Research Article, J Soil Sci Plant Health Vol: 2 Issue: 1
A Combined Condition of Hydroponic Effects on Growth and Cadmium Distribution of Impatiens (Impatiens Walleriana)
Hung-Yu Lai*
Department of Soil and Environmental Sciences, National Chung Hsing University, Taiwan
*Corresponding Author : Hung-Yu Lai
Department of Soil and Environmental Sciences, National Chung Hsing University, 145 Xinda Rd., South Dist., Taichung City 402, Taiwan
Tel: +886-4-22840373 ext.4406
Fax: +886-4-22851969
E-mail: soil.lai@nchu.edu.tw
Received: October 11, 2017 Accepted: November 02, 2017 Published: January 10, 2018
Citation: Lai H (2018) A Combined Condition of Hydroponic Effects on Growth and Cadmium Distribution of Impatiens (Impatiens Walleriana). J Soil Sci Plant Health 2:1.
Abstract
Impatiens can be bred from cuttings, a method which has been shown to lead to a high concentration of cadmium in its shoots. In order to determine the best breeding conditions, a hydroponic experiment was conducted with five different cadmium concentrations, two lighting periods, and two solution intensities. The experimental results show that differences in treatments did not affect growth exhibition significantly. Impatiens grown in 50% solution intensity accumulates significantly higher cadmium concentrations, however, when compared with 100% solution intensity. The maximum cadmium concentrations in the roots and shoots were more than 2600 and 1400 mg/kg, respectively. Changes in the lighting period and the solution intensity affected the subcellular distribution of cadmium but not its chemical form. Cadmium was found to be associated primarily with pectate/protein in most of the treatments, regardless of the conditions. When considering optimum growth exhibition and cadmium fraction in impatiens bred from rooted cuttings, 100%-12HCd20 was shown to be the best hydroponic condition.
Keywords: Cadmium; Chemical form; Impatiens; Phytoextraction; Subcellular distribution
Introduction
Increased industrial and agricultural activities have released cadmium (Cd) into the soil environment, thus leading to contamination [1]. As this situation can lead to contamination in the food chain, there is a risk of Cd affecting human health negatively. Soil washing has been conducted using chemicals in order to decrease the Cd concentration in contaminated soil [2,3]. This technique can affect the population of microorganisms and the activity of enzymes, however, in the soil [4,5]. Phytoremediation is an environmentally friendly technique that uses plants to treat contaminants in the soil and can be utilized in addition to engineering techniques. The benefits of phytoremediation are that it does not affect the soil quality, it can be applied to a large area of contaminated soil, and it is cheaper than other techniques. A longer period of time is needed, however, when using phytoremediation for decontamination purposes. Some hyperaccumulating plants have been reported to accumulate high concentrations of heavy metals [6]. When assessing hyperaccumulation, in addition to determining the concentration in the shoot of a plant, the translocation factor (TF = shoot concentration / root concentration) should also be established.
In previous studies, indigenous plants have been placed in situ at contaminated sites in northern and central Taiwan in order to test the phytoextraction potential of the plants [7,8]. The experimental results have shown that impatiens (Impatiens walleriana) has a high phytoextraction potential for Cd, amongst other contaminants. Lin et al. [9] planted marketable impatiens seedlings in artificially Cdcontaminated soils, finding the TF to be 1.0–1.7. Wei et al. [10] also planted marketable impatiens seedlings in artificially Cd-contaminated soil with a maximum Cd concentration of 80 mg/kg. The shoots of the impatiens plants accumulated 280–1170 mg/kg of Cd, and the TF reached 1.7–2.6. It has also been found, however, that the TF is lower in rooted cuttings of impatiens [11,12].
Compartmentalization plays an important role in the migration and reduction of the toxicity of heavy metals [13,14]. Zhang et al. [15] have shown, for example, that compartmentalization varies between different paddy rice species. They suggest that the Cd accumulated is compartmentalized mainly in the cell wall and the vacuole. Wu et al. [16] have also demonstrated that Cd compartmentalization in the cell wall and the vacuole is important for the tolerance of different barley genotypes. Furthermore, they indicate that the toxicity and migration of Cd are affected not only by the subcellular distribution of the Cd, but also by the chemical form. Among the various chemical forms, water-soluble and ethanol-extractable Cd exhibit higher translocation capacity, compared with other chemical forms [17]. Fu et al. [13] have found that most of the root Cd in Phytolacca americana L. is in an inorganic form and translocates to the plant’s shoots.
The harvesting of impatiens can continue even when the plant is growing on contaminated sites because the shoots of impatiens have a high generating capacity after harvesting. Similarly to many herb plants, impatiens can be bred using cuttings, and the rooted cuttings can be planted in contaminated soils to reduce remediation costs. In this study, in order to obtain suitable rooted cuttings with a high tolerance level for Cd and which could be planted in Cd-contaminated soils, rooted cuttings of impatiens were planted hydroponically in Cd-containing solutions. The Cd concentrations, lighting periods, and solution intensities were varied so that the optimum conditions could be determined. The subcellular distribution and chemical forms of Cd in the various organs of impatiens were analyzed, as was growth exhibition. The objective of this study was to examine growth exhibition, subcellular distribution, and the chemical forms of Cd in order to determine which hydroponic breeding conditions are best for the seedlings.
Methods
This hydroponic experiment was carried out in a phytotron (110 μmol sec–1 m–2, day/night = 30/25°C, relative humidity: 80.7 ± 10.1%) with two lightning periods of 12 h and 20 h (coded as 12H and 20H). All the solutions were prepared following the method described by Yoshida et al. [18] but with solution intensities of 100% and 50% (in the latter, the concentrations of the macroelements were reduced by 50%. The two concentrations were coded as 100% and 50%, respectively). The solutions were spiked artificially with cadmium (CdCl2), with the final concentrations being 0, 5, 10, 20, and 40 μM (coded as Cd0, Cd5, Cd10, Cd20, and Cd40, respectively), and the pH being 5.5. Rooted cuttings of 15-day-old seedlings of similar appearance were planted in these various treatments, with three replicates of each. The solutions were replaced every three days. The plants were harvested after 50 days of growth. At this time, the root length, dry weight, and leaf area were measured for each plant using a LI-COR LI-3000 portable area meter. The chlorophyll content of the leaves was also measured using a Konica Minolta SPAD-502 (Konica Minolta, Tokyo, Japan).
The harvested plants were rinsed with tap water and then divided into roots, stems, and leaves. The roots were then soaked in solutions of 20 mM Na2-EDTA for 15 min to remove adsorbed Cd. With the exception of the fresh tissues required for the analysis of subcellular distribution and chemical form, all the other parts of the plants were oven dried at 65 °C for 72 h before being ground using a grinder, digested with HNO3/HClO4 (v/v = 3/1), quantified with deionized water (DI water) to 25 mL, and then filtered with filter papers (Whatman No. 42). The Cd concentrations in the digestant were determined using a flame atomic absorption spectrophotometer (Perkin Elmer AAnalyst 200).
Subcellular distribution analysis was conducted in accordance with the method used by Lai [11]. Three fractions were utilized: a cell wall fraction (Fcw), an organelle-containing fraction (Foc), and a soluble fraction (Fs). The chemical form of Cd in the different organs of impatiens was analyzed following the approach of Lai [11]. Six chemical forms were extracted: inorganic Cd (FE), water-soluble Cd (FW), pectate- and protein-integrated Cd (FNaCl), undissolved Cd phosphate (FHAc), Cd oxalate (FHCl), and residual Cd (FR).
The concentrations for the three subcellular Cd fractions and the six chemical forms were totaled separately. Recovery rates were calculated according to the ratio between the forgoing sum and the total Cd concentration, and the data were considered valid when the recovery rate was in the 90–110% range. Statistical analysis was performed using SPSS (Statistical Package for Social Science, Armonk, NY, USA). Analysis of variance (ANOVA) was used to test the effect of various treatments on the root length, leaf area, fresh weight, SPAD reading, TF, and Cd accumulation in the impatiens plants. The least significant difference (LSD) test was used to compare the differences between treatments (p = 0.05).
Results
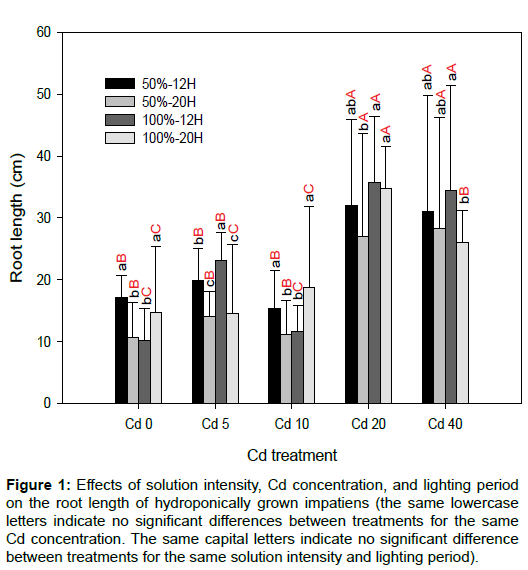
Figure 1 demonstrates the changes to root length under the different solution intensities, Cd concentrations, and lighting periods. It can be seen that, relative to Cd0, the various Cd concentrations do not inhibit the root growth of impatiens. Cuttings of impatiens grown in Cd20 and Cd40 had longer roots, compared with the other Cd treatments. For these two Cd treatments, a longer lighting period (20H) also led to shorter roots, when compared with the shorter lighting period (12H). The root is the first organ that attracts the Cd solution, and the root length was used to assess the response of the plant to various pollutants [19,20]. With the exception of 100%-20H-Cd40, the root length was significantly higher for the impatiens plants grown in Cd20 and Cd40 than for the other Cd treatments (p < 0.05; Figure 1). The experimental results reveal, therefore, that a higher Cd concentration in the solution is beneficial for the elongation of impatiens roots.
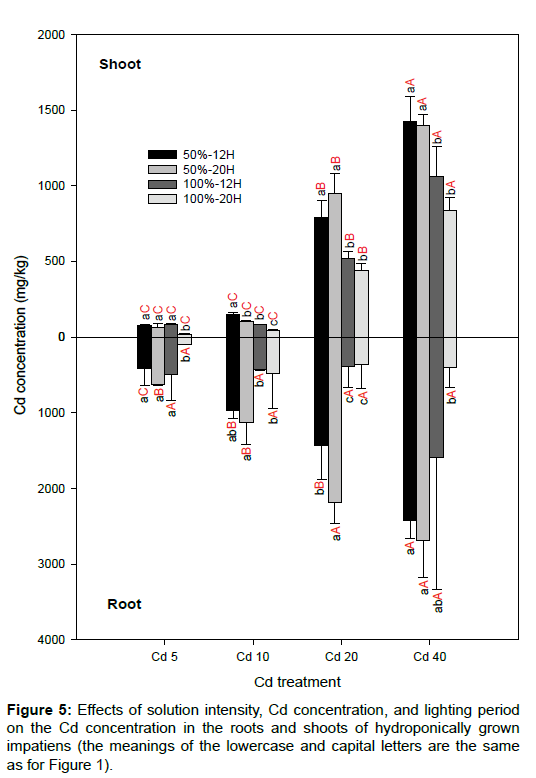
Figure 1: Effects of solution intensity, Cd concentration, and lighting period on the root length of hydroponically grown impatiens (the same lowercase letters indicate no significant differences between treatments for the same Cd concentration. The same capital letters indicate no significant difference between treatments for the same solution intensity and lighting period).
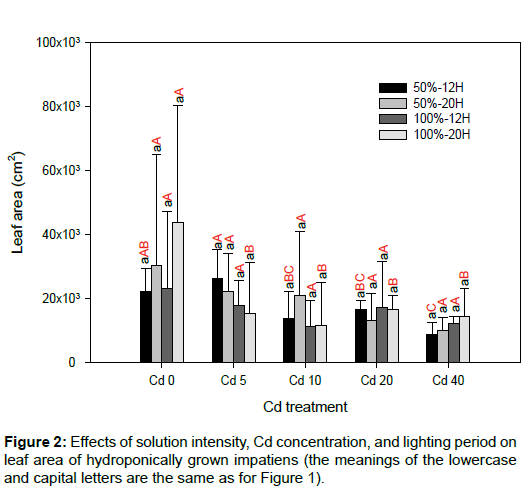
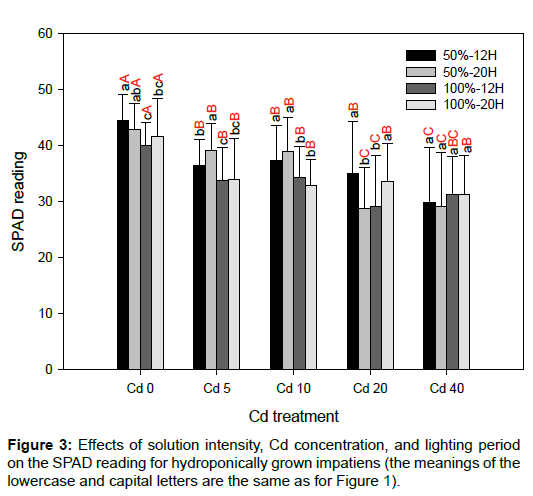
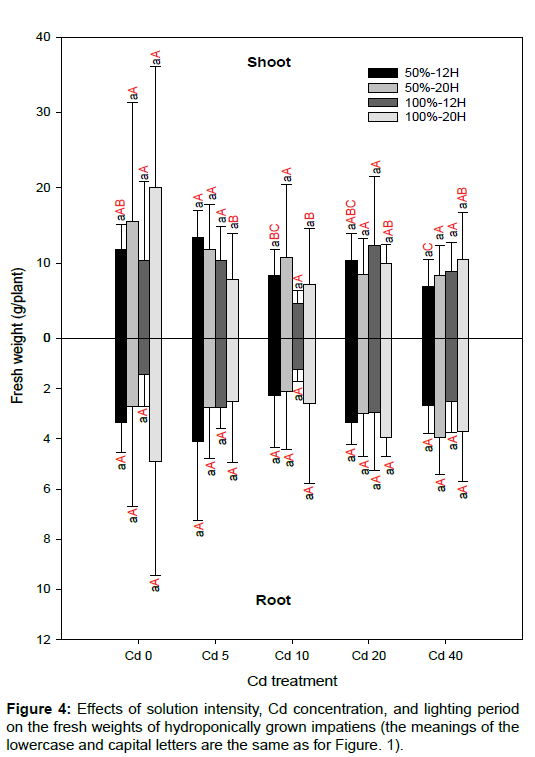
There were no significant differences in leaf area between the various solution intensities and lighting periods for the same Cd concentration (Figure 2). Relative to Cd0, the SPAD readings decreased significantly for the other Cd treatments (p<0.05; Figure 3). This finding is in agreement with previous studies. There was no specific trend regarding the effects of solution intensity and lighting period on SPAD readings. As cuttings of impatiens were used and the initial fresh weight of each cutting was not constant, there were large variations for each Cd treatment. In addition, differences between solution intensities and lighting periods did not affect the fresh weights of roots and shoots significantly (Figure 4). Shi and Cai [21] and Xu et al. [22] have reported that Cd has a negative effect on the leaf area and chlorophyll content in plants. Relative to Cd0, leaf area also decreased significantly for most of the impatiens grown in 50%-12H and 100%-12H (p<0.05; Figure 2). As rooted cuttings of impatiens were used in this study, the Cd treatments did not affect the fresh weights of the shoots significantly, nor did they influence the fresh weights of the roots in most cases. In addition, the different solution intensities and lighting periods had no significant effects on the fresh weights of plants in the same Cd concentration because of the large levels of variation (Figure 4).
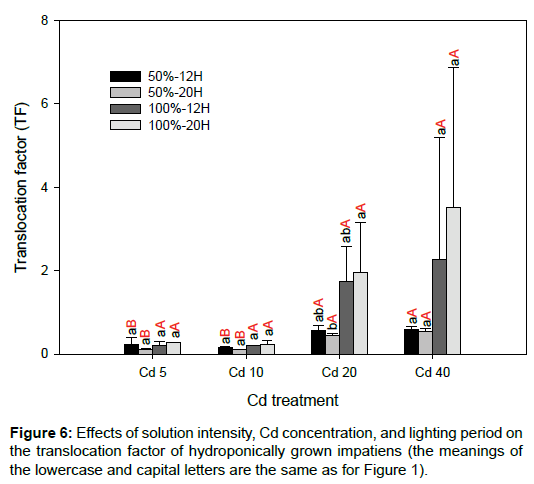
The Cd concentrations accumulated in the roots and shoots of the impatiens plants increased as the solution of the Cd concentrations increased (Figure 5). For most of the treatments, impatiens grown in 50% solution intensity accumulated significantly higher concentrations of Cd than those grown in 100% solution intensity. The maximum Cd concentration in the roots and shoots reached 2686 ± 495 and 1424 ± 166 mg/kg, respectively. Even though the impatiens plants could accumulate high concentrations of Cd in their tissues, most of the Cd was accumulated in the roots. Even though a hydroponic experiment was conducted, the TF was less than unity for other treatments, except in the Cd20 and Cd40 samples with 100% solution intensity (Figure 6). This phenomenon is in agreement with Lai [11], who states that the one drawback of using rooted impatiens cuttings is the lower translocation capacity of Cd from root to shoot. When using marketable seedlings grown in Cd-contaminated soils with total concentration levels of 20 to 80 mg/kg, the TF can reach 1.7–2.6 [10]. In this study, the TF ranged from 1.6 to 3.6 in Cd20 and Cd40 with 100% solution intensity only.
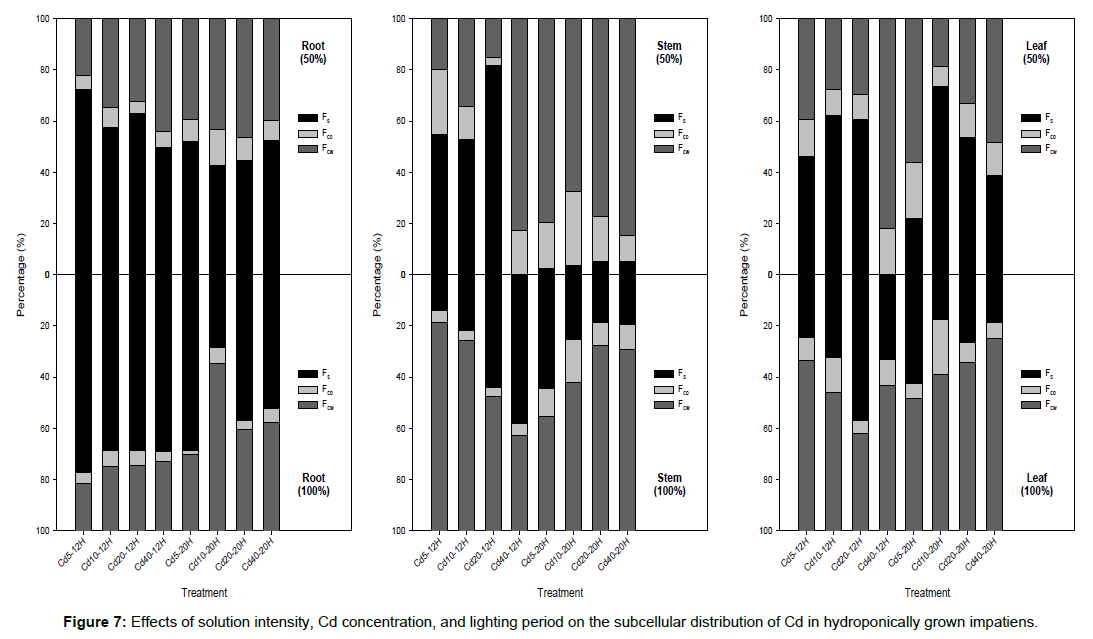
Fs was the main fraction of Cd compartmentalization in the roots for most of the treatments (Figure 7). Except for Cd10-20H-100%, approximately 42–78% of the accumulated Cd in the roots was compartmentalized in Fs regardless of the lighting period, Cd concentration, or solution intensity. For 50%-20H, approximately 40– 85% of the accumulated Cd in the impatiens roots and stems was in Fcw. When the solution intensity was increased to 100%, the accumulated Cd in the roots and stems was primarily in Fcw and accounted for 44- 75% of the total accumulated Cd.
Subcellular distribution is important for the detoxification and tolerance of heavy metals [23-25]. Previous research has revealed that the negative sites provided by the cell wall’s functional groups can bind with heavy metals and thus restrict transportation [26]. Xin et al. [27] found that a low-Cd cultivar of water spinach was able to sequester Cd in the cell wall of roots, thus decreasing its translocation to other organs. In agreement with Su et al. [28] and Lai [11], our results showed that Cd in the roots is accumulated primarily in Fs, regardless of the treatment (Figure 7). Cd in Fs has a higher level of mobility and translocates upward to the shoots by transpiration more easily. In contrast, Cd compartmentalized in Fcw is beneficial for the plant, in terms of decreasing Cd toxicity. This means that phytoextraction plants will have a high percentage of Cd compartmentalized in Fs and Fcw in the root and shoot, respectively. It was found that, in the same Cd solution intensity and concentration, increasing the lighting period increases the percentage of Fcw in the roots (Figure 7). At the same time, the percentage of Fs decreased, where most of the treatments were concerned. A 12H lighting period is better, therefore, than a 20H lighting period, when seeking to increase the phytoextraction capacity of rooted cuttings of impatiens.
With a 12H lighting period and the same Cd concentration, a solution intensity of 100% increased the Cd compartmentalized in the Fs of roots, when compared with the results for 50% solution intensity. The amount of Cd in the Fcw of stems and leaves also increased in the Cd5, Cd10, and Cd20 treatments in these conditions. With a 20H lighting period and the same Cd concentration, a solution intensity of 100% increased the Cd in the Fs of roots and the Fcw of leaves, when compared with the results for 50% solution intensity. The percentage of Cd in the Fcw of stems decreased, however, under these conditions. As Fcw is the tolerance and detoxification mechanism for plants [16,26], a 20H lighting period can thus be deemed unsuitable. The experimental results reveal, therefore, that a solution intensity of 100%, a 12H lighting period, and Cd concentrations of 5 to 20 μM are suitable conditions for the breeding of cuttings suitable for use in the phytoextraction of Cd contaminated soils.
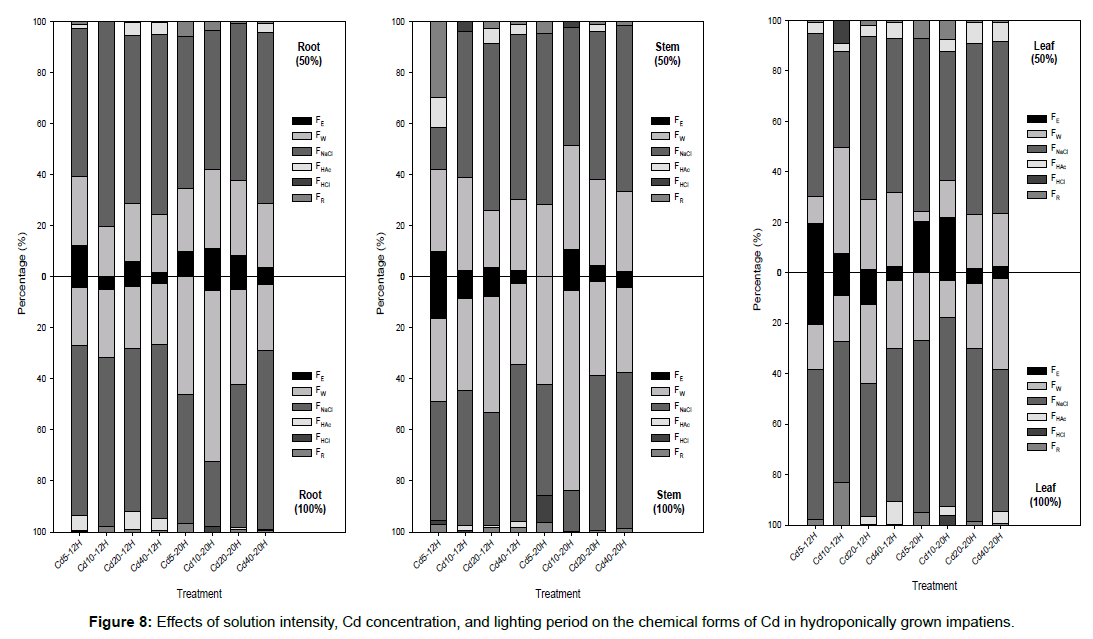
With the exception of Cd10-20H-100%, FNaCl was found to be the primary chemical form of Cd in the roots of the impatiens plants, regardless of the solution intensity, lighting period, or Cd concentration (Figure 8). More than 50% of the accumulated Cd in the roots was compartmentalized in FNaCl. A similar phenomenon has been observed in the leaves of impatiens grown in various treatments. Approximately 38–75% of the total Cd has been found to be compartmentalized in the FNaCl of leaves. Of the six chemical forms, FE and FW have higher mobility and translocate to aerial organs more easily [17]. The Cd in FNaCl can be categorized as an insoluble fraction or a detoxification mechanism [29,30]. It is fixed mainly by proteins or exists as Cd phosphate [14]. The results of previous studies have shown that the majority of Cd is found in FNaCl, with the next largest amount being in FHAc [14,23,30]. He et al. [25] report, however, that the Cd distribution in different chemical forms is dependent on the plant species. For the cuttings of impatiens used in this study, FNaCl was the primary chemical form in different organs, with FW being second (Figure 8). Increasing the solution intensity from 50% to 100% raised the percentage of FE in the stems and leaves of impatiens. When compared with 50%- 20H, treatments of 100%-12H provided better breeding conditions because approximately 44–69% of the total Cd was found in FNaCl in every case.
The subcellular analysis and chemical-form results indicate that a solution intensity of 100% and a lighting period of 12 hours provide the optimum situation for breeding impatiens cuttings with a high tolerance for Cd and high detoxification capabilities. We also used the root length, leaf area, fresh weight of shoots, and SPAD readings, totaling the figures in order to determine the best conditions for growth exhibition in various Cd concentrations. The highest score was found for the Cd20 treatment. Overall, when growth exhibition, tolerance, and detoxification are considered simultaneously, 100%-12H-Cd20 can be considered the best situation for breeding cuttings of impatiens to be planted in the Cd-contaminated soils.
Conclusions
Growth exhibition and Cd fraction for impatiens were used to determine suitable hydroponic conditions for breeding rooted cuttings. The experimental results indicate that 100%-12H-Cd20 provides the optimum conditions. In future studies, these root cuttings must be planted in Cd-conditioned soils in order to examine their phytoextraction capacities.
Acknowledgements
The author would like to thank the Ministry of Science and Technology of the Republic of China for financial support of this research under Contract No. MOST 105-2313-B-005-044.
References
- Kabata-Pendias A, Pendias H (2000) Trace elements in plants, CRC Press, Florida, USA.
- Lai HY, Lin YC, Wang YS, Fu BW (2016) Removal of Cadmium from contaminated soils by multiple washing with iron (III) chloride. Soil Sediment Contam 25: 624-636.
- Makino T, Maejima Y, Akahane I, Kamiya T, Takano H, et al. (2016) A practical soil washing method for use in a Cd-contaminated paddy field, with simple on-site wastewater treatment. Geoderma 270: 3-9.
- Ultra Jr. VU, Yano A, Iwasaki K, Tanaka S, Mei KY, et al. (2005) Influence of chelating agent addition on the copper distribution and microbial activity in soil and copper uptake by brown mustard (Brassica juncea). Soil Sci Plant Nutr 51: 193-202.
- Epelde L, Hernández-Allica J, Becerril JM, Blanco F, Garbisu G (2008) Effects of chelates on plants and soil microbial community: Comparison of EDTA and EDDS for lead phytoextraction. Sci Total Environ 401: 21-28.
- Broadley MR, Willey NJ, Wilkins JC, Baker AJM, Mead A, et al. (2001) Phytogenetic variation in heavy metal accumulation in angiosperms. New Phytol 152: 9-27.
- Iskandar IK, Adriano DC (1997) Remediation of Soils Contaminated with Metals, Science Reviews, North wood, UK.
- Lai HY, Chen ZS (2009) In-situ selection of suitable plants for phytoremediation of multi-metals contaminated sites in central Taiwan. Int J Phytoremediat 11: 235-250.
- Lin CC, Lai HY, Chen ZS (2010) Bioavailability assessment and accumulation by five garden flower species grown in artificially cadmium-contaminated soils. Int J Phytoremediat 12: 454-467.
- Wei JL, Lai HY, Chen ZS (2012) Chelator effects on bioconcentration and translocation of cadmium by hyperaccumulators, Tagetes patula and Impatiens walleriana. Ecotox Environ Safe 84: 173-178.
- Lai HY (2015) Subcellular distribution and chemical forms of cadmium in Impatiens walleriana in relation to its phytoextraction potential. Chemosphere 138: 370-376.
- Lai HY, Lam CM, Wang WZ, Ji YJ (2017) Cadmium uptake by cuttings of Impatiens walleriana in response to different cadmium concentrations and growth periods. Bull Environ Contamin Toxicol 98: 317-322.
- Fu X, Dou C, Chen Y, Chen X, Shi J (2011) Subcellular distribution and chemical forms of cadmium in Phytolacca americana L. J Hazard Mater 186: 103-107.
- Xin J, Zhao XH, Tan QL, Sun XC, Hu CX (2017) Comparison of cadmium adsorption, translocation, subcellular distribution and chemical forms between two radish cultivars (Raphanus sativus L.) Ecotox Environ Safe 145: 258-265
- Zhang J, Sun WC, Li ZJ, Liang YC, Song A (2009) Cadmium gate and tolerance in rice cultivars. Agron 29: 483-490.
- Wu FB, Dong J, Qian QQ, Zhang GP (2005) Subcellular distribution and chemical form of Cd and Ca-Zn interaction in different barley genotypes. Chemosphere 60: 1437-1446.
- Yu H, Xiang ZX, Zhu Y, Wang JL, Yang ZJ, et al. (2012) Subcellular and molecular distribution of cadmium in two rice genotypes with different levels of cadmium accumulation. J Plant Nutr 35: 71-84.
- Yoshida S, Forna DA, Cock JH, Gomez KA (1976) Laboratory manual for physiological studies of rice, International Rice Research Institute, Los Banos, Philippines.
- Liu X, Zhang S, Shan XQ, Christie P (2007) Combined toxicity of cadmium and arsenate to wheat seedlings and plant uptake and antioxidative enzyme responses to cadmium and arsenate co-contamination. Ecotox Environ Safe 68: 305-313.
- Juang KW, Lee YI, Lai HY, Wang CH, Chen BC (2012) Copper accumulation, translocation, and toxic effects in grapevine cuttings. Environ Sci Pollut R 19: 1315-1322.
- Shi G, Cai Q (2009) Leaf plasticity in peanut (Arachis hypogaea L.) in response to heavy metal stress. Environ Exp Bot 6: 112-117.
- Xu D, Chen Z, Sun K, Yan D, Kang M, et al. (2013) Effect of cadmium on the physiological parameters and the subcellular cadmium localization in the potato (Solanum tuberosum L.). Ecotox Environ Safe 97: 147-153.
- Wang JL, Yuan JG, Yang ZY, Huang BF, Zhou YH, et al. (2009) Variation in cadmium accumulation among 30 cultivars and cadmium subcellular distribution in 2 selected cultivars of water spinach (Ipomoea aquatica Forsk). J Agric Food Chem 57: 8942-8949.
- He SY, Wu QL, He ZL (2013) Effect of DA-6 and EDTA alone or in combination on uptake, subcellular distribution and chemical form of Pb in Lolium perenne. Chemosphere 93: 2782-2788.
- He SY, Wu QL, He ZL (2014) Synergetic effects of DA-6/GA3 with EDTA on plant growth, extraction and detoxification of Cd by Lolium perenne. Chemosphere 117: 132-138.
- Gallego SM, Pena LB, Barcia RA, Azpilicueta CE, Iannone MF, et al. (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83: 33-46.
- Xin JL, Huang BF, Yang ZY, Yuan JG, Zhang YD (2013) Comparison of cadmium distribution in different organs of two spinach (Ipomoea aquatica Forsk.) cultivars. Plant Soil 372: 431-444.
- Su Y, Liu JL, Lu ZW, Wang XM, Zhang Z, et al. (2014) Effects of iron deficiency on subcellular distribution and chemical forms of cadmium in peanut in relation to its translocation. Environ Exp Bot 97: 40-48.
- Ding P, Zhuang P, Li Z, Xia HP, Lu HP (2013) Accumulation and detoxification of cadmium by larvae of Predenia litura (Lepidoptera: Noctuidae) feeding on Cd-enriched amaranth leaves. Chemosphere 91: 28-34.
- Mwamba TM, Li L, Gill RA, Islam F, Nawaz A, et al. (2016) Differential subcellular distribution and chemical forms of cadmium and copper in Brassica napus. Ecotox Environ Safe 134: 239-249.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi