and voriconazole. However, because N. fowleri infections are so uncommon, there aren’t many clinical trials assessing treatment plans. Avoiding freshwater, wearing nose clips when in the water, and cleaning the nose with clean water following exposure are all preventative strategies. The danger of infection is also decreased by boiling or filtering water.
Overall, accurate diagnosis, treatment, and prevention of this terrible disease depend on a thorough understanding of N. fowleri and PAM.
References
- Siddiqui R, Khan NA (2014) Primary Amoebic Meningoencephalitiscaused By Naegleria Fowleri: An Old Enemy Presenting New Challenges. PLoS Negl Trop Dis; 8(8): e3017.
- Pook S (2020). If We Only Had A Brain: Toothless Aquatic Code Allows Deadly, Brain-Eating Zombie Amoeba To Flourish In Arizona Splash Pads And Water Playgrounds. Ariz St Lj; 52: 311.
- Marciano-Cabral F, Cabral GA (2007) The Immune Response to Naegleria Fowleri Amebae And Pathogenesis of Infection. FEMS Immunol Med Microbiol; 51(2): 243-259.
- Grace E, Asbill S, Virga K (2015). Naegleria Fowleri: Pathogenesis, Diagnosis, and Treatment Options. Antimicrob Agents Chemother; 59(11): 6677-6681.
- Tillery L, Barrett K, Goldstein J, Lassner JW, Osterhout B, et al. (2021) Naegleria Fowleri: Protein Structures To Facilitate Drug Discovery For The Deadly, Pathogenic Free-Living Amoeba. PLoS One; 16(3):e0241738.
- Martinez AJ (2019) Free-Living Amebas: Natural History, Prevention, Diagnosis, Pathology, And Treatment Of Disease. Crc Press.
- Barnett ND, Kaplan AM, Hopkin RJ, Saubolle MA, Rudinsky MF (1996). Primary Amoebic Meningoencephalitis With Naegleria Fowleri: Clinical Review. Pediatric Neurology; 15(3): 230-234.
- De Jonckheere JF (2011) Origin and Evolution of the Worldwide Distributed Pathogenic Amoeboflagellate Naegleria Fowleri. Infect Genet Evol; 11(7): 1520-1528.
- Jarolim KL, McCosh JK, Howard MJ, John DT (2000) A Light Microscopy Study Of The Migration Of Naegleria Fowleri From The Nasal Submucosa To The Central Nervous System During The Early Stage Of Primary Amebic Meningoencephalitis In Mice. J Parasitol; 86(1):50-55.
- Visvesvara GS, Moura H, Schuster F (2007) Pathogenic and Opportunistic Free-Living Amoebae: Acanthamoeba spp., Balamuthia Mandrillaris, Naegleria Fowleri, and Sappinia Diploidea. FEMS Immunol Med Microbiol; 50(1): 1-26.
- Craun GF. Microbiology--Waterborne Outbreaks. J Water Pollut Control Fed; 1378-1397.
- John DT, Cole Jr TB, Bruner RA (1985) Amebostomes of Naegleria Fowleri1. J Protozool; 32(1): 12-19.
- Martinez AJ (2019) Free-Living Amebas: Natural History, Prevention, Diagnosis, Pathology, and Treatment Of Disease. Crc Press.
- S Matthews, D Ginzl, D Walsh, K Sherin, J Middaugh, et al. (2008). Primary Amebic Meningoencephalitis--Arizona, Florida, and Texas, 2007. MMWR. Morb Mortal Wkly Rep; 57(21): 573-577.
- Hebbar S, Bairy I, Bhaskaranand N, Upadhyaya S, Sarma MS, et al. (2005) Fatal Case Of Naegleria Fowleri Meningo-Encephalitis In An Infant: Case Report. Ann Trop Paediatr; 25(3): 223-226.
- Kim JH, Lee YJ, Sohn HJ, Song KJ, Kwon D, et al. (2008) Therapeutic Effect Of Rokitamycin In Vitro And On Experimental Meningoencephalitis Due To Naegleriafowleri. Int J Antimicrob Agents; 32(5):411-417.
- Debnath A, Nelson AT, Silva-Olivares A, Shibayama M, Siegel D, et al. (2018) In Vitro Efficacy Of Ebselen And BAY 11-7082 Against Naegleria Fowleri. Front Microbiol; 9: 414.
- Goswick SM, Brenner GM (2003) Activities of Azithromycin and Amphotericin B Against Naegleria Fowleri In Vitro And In A Mouse Model Of Primary Amebic Meningoencephalitis. Antimicrob Agents Chemother; 47(2): 524-528.
- Kim JH, Jung SY, Lee YJ, Song KJ, Kwon D, et al.(2008) Effect of Therapeutic Chemical Agents In Vitro And On Experimental Meningoencephalitis Due To Naegleria Fowleri. Antimicrob Agents Chemother; 52(11): 4010-4016.
- Grace E, Asbill S, Virga K (2015). Naegleria Fowleri: Pathogenesis, Diagnosis, and Treatment Options. Antimicrob Agents Chemother; 59(11): 6677-6681.
- Hannisch W, Hallagan LF (1997) Primary Amebic Meningoencephalitis: A Review of The Clinical Literature. Wilderness Environ Med, 8(4), 211-213.
- Ferrante A (1982). Comparative Sensitivity of Naegleria Fowleri To Amphotericin B And Amphotericin B Methyl Ester. Trans R SocTrop Med Hyg; 76(4): 476-478.
- Stevens AR, Shulman ST, Lansen TA, Cichon MJ, Willaert E (1981) Primary Amoebic Meningoencephalitis: A Report of Two Cases and Antibiotic and Immunologic Studies. J Infect Dis; 143(2): 193-199.
- Debnath A, Tunac JB, Galindo-Gómez S, Silva-Olivares A, Shibayama M, et al. (2012) Corifungin, a New Drug Lead against Naegleria, Identified From a High-Throughput Screen. Antimicrob Agents Chemother; 56(11): 5450-5457.
- Singh P, Kochhar R, Vashishta RK, Khandelwal N, Prabhakar S, et al. (2006) Amebic Meningoencephalitis: Spectrum Of Imaging Findings. AJNR Am J Neuroradiol; 27(6): 1217-1221.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi